Paper Menu >>
Journal Menu >>
 Advances in Bioscience and Biotechnology, 2010, 1, 367-371 ABB doi:10.4236/abb.2010.15049 Published Online December 2010 (http://www.SciRP.org/journal/abb/). Published Online December 2010 in SciRes. http://www.scirp.org/journal/ABB Quantitative determination of residual 2-(2-chloroethoxy) ethanol (CEE) in quetiapine fumarate by gas chr omatogaraphy Pravish Tiwari, Ravi Yadav, Padmakar Sathe, Deepali Gangrade Department of chemistry, Ramnarain Ruia College, Matunga, Mumbai, India. Email: pravishkumar1981@yahoo.com Received 6 July 2010; revised 19 July 2010; accepted 26 July 2010. ABSTRACT A simple and specific gas chromatographic method developed and validated for the determination of 2-(2-chloroethoxy) ethanol in Quetiapine Fumarate. The method is carried out with a flame ionization detector and DB-FFAP capillary column. The linear- ity was established over a range of 40-150 µg ml-1 and correlation coefficient is more than 0.999. Keywords: GC; 2-(2-Chloroethoxy) Ethanol 1. INTRODUCTION Quetiapine is an antipsychotic drug belonging to the group of the dibenzothiazepines and used for the treat- ment of schizophrenia and other psychotic syndromes [1,2]. Quetiapine is used in a form of tablets containing 50, 100, 200 and 300 mg of the active substance.The starting material which is used in the synthesis of Quetiapine fumarate is 2-(2-chloroethoxy) ethanol. 2-(2-chloroethoxy)-ethanol: CEE, structural formula C4H9ClO2; CAS number 628-89-7, boiling point 200℃ at 101.3 kPa. The starting material are often not totally removed by practical manufacturing techniques, and consequently low levels are present in most pharmaceu- ticals. An acceptable level of CEE is unclassified but it is known impurity so was specified with acceptance crite- rion of 0.01% [3]. {Based on NOEL and PDE Value of CEE and Quetiapine}The European Pharmacopoeia (Eur. Ph.) included this guideline in the chapter Residual Sol- vents [4,5] and described a general procedure for identi- fication and control of residual solvents in drug sub- stances. Some problems have been overcome, for in- stance quantitative determination of non-volatile sol- vents such as 2-(2-chloroethoxy)-ethanol (CEE). In the literature there is no information about the methods for determination of CEE in the Quetiapine. In the present study, a gas chromatographic method with direct injec- tion for the determination of CEE in the activ e substance has been develope d. The separation was obtained on a DB-FFAP column (30 m × 0.32 mm i.d. × 1.0 µm coating thickness). 2. EXPERIMENTAL The active substance was synthesized by P recise pharmaceutical Ltd (Pharmaceutical Research Centre, Mumbai, India). Acetonitrile was purchased by J.T baker (USA), Hydrochloride was purchased by S.D. Fine chem. (India), Water was purchased by Lab chem. (India), and 2-(2-chloroethoxy) ethanol was purchased by Sigma Aldrich, (Germany). 2.1. Preparation of Solution Quantitative standard solution of CEE Standard solu- tions was prepared from standard stock solutions. Stan- dard stock solutions were prepared in Diluent {0.2N Hydrochloride in Aceton itrile: Water (70:30 v/v)}. Stan- dard stock solution A: containing 1mg ml-1 of CEE; Standard solution: containing 0.01mg ml-1 of CEE which corresponds to 0.1mg ml-1 of CEE in the tested sub- stance. Qualitative standard solution of CEE for system suitability: Selectivity solution was prepared to check Eur. Ph. system suitability requirements. A total of 7 solvents were included in this standard solution. Selec- tivity solution contained 300 ppm of methanol, 500ppm of Ethanol, 500ppm of Acetone, 500 ppm of Isopropyl alcohol, 89 ppm of Toluene, 88 ppm of N, N-dimethyl formamide, 60 ppm of Dichloromethane and 10 ppm of CEE [6]. Test solution was prepared solution 1 contain- ing 100mg ml-1. A b lank was prep ared using the dilu ent, but without sample or standard solution. 2.2. Instrumentation and Operating Condition The experiments were performed on an Agilent 7890A gas chromatograph (GC) equipped with a CTC combipal autosampler and a flame ionization detect or. A DB -FFAP column (phase composition: Nitroterepthalic acid modi- 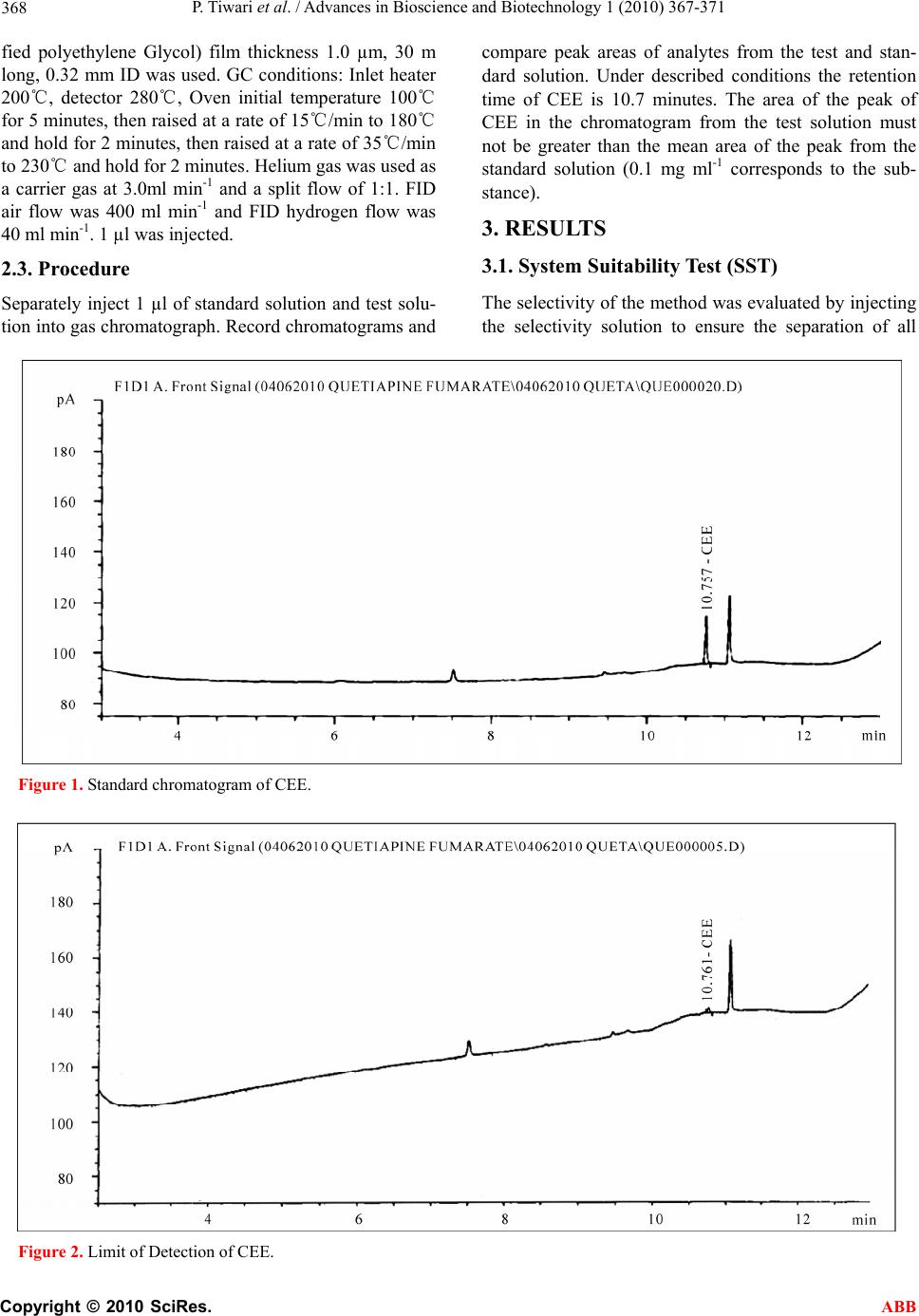 P. Tiwari et al. / Advances in Bioscience and Biotechnology 1 (2010) 367-371 Copyright © 2010 SciRes. ABB 368 fied polyethylene Glycol) film thickness 1.0 µm, 30 m long, 0.32 mm ID was used. GC conditions: Inlet heater 200℃, detector 280℃, Oven initial temperature 100℃ for 5 minutes, then raised at a rate of 15℃/min to 180℃ and hold for 2 minutes, th en raised at a rate of 35℃/min to 230℃ and hold for 2 minutes. Helium gas was used as a carrier gas at 3.0ml min-1 and a split flow of 1:1. FID air flow was 400 ml min-1 and FID hydrogen flow was 40 ml min-1. 1 µl was injected. 2.3. Procedure Separately inject 1 µl of standard solution and test solu- tion into gas chro matograph . Record chromatograms and compare peak areas of analytes from the test and stan- dard solution. Under described conditions the retention time of CEE is 10.7 minutes. The area of the peak of CEE in the chromatogram from the test solution must not be greater than the mean area of the peak from the standard solution (0.1 mg ml-1 corresponds to the sub- stance). 3. RESULTS 3.1. System Suitability Test (SST) The selectivity of the method was evaluated b y injecting the selectivity solution to ensure the separation of all Figure 1. Standard chromatogram of CEE. Figure 2. Limit of Detection of CEE.  P. Tiwari et al. / Advances in Bioscience and Biotechnology 1 (2010) 367-371 Copyright © 2010 SciRes. ABB 369 analytes. The selectivity solution contained: Methanol, Ethanol, Acetone, Dichloromethane, toluene, Isopropyl alcohol, N, N-Dimethyl formamide, CEE. Resolution was calculated directly by the software: Chemstation solution ver. Rev.13.03.02 [341]. Chromatogram of se- lectivity solution is shown in Figure 1; the results are presented in Figure 1 (inset).Good separation was ob- tained between CEE and other solvents used in the syn- thetic route of the active substance. For the drug sub- stance excellent recoveries of 92-95% were obtained at 0.1 mg ml-1 corresponds to the substance. 3.2. Validation of Method for CEE in API Full validation data was required for API as it was in last stage development. 3.3. Limit of detection and limit of Quantification for CEE The LOD and LOQ were calculated form S/N data gen- erated from six injection of CEE (with API) containing Figure 3. Limit of Quantification of CEE. Figure 4. Accuracy at Limit of quantification level.  P. Tiwari et al. / Advances in Bioscience and Biotechnology 1 (2010) 367-371 Copyright © 2010 SciRes. ABB 370 0.1 mg ml-1 with respect to an API sample concentration 100mg ml-1. A LOQ of 0.04mg ml-1 is typical for the CEE with a LOD approximately three times less than LOQ. LOD and LOQ chromatograms are shown in the Figures 2 & 3. 3.4. Recovery of CEE in API The accuracy of the method was evaluated in triplicate at LOQ level in bulk drug sample. The percentage recov- eries were calculated. A satisfactory recovery value of CEE (90-92%) was obtained. At such low levels these recoveries and %RSD were satisfactory. Accuracy at LOQ and STD chromatogram was shown in Figures 4 & 5. 3.5. Linearity of the CEE on Gas Chromatography The linearity of CEE was satisfactorily demonstrated with six point calibration graph between LOQ to 150% of analyte concentrations (LOQ, 50, 75,100,125 & 150). The peak area versus concentration data was performed by least-squares linear regression analysis. The calibra- tion curve was produced by plotting the average of trip- licate CEE injections against the concentration expressed in percentage. Correlatio n coefficient for CEE was 0.99. Linearity of the CEE chromatogram was shown in the Figure 5. Accuracy at Standard level. Figure 6. Linearity at standard level CEE.  P. Tiwari et al. / Advances in Bioscience and Biotechnology 1 (2010) 367-371 Copyright © 2010 SciRes. ABB 371 Figure 6. 4. DISCUSSION AND CONCL USION In this study, a GC analytical method was developed for control of residual 2-(2-chloroethoxy)ethanol (CEE) in the active substance. Sample solvent diluent was se- lected to obtain good selectivity and sensitivity for CEE. The sample dilution factor was adapted to detect unclas- sified solvent CEE at known impurity levels (100 ppm) by FID. This GC method is suitable for its intended purpose. REFERENCES [1] Richelson, E. (1999) J. Clin. Psychiatry, 60(Suppl 10), 5. [2] Caley, C.F. and Rosenbaum, S. (1998) Formulary, 33, 105. [3] Proceedings of International Conference on Harmonisa- tion of Technical Requirements for Registration of Phar- maceuticals for Human Use (ICH), Tripartite harmonised guideline Q3A (R) Impurities in new drug substancesî, 2002. [4] The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use, ICH Topic Q3C (M) Maintenance of Note for guidance on Impurities: Residual solvents (CPMP/ICH/283/95), Per- missible Daily Exposure (PDE) for Tetrahydrofuran and N-Methylpyrrolidoneî. [5] Residual solvents (5.4) (2004) European Pharmacopoeia, Supplement 4.6., Directorate for the Quality of Medi- cines of the Council of Europe, Strasbourg, 4th Edition, 3911. [6] Proceedings of International Conference on Harmoniza- tion of Technical Requirements for Registration of Phar- maceuticals for Human Use (ICH), Tripartite harmonised guideline Q3C Impurities: Residual Solvent’s, 1997. |

