Paper Menu >>
Journal Menu >>
 Advances in Nanoparticles, 2013, 2, 247-258 http://dx.doi.org/10.4236/anp.2013.23035 Published Online August 2013 (http://www.scirp.org/journal/anp) Influence of Modified ZnO Quantum Dots and Nanostructures as New Antibacterials Zahra Fakhroueian1*, Faraz M. Harsini2, Firoozeh Chalabian3, Fa temeh Katouzi an4, Azizollah Shafiekhani5,6, Pegah Esmaeilz adeh7 1School of Chemical Engineering, College of Engineering, University of Tehran, Tehran, Iran 2Department of Biotechnology, School of Chemical Engineering, University of Tehran, Iran 3Department of Biology, Azad University, Tehran, Iran 4Department of Microbiology, Azad University, Tehran, Iran 5Physics Department, Alzahra University, Tehran, Iran 6School of Physics, IPM, Tehran, Iran 7Biomedical Material Department, Institute of Pharmacy, Martin Luther University, Halle-Wittenberg, Germany Email: *fakhroueian@ut.ac.ir Received April 25, 2013; revised May 25, 2013; accepted June 4, 2013 Copyright © 2013 Zahra Fakhroueian et al. This is an open access article distributed under the Creative Commons Attribution Li- cense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Antibacterial activities of various spherical zinc oxide nanoparticles and nano special morphological structures includ- ing quantum dots, nanorod arrays, nanoporous shapes and needle-like crystals had been investigated as new nanomedi- cine compounds. Also antibacterial activity based on minimal inhibitory concentration and the growth inhibitory zone (well method) was evaluated. ZnO nanostructures were fabricated by novel hydrolysis sol-gel-hydrothermal process followed with rapid quenching as new technique using glycerine, vegetable fatty esters such as coconut, sunflower and Lauric alcohol ethoxylated as organic templates soluble in eco-friendly nanofluids. The results showed that Bacillus anthracis and Pseudomonas aerogenes were extremely sensitive to treatment with unique ZnO nanostructured. Their growth inhibitory zone presented 30 mm and 25 mm inhibition zone with better inhibitory effect compared to the Gen- tamicin antibiotic standard. ZnO nanostructures had also been indicated to have a wide range of antibacterial activities against both Gram-positive and Gram-negative bacteria especially more effective on (gr+) species using the growth inhibitory zone. We could design and make significant formulations of fatty acids and esters-capped ZnO quantum dots nanofluids which created high promising agents for controlling Anthrax, Staphylococcus epidermidis and their influ- ences in antimicrobial properties with low cost for future. Keywords: Nanobiotechnology; Antibacterial Activity; Hydrolysis Sol-Gel-Hydrothermal; ZnO Quantum Dots; MIC and Well Method; Complex Defects 1. Introduction Strong luminescence material zinc oxide including hex- agonal wurtzite crystal is a wide band gap (3.37 eV) semiconductor with a large excitation binding energy and an exciton Bohr radius in the range of 1.4 - 3.5 nm [1] and is a commercially important material used in paints, rubbers, concrete, electronics, lasers, transistors, photo- detectors, gas and biosensors, piezoelectric and solar cells, optoelectronics, photocatalysts, cosmetic, biomedi- cine, food industry, anticorrosive coating, antibacterial and antifungal agents. These wide varieties of prominent applications require the fabrication of special morpho- logical and functionalization of ZnO nanostructures sur- face [2-4]. In fact, if we are able to modify the surface of ZnO nanoparticles, the most of their excellent properties will wonderful be obvious. Many efforts have been made to synthesize ZnO with various morphologies, including nanorods, nanowires, nanorings, nanoflowers, nanospheri- cal, nanotubes, nanodisks, nanodumbbells, nanoneedles, nanowhiskers, nanonail, nanobelts, nanosheets, nanosprings, nanoribbon and many more by self-assembly of nano- scaled building blocks [5,6]. To achieve these interesting morphologies there are many different preparing tech- niques for synthesis of ZnO nanostructures such as direct precipitation, spray pyrolysis, microemulsion, the hydro- thermal treatment, sol-gel process using surfactant addi- tive as templates (chemical hydrolysis), wet-chemical *Corresponding autho r . C opyright © 2013 SciRes. ANP  Z. FAKHROUEIAN ET AL. 248 procedure, audible sound method, hydrothermal micro- wave heating, flame spray pyrolysis (FSP), high-tem- perature methods, chemical vapor deposition (CVD), mo- lecular beam epitaxy (MBE) [7], and finally the particu- lar rapid cold or hot annealing quenching sol-gel method (cold and thermal shock) for producing of new quantum dots ZnO nanostructures. Quantum-sized nanodots ZnO represents a unique class of zero-dimensional nanostructures which can generate novel properties that differ from those of their bulk crys- tals due to their small sizes and large surface-to-volume ratios [8]. In the quantum-size region, the absorption of UV or visible light strongly depends on their size, shape, kind of fabrication conditions and techniques, tempera- ture of calcination, annealing condition and aging time, presence of dopants and on PVP (polyvinylpyrrolidone) or green fatty acids coating. Actually, these key factors are able to produce n-type semiconductor containg the measured of direct band gap in quantum-dots ZnO nano- particles. On the other hand, the visible emission is mainly attributed to the surface or structural defects of the crystal, resulting in large variations in the emission peaks [8]. The existence of certain defect complexes such as VZn-Hi, O-H, and Zn-HO has advantages over the pure semiconductor and quantum dots ZnO nanostructures [9] and a wide visible-emission at the deep level defect in the ZnO nanoparticles (NPs) are due to the existence of cationic Zn vacancies (VZn), oxygen vacancies (VO), Zn interstitials (Zni), charge defects, surface defects and their complex defects [10]. Semiconductor quantum dots (QDs) have shown unique optical properties, strong photoluminescence (PL) emis- sion and potential applications in biological fluorescent labels like CdSe and CdTe QDs [11] and ZnO as a non- toxic and cheap luminescent material is a promising can- didate as antibacterial agent and several mechanisms have been proposed for this evidence [12] which is considered to be due to the induction of intercellular reactive oxygen species, including (H2O2) from its surface as a strong oxidizing agent harmful to bacterial cells which can pene- trate into the cell membrane [13,14]. The high rate of gen- eration of surface oxygen species from ZnO leads to the death of the bacteria. There are some reports on the con- siderable antibacterial activity of TiO2, MgO, CaO, SiO2 and ZnO [15] which is attributed to the generation of reactive oxygen species on the surface of these inorganic oxides owing to they contain mineral elements essential to humans and exhibit strong activity even when admin- istered in small amounts. Another possible mechanism for ZnO antibacterial activity is the release of Zn2+ ions. It is well known that ZnO normally becomes unstable in the solution, and when H2O2 is produced, the Zn2+ ion concentration is increased as a result of ZnO decomposi- tion [16]. Quantum dots (QDs) ZnO nanoparticles have shown strong activity against some of Gram- positive and Gram-negative bacteria and biocompatibility with colloidal semiconductor luminescent inorganic materials [17] and a promising member of the Cd-free QD family is ZnO nanoparticles. Functionalization and treat- ment of ZnO quantum Dots with polymers, organosilanes and vegetable fatty acids is able to modify the surface of QDs ZnO. Nanospherical, nanorods, nano-porous ZnO and nanowire morphologies could be used as effective bacte- ricidal materials against both Gram-positive and Gram- negative bacteria. ZnO quantum dot nanoparticles con- taining polymer templates (PVP, PEG, PVA, polystyrene) and oleic acid treatment will be promising candidate as interesting nanodrug-carriers and also new antibacterial agents. ZnO is one of five zinc compounds that are cur- rently listed as generally recognized as safe by the U.S. Food and Drug Administration (21CFR182.8991). An- timicrobial efficacy of zinc oxide quantum dots contain polystyrene and PVP as active antibacterials against some of bacteria was investigated in culture media and liquid egg white [3] and was found that the functionalization and treatment of surface can produce significant results. The availability of ZnO QDs in media was important for antibacterial efficiency. They even have antibacterial ac- tivity against spores that are resistant to high temperature and high pressure and parameters such as size of ZnO nanoparticles, larger the surface area, crystalline struc- ture, particle shape, concentration, time, temperature and combination with other bacteriocins (synergistic effect) will be the focus of further study. However, the antican- cer property of ZnO nanoparticles is an undeveloped area that is of potential medical interest in future [18-22]. In the present study, three various groups of ZnO na- nostructures such as nanoparticles, quantum dots, and modified surface compounds using polymers, vegetable fatty acids, coconut, sunflower esters have been synthe- zied and also ZnO nanoparticles doped silica-substrate was made. We believe that they can generate strong nanoporous surfaces liable to do chemical reactions with live bacteria. Also the types of nanospherical, nanorods and nanoporous ZnO morphologies could be character- ized. It was also found that the size of the quantum dots could be tailored by controlling the process parameters aligned with additional surfactants, stabilizers, suitable solvents or capping agents. Audible sound method and sol-gel hydrothermal microwave process were carried out for synthesis of ZnO nanostructure and antibacterial ac- tivities against both gr+ and gr− bacteria were success- fully investigated by well method and MIC test. Potential applications of ZnO QDs and treatment products were observed for most of both strong microorganisms for the first time. Copyright © 2013 SciRes. ANP  Z. FAKHROUEIAN ET AL. 249 2. Experimental Details All chemicals are analytical grade and are used as re- ceived without further purification. Preparation of ZnO Nanostructures by Sol-Gel Process, Wet-Chemical Procedure, Audible Sound and Hydrothermal Microwave Method Sample 1: 0.03 mole zinc acetate dihydrate Zn (CH3CO2)2·2H2O in 15 - 20 ml isopropanol solution was dissolved (solution A). 10 ml coconut fatty glycerine ester-PEG was added and heated. The resultant cold- mixture containg of isopropanol, glycerine and LA7EO (Lauryl alcohol 7 mole ethoxylate) was prepared (solu- tion B). Solution A was added to solution B under the rapid cold quenching and was hydrolyzed with 20% NaOH solution under vigorous stirring and was kept at 0˚C. After the mixing, they were heated to reflux quickly at 70˚C - 85˚C in the hot bath. The product was washed and filtered and finally dried at 100˚C in oven and sub- sequently calcined at 850˚C. Sample 2: The sample 1 was washed and filtered sev- eral times with H2O/ethanol (1:1 v/v) solution. The ob- tained sol was dried in hot air oven at 90˚C for 48 h fur- ther the white powder was calcined at 700˚C again for 6 h to form ZnO nanocrystals. Sample 3: This product is the same as the sample 1, but we used cold 1, 2-propylene oxide (C3H6O) nonionic surfactant instead of coconut fatty glycerine ester-PEG and 0.6 gr. hydroxy propyl cellulose (J-type) as stabilizer with 2 gr. starch in white powder before of calcination process. Sample 4: Audible sound is produced by sound pres- sure applied to a listener’s ear. The pressure is initiated by some mechanical devices like speakers that create a series of pulses of energy which cause air molecules to vibrate. The sol-gel method was made using of zinc ace- tate dihydrate which hydrolyzed by cold NaOH solution like sample 3, then audible sound method was carried out under the specific frequency of 11,100 Hz and intensity of 115 dB in hot bath at 70˚C - 80˚C during 3 days at sonic condition. This frequency was selected to produce the maximum intensity which is possible for our devices. The audible sound with this condition was performed above the nanoparticles suspension vessel. Sample 5: 0.0095 mole zinc acetate dihydrate merck company was hydrolyzed by 4.5 gr. KOH solution con- taing 200 ml mixture of isopropanol, methanol, ethanol and water. Then the solution of PEG-6000 and 1 gr. PVP (polyvinyl pyrrolidone) was added meanwhile the hy- drolyzing reaction was occurring. The yellow precipita- tion appeared after stirring for 2 h at 70˚C - 85˚C. Then the product was evaporated and dried in oven. Final product dissolved in mixture of hexane and alcohols and was kept at 0˚C until the ZnO QDs were fully precipi- tated and settled. After the removal of the supernatant it was ready to be calcined at 850˚C. The obtained white product was washed with the mixture of alcohols several times and filtered and dried at 85˚C and was annealed at 700˚C again. Sample 6: This product was fabricated the same as sample 5 using of audible sound method including the frequency of 150 Hz and the intensity of 94 dB This spe- cial frequency had been chosen because of its high ability to produce visible vibration at nanoparticles suspension. Finally the suspensions were left for 7 days under sonic exposure. Sample 7: The surface of ZnO nanoproduct was mo- dified by doped silica-substrate using coprecipitation me- thod. The mixture of 0.2 mole zinc acetate dihydrate and 0.8 mole TEOS (tetraethyl orthosilicate) were dissolved in alcohol solution including oleic acid as stabilizer. The pH was adjusted at 10 - 11 with cold NaOH and ethylene diamine solution by drop wise until the solution reaches a suitable pH. After the hydrothermal process at 90˚C for 4 days, the yellow powder precursor became ready to cal- cine at 850˚C. Sample 8: Oleic acid-capped ZnO Q-Dots was fabri- cated by sol-gel method using zinc acetate dihydrate (1.2 mmole) in ethanol, LA3 (Lauryl alcohol 3 mole ethoxy- lated) as surfactant was mixed below 50˚C under vigor- ous stirring. Around the 10 - 20 gr. oleic acid in alcohol was added during the reflux of mixture. The hydrolysis reaction was carried out with TBAH reagent (tetrabu- tylammonium hydroxide) in ice bath at 0˚C. After dis- solving the precipitation, pH value was changed to acidic and supernatant was evaporated at 60˚C, and the soluble product in oleic acid was appeared. Samples 9, 10 and 11: In this synthesis the sol-gel method was carried out for hydrolysis of 0.2 M zinc ace- tate dihydrate in isopropyl alcohol with 0.4 M NaOH solution at pH 10 - 11 in appearance of TEA (three etha- nolamine), EDA (ethylene diamine) and citric acid as template-assisted. The mixture was heated to reflux at 85˚C for 8 h. The white crystalline ZnO nanoparticles appeared after the filtration, washing, drying and calcina- tion at 850˚C. Samples 12, 13 and 14: Sample 12 was fabricated us- ing LA3EO (Lauryl Alcohol 3 moles EO) and nonylphe- nol 10-EO by sol-gel method and further cold quenching. sample 13 was synthesized by sol-gel, hydrothermal pro- cess including PEG6000 and PVP as capped polymer templates by fast cold quenching method and calcined at 850˚C only once. Finally, sample 14 was synthesized by cold sol-gel method in alkaline condition using tetra bu- tyl ammonium hydroxide (TBAH) containing NON9EO (nonylphenol 9 moles ethoxylated) contains 1,2-propy- lene oxide as nonionic surfactant. Copyright © 2013 SciRes. ANP  Z. FAKHROUEIAN ET AL. Copyright © 2013 SciRes. ANP 250 3. Results and Discussion 3.2. XRD Patterns of ZnO Nanostructures (Samples 2, 5 and 11) 3.1. SEM Pictures of QDs and ZnO Nanoparticles According to the Symbol Samples The XRD patterns of the three products demonstrate that all of the diffraction peaks can be indexed as typical hexagonal phase of ZnO for samples 2 and 5 including space group p63 mc, but the sample 11 showed Wurtzite structure with hexagonal phase according to lattice con- stant of a = b = 3.249 Å and c = 5.206 Å with JCPDS card no. 36 - 1451. These peaks at scattering angles (2θ) correspond to the reflection from: (100), (002), (101), (102), (110), (103), (200) and finally (112) crystal planes respectively. Figure 7 shows XRD patterns of ZnO na- noparticles for nanorods of samples 2 and 5 and nano- spherical for sample 11 [2,7,23-25]. For sample 1 in Figur e 1. For sample 2 in Fi gu re 2. For samples (a) extended nanoparticles of 3 and (b) nanosphericals 4 in Fig ure 3. For nanorods as nanowhiskers or nanoflowers ZnO QDs samples 5 and 13 in Figure 4. For samples (a) ZnO nanoparticles of sample 6 and (b) nanoparticles of sample 7 in Figure 5. For samples (a) ZnO nanorods which are dispersed in oleic acid for sample 8, (b) sample 10, (c) very homoge- neous nanospherical of sample 11 and (d) ZnO QDs of sample 12 in Figure 6. FTIR Spectroscopy of Three ZnO Nanostructures FTIR spectrum of ZnO in KBr matrix showed a broad Nanorods Figure 1. SEM images of QDs ZnO nanorods for sample 1. Nanorods Figure 2. SEM images of QDs ZnO nanorods for sample 2. (a) (b) Figure 3. SEM images of QDs ZnO nanoparticles for (a) sample 3 and (b) sample 4.  Z. FAKHROUEIAN ET AL. 251 (a) (b) Figure 4. SEM images of nice formation of QDs ZnO nanorods for (a) sample 5 and (b) nanorods-nanoflowers mixture sam- ple 13. (a) (b) Figure 5. SEM images of two ZnO nanoparticles for (a) homogeneous of sample 6 and (b) sample 7. (a) (b) (c) (d) Figure 6. SEM images of various ZnO nanostructures for (a) ZnO QDs of sample 8; (b) sample 10; (c) sample 11 and (d) ZnO QDs as nanorods (nanotubes) for sample 12. band with very low intensity at 3326.83 cm−1 corre- sponding to the vibration mode of water –OH group, the band at 1653.19 cm−1 is due to the OH bending of water. A strong band at 689 cm−1 is attributed to the Zn-O stretching band which is indicated in Figure 8. Two sharp peaks at 920 and 956 cm−1 showed OH twisting vibrations and lattice Zn-HO for samples 2 and 5. These sharp peaks are related to substitutional hydrogen at oxygen site HO bond to the lattice Zn site (Zn-HO) [9]. The strong peak at 1374 cm−1 is also visible, indicating that -COO groups are not completely removed in sample 11 [26]. The low frequency at 600 - 800 cm−1 is attrib- uted to hydride Zn-H bending modes for sample 11 (ZnO citric acid). Copyright © 2013 SciRes. ANP  Z. FAKHROUEIAN ET AL. 252 Figure 7. XRD patterns of three ZnO nanoparticles for nanorods of samples 2 and 5 and nanospherical of sample 11. Figure 8. FTIR spectra of three ZnO nanoparticles for nanorods of samples 5 and 2 and nanospherical of sample 11 respectively. 3.3. UV-Vis Absorbance Spectrum for Several of ZnO Nanoparticles Optical absorption studies of the prepared crystalline se- ries of new nanoparticles colloids were carried out as very intense peaks and expanded portion of them at in- terval of 350 - 372 nm and their unique trapping states at 400 - 700 nm which are usually attributed to the point defects similar to complex or structural defects for in- stance of singly ionized oxygen vacancies, zinc vacan- cies, and surface defects in especial structures of QDs- ZnO (Figure 9) [8,23,27]. It seems that surface trapping states were occupied by positively charged oxygen vacancy defects during high annealing temperatures. Therefore, both absorption spec- tra and emission shows a distinct blue shift under reac- tion conditions (increase of calcination temperatures dur- ing prolonged aging time) [28]. Figure 10 was shown the typical analysis and feature enlargement of UV-Vis ab- sorption spectrum for four QDs ZnO NPs at limited wa- velength of 635 - 665 nm individually. In such circum- stance the excitation of electrons from valance band to conduction band happened through these trapping states. 3.4. Room Temperature Photoluminescence Including Distinct Broad and Deep Level Blue-Shift Spectrum Figures 11 and 12 show photoluminescence spectra and unique ZnO QDTs nanostructures area. Figure 9. Optical absorption spectra of various QDs ZnO nanorods (samples 5, 13, 2, 12) and nanosphe ricals (sample s 3, 11). Figure 10. Expansion and analysis of UV-Vis absorption spectra for some QDs ZnO NPs. Copyright © 2013 SciRes. ANP 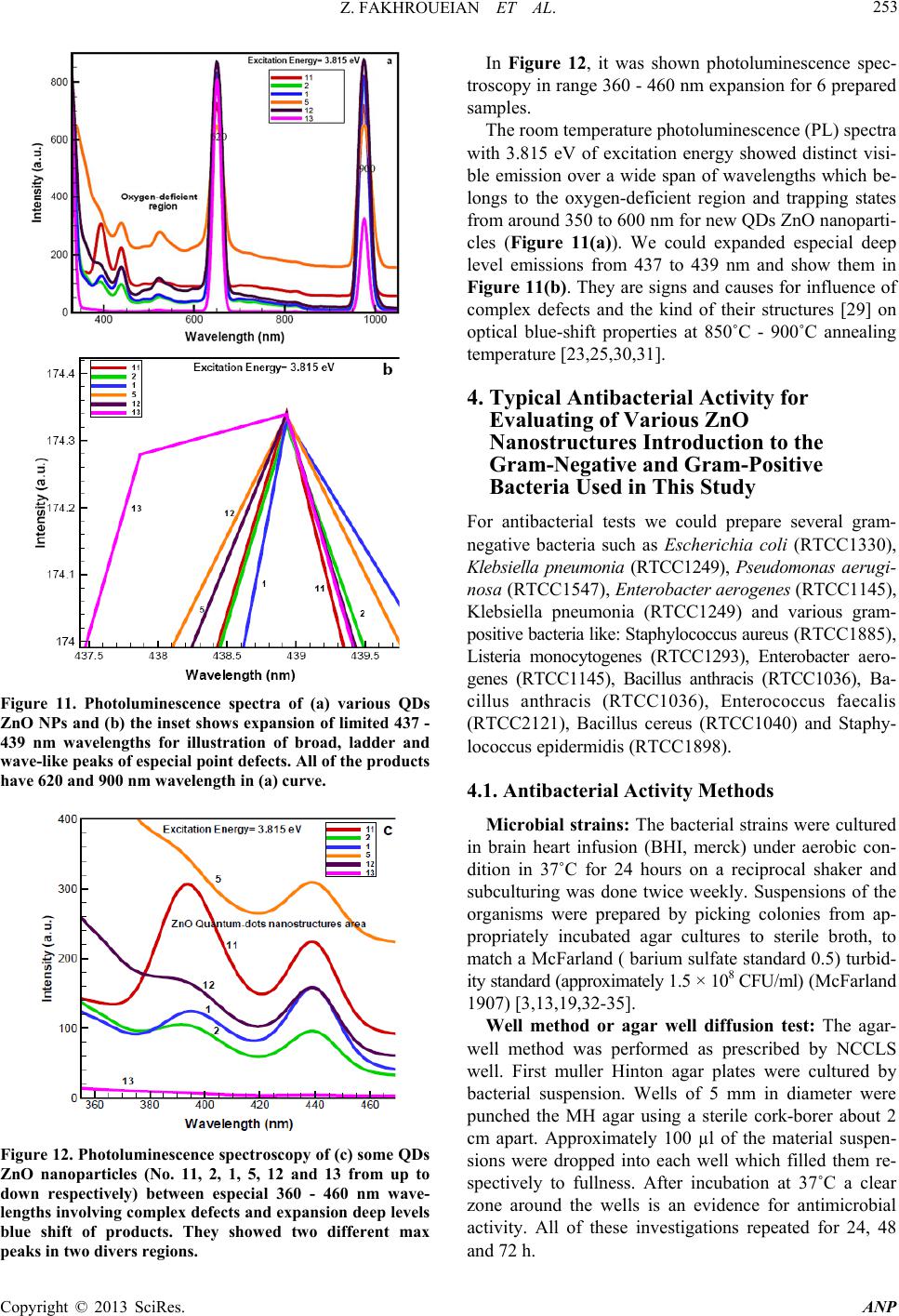 Z. FAKHROUEIAN ET AL. 253 Figure 11. Photoluminescence spectra of (a) various QDs ZnO NPs and (b) the inset shows expansion of limited 437 - 439 nm wavelengths for illustration of broad, ladder and wave-like peaks of especial point de fects. All of the pr oducts have 620 and 900 nm wavelength in (a) curve. Figure 12. Photoluminescence spectroscopy of (c) some QDs ZnO nanoparticles (No. 11, 2, 1, 5, 12 and 13 from up to down respectively) between especial 360 - 460 nm wave- lengths involving complex defects and expansion de ep levels blue shift of products. They showed two different max peaks in two divers regions. In Figure 12, it was shown photoluminescence spec- troscopy in range 360 - 460 nm expansion for 6 prepared samples. The room temperature photoluminescence (PL) spectra with 3.815 eV of excitation energy showed distinct visi- ble emission over a wide span of wavelengths which be- longs to the oxygen-deficient region and trapping states from around 350 to 600 nm for new QDs ZnO nanoparti- cles (Figure 11(a)). We could expanded especial deep level emissions from 437 to 439 nm and show them in Figure 11(b). They are signs and causes for influence of complex defects and the kind of their structures [29] on optical blue-shift properties at 850˚C - 900˚C annealing temperature [23,25,30,31]. 4. Typical Antibacterial Activity for Evaluating of Various ZnO Nanostructures Introduction to the Gram-Negative and Gram-Positive Bacteria Used in This Study For antibacterial tests we could prepare several gram- negative bacteria such as Escherichia coli (RTCC1330), Klebsiella pneumonia (RTCC1249), Pseudomonas aerugi- nosa (RTCC1547), Enterobacter aerogenes (RTCC1145), Klebsiella pneumonia (RTCC1249) and various gram- positive bacteria like: Staphylococcus aureus (RTCC1885), Listeria monocytogenes (RTCC1293), Enterobacter aero- genes (RTCC1145), Bacillus anthracis (RTCC1036), Ba- cillus anthracis (RTCC1036), Enterococcus faecalis (RTCC2121), Bacillus cereus (RTCC1040) and Staphy- lococcus epidermidis (RTCC1898). 4.1. Antibacterial Activity Methods Microbial strains: The bacterial strains were cultured in brain heart infusion (BHI, merck) under aerobic con- dition in 37˚C for 24 hours on a reciprocal shaker and subculturing was done twice weekly. Suspensions of the organisms were prepared by picking colonies from ap- propriately incubated agar cultures to sterile broth, to match a McFarland ( barium sulfate standard 0.5) turbid- ity standard (approximately 1.5 × 108 CFU/ml) (McFarland 1907) [3,13,19,32-35]. Well method or agar well diffusion test: The agar- well method was performed as prescribed by NCCLS well. First muller Hinton agar plates were cultured by bacterial suspension. Wells of 5 mm in diameter were punched the MH agar using a sterile cork-borer about 2 cm apart. Approximately 100 µl of the material suspen- sions were dropped into each well which filled them re- spectively to fullness. After incubation at 37˚C a clear zone around the wells is an evidence for antimicrobial activity. All of these investigations repeated for 24, 48 and 72 h. Copyright © 2013 SciRes. ANP  Z. FAKHROUEIAN ET AL. Copyright © 2013 SciRes. ANP 254 Determination of minimum inhibitory concentra- tion (MIC): The minimum inhibitory concentration (MIC) of the extracts was determined according to methods described by CLSI 2006. ZnO suspensions were diluted to concentrations ranging from 100 to 0.78 mg/ml in Mueller Hinton broth. To each dilution tubes, 0.1 ml of the bacterial inoculum was seeded. Control tubes with no bacterial inoculation were simultaneously maintained. Tubes were incubated aerobically at 37˚C for 24 hours. The lowest concentration of the extract that produced no visible bacterial growth (turbidity) was recorded as the MIC (CLSI, 2006). To estimate the MIC of the bacteria suspensions more precisely and for confirmation of the results, a more precise concentration in agar dilution method was used [3,16,20,21,36]. 4.2. Evaluation of Methodologies to Determine Antibacterial Activity for Six Q-Dots ZnO Nanoparticles by W. M and MIC Methods Compared with Gentamicin Antibiotic Standard Tables 1 and 2 show performance of five and seven vari- ous QDs ZnO nanoparticles including nanorods and nano- sphericals structutres against examined Gram negative bacterial strains and the efficiency was compared with standard antibiotic using well diffusion method. Figure 13 indicates the representation of some agar plates in well method test for sample 5 (Q-ZnO523) against B. cer. (25 mm zone diameter) and sample 8 (ZnO19) against (b) B. ant (30 mm), (c) St. epi (40 mm) and finally (d) St. au (30 mm). The presence of an inhibi- tion zone clearly exhibited the significant antibacterial effect of new QDs ZnO nanostructures on these micro- bes. Sample 1 shows strong antibacterial activity against E. coli. Sample 3 and sample 5 shows excellent biological activity against Klebsiella pneumonia and also sample 3 is very effective agent against Pseudomonas aeruginosa and all of gr-bacterial strains in Table 1. The results in Table 2 indicates that sample 2 and 3 in the first row shows excellent activity against Bacillus anthra cis, and sample 4 illustrates high biological activity against Staphy- lococcus aureus, and sample 3 showed high activity on the Bacillus cereus. Sample 13 is sensitive to Staphylo- coccus aureus but sample 5 does not show any influence Table 1. Effect of five QDs ZnO NPs samples against four Gram negative bacteria with gentamicin standard. Gram Negative Bacteria Method Samples Reference 1 2 3 4 5 W.M 20 10 15 15 - Escherichia coli MIC 15 mm 25 100 50 50 - W.M - - 20 20 20 Enterobacter aerogenes MIC 10 - - 25 25 25 W.M - - 25 15 25 Klebsiella pneumonia MIC 10 - - 12.5 50 12.5 W.M 15 15 25 NT* NT* Pseudomonas aeruginosa MIC 10 50 50 12.5 NT NT NT* = was not done. Table 2. Illustration of seven QDs ZnO NP against six Gram positive bacteria. Gram Positive Bacteria Method Samples Reference 1 2 3 4 5 13 14 W.M 20 30 30 15 15 - 15 Bacillus anthracis MIC 15 mm 25 6.25 6.25 50 50 - 50 W.M 20 - 15 25 10 20 20 Staphylococcus aureus MIC 20 25 - 50 12.5 100 25 25 W.M - - - - - - - Listeria monocytogenes MIC 20 - - - - - - - W.M - 20 NT* - - NT* - Enterococcus faecalis MIC 10 - 25 NT - - NT - W.M 20 10 25 - 25 10 - Bacillus cereus MIC 20 25 100 12.5 - 12.5 100 - W.M - - 20 15 - - - Staphylococcus epidermidis MIC 20 - - 25 50 - - - NT* = was not done.  Z. FAKHROUEIAN ET AL. 255 Figure 13. Agar plate test (well method) for (a) sample 5 (with symbol QZnO523) and (b)-(d) sample 8 (with symbol QZnO19) films showing excellent inhibition zone around the films. ZnO16P symbol is the same as sample 8 with polystyrene-capped which it was not successful dur ing the growth process in well te st. against it, whereas they are similar to each other in pro- duction stages but are different in purification and calci- nation process. It was found that, very homogenous nano- spherical sample 14 exhibited proper sensitivity related to both Bacillu s anthracis and Staphylococcus aureus bacteria. Sample1displaces identical permanent sensitiv- ity responses of the Bacillus anthracis, Staphylococcus aureus and Bacillus cereus. It is promising that we will be able to do treatment and coating its surface owing to bind more strongly to microorganisms. Therefore, by synthesis of these antimicrobial QDs ZnO nanoparticles, we found ideal candidates as antimicrobial agents. Also mechanism of action of ZnO nanoparticles highlighted that they are capable to kill bacteria through various mechanisms, such as by binding to intracellular proteins and inactivating them, generation of radical and reactive oxygen species including hydrogen peroxide (H2O2), electrostatic interaction between the ZnO NPs surface and bacteria membranes (cell surfaces) and penetration in and finally via direct damage to cell wall and membrane. Moreover, the key factor is the formation of desired structure and manufacturing of ZnO NPs involve active surfaces with much greater surface area to volume ratio (high BET) to generate defect nanoporous surface (like- nanocatalyst) because imperfection nanopores can create active radicals with enough activation energy without dependence upon the crystal size of ZnO NPs and this is very important and notable point in typical present inves- tigation. In Tables 3 and 4, performance of ZnO NPs groups including audible sound, pretreatments with assistant agents and oleic acid-capped ZnO Q-Dots products which were tested with gr− and gr+ bacteria is presented. It is remarkable that three samples are effective against Enterobacter aerogenes in comparison with gentamicin antibiotic reagent in Table 3. Sample 8 illustrated very activity against Klebsiella pneumonia that was modified by oleic acid. Nanospherical of ZnO which was treated with silica nanoparticles like-square plates morphology in sample 7 showed excellent inhibiting effect against the growth of Pseudomonas aeruginosa and others are sensi- tive comparing to standard antibiotic. In fact this modifi- cation method could increase the surface activity of ZnO nanoparticles to induce proper interactions with micro- organisms. Table 4 is capable to report the performance of modified ZnO NPs by different conditions. Among all samples in Table 4, sample 8 has high pre- vent effect on life of Staphylococcus epidermidis and showed the most impressive antibacterial property against mostly Gram Positive bacteria and increased the inhibi- tion zone diameters against all microbes compared with standard antibiotic (W.M = 20 mm for reference and 40 mm for sample 8) and good biocompatibility. It seems that the appearance of oleic acid as a development of reliable processes using polymer template type could improve the activity of large surface area available for interaction, binding and diffusion in organ of microbes better resulting into cell death [34]. In addition, sample 7 also showed much activity against the Enterococcus fae- calis and Staphylococcus epiderm idis and demonstrated excellent activity as an antibacterial agent. Sample 6 is strong antibacterial against Staphylococcus epidermidis. Overall, the results of ZnO nanoparticles containg or- ganic templates such as TEA, EDA and citric acid were appeared which were not highly against some of the tested pathogens and did not undergo obvious modifica- tion on the surface of the ZnO nanostructures. 5. Conclusion In this study, ZnO nanoparticles and QDs nanostructures as nanospherical and nanorods arrays were fabricated by sol-gel, wet-chemical, and hydrothermal methods. Audi- ble sound including microwave process as unreported and new procedure for biosynthesis of zinc oxide nanopar- ticles (ZnO NPs) was used as novel method. PVP and oleic acid as capping agent showed that they could mo- dify and activate the wide surface of nanoZnO structures. In this case, they can have more contact with bacteria and the efficiency will enhance. These studies demonstrate that the especial ZnO QDs nanoparticles including blue shifts spectrums and complex defects on surfaces exhibit Copyright © 2013 SciRes. ANP 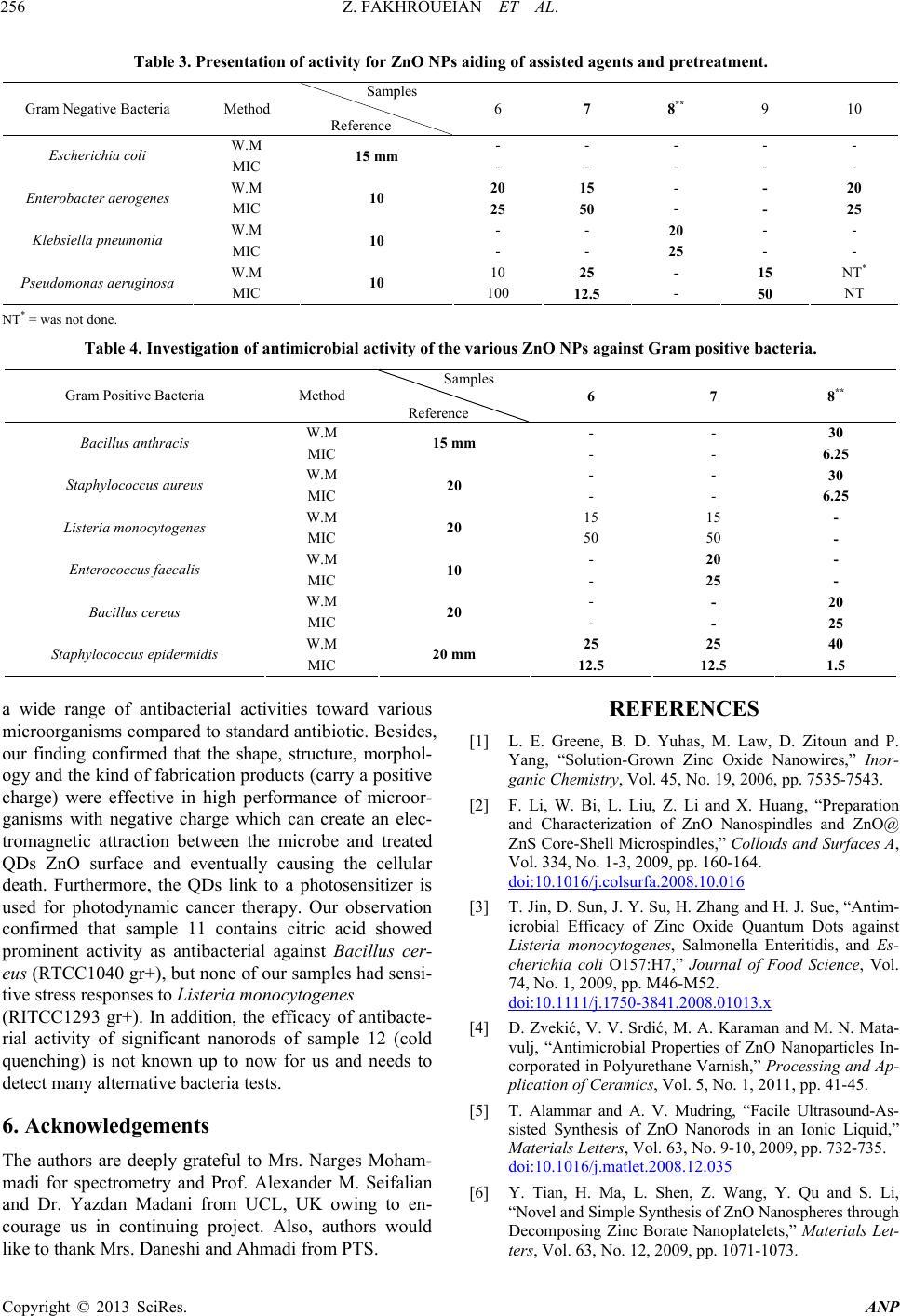 Z. FAKHROUEIAN ET AL. 256 Table 3. Presentation of activity for ZnO NPs aiding of assisted agents and pretreatment. Gram Negative Bacteria Method Samples Reference 6 7 8** 9 10 W.M - - - - - Escherichia coli MIC 15 mm - - - - - W.M 20 15 - - 20 Enterobacter aerogenes MIC 10 25 50 - - 25 W.M - - 20 - - Klebsiella pneumonia MIC 10 - - 25 - - W.M 10 25 - 15 NT* Pseudomonas aeruginosa MIC 10 100 12.5 - 50 NT NT* = was not done. Table 4. Investigation of antimicrobial activity of the various ZnO NPs against Gram positive bacteria. Gram Positive Bacteria Method Samples Reference 6 7 8** W.M - - 30 Bacillus anthracis MIC 15 mm - - 6.25 W.M - - 30 Staphylococcus aureus MIC 20 - - 6.25 W.M 15 15 - Listeria monocytogenes MIC 20 50 50 - W.M - 20 - Enterococcus faecalis MIC 10 - 25 - W.M - - 20 Bacillus cereus MIC 20 - - 25 W.M 25 25 40 Staphylococcus epidermidis MIC 20 mm 12.5 12.5 1.5 a wide range of antibacterial activities toward various microorganisms compared to standard antibiotic. Besides, our finding confirmed that the shape, structure, morphol- ogy and the kind of fabrication products (carry a positive charge) were effective in high performance of microor- ganisms with negative charge which can create an elec- tromagnetic attraction between the microbe and treated QDs ZnO surface and eventually causing the cellular death. Furthermore, the QDs link to a photosensitizer is used for photodynamic cancer therapy. Our observation confirmed that sample 11 contains citric acid showed prominent activity as antibacterial against Bacillus cer- eus (RTCC1040 gr+), but none of our samples had sensi- tive stress responses to Listeria monocytogenes (RITCC1293 gr+). In addition, the efficacy of antibacte- rial activity of significant nanorods of sample 12 (cold quenching) is not known up to now for us and needs to detect many alternative bacteria tests. 6. Acknowledgements The authors are deeply grateful to Mrs. Narges Moham- madi for spectrometry and Prof. Alexander M. Seifalian and Dr. Yazdan Madani from UCL, UK owing to en- courage us in continuing project. Also, authors would like to thank Mrs. Daneshi and Ahmadi from PTS. REFERENCES [1] L. E. Greene, B. D. Yuhas, M. Law, D. Zitoun and P. Yang, “Solution-Grown Zinc Oxide Nanowires,” Inor- ganic Chemistry, Vol. 45, No. 19, 2006, pp. 7535-7543. [2] F. Li, W. Bi, L. Liu, Z. Li and X. Huang, “Preparation and Characterization of ZnO Nanospindles and ZnO@ ZnS Core-Shell Microspindles,” Colloids and Surfaces A, Vol. 334, No. 1-3, 2009, pp. 160-164. doi:10.1016/j.colsurfa.2008.10.016 [3] T. Jin, D. Sun, J. Y. Su, H. Zhang and H. J. Sue, “Antim- icrobial Efficacy of Zinc Oxide Quantum Dots against Listeria monocytogenes, Salmonella Enteritidis, and Es- cherichia coli O157:H7,” Journal of Food Science, Vol. 74, No. 1, 2009, pp. M46-M52. doi:10.1111/j.1750-3841.2008.01013.x [4] D. Zvekić, V. V. Srdić, M. A. Karaman and M. N. Mata- vulj, “Antimicrobial Properties of ZnO Nanoparticles In- corporated in Polyurethane Varnish,” Processing and Ap- plication of Ceramics, Vol. 5, No. 1, 2011, pp. 41-45. [5] T. Alammar and A. V. Mudring, “Facile Ultrasound-As- sisted Synthesis of ZnO Nanorods in an Ionic Liquid,” Materials Letters, Vol. 63, No. 9-10, 2009, pp. 732-735. doi:10.1016/j.matlet.2008.12.035 [6] Y. Tian, H. Ma, L. Shen, Z. Wang, Y. Qu and S. Li, “Novel and Simple Synthesis of ZnO Nanospheres through Decomposing Zinc Borate Nanoplatelets,” Materials Let- ters, Vol. 63, No. 12, 2009, pp. 1071-1073. Copyright © 2013 SciRes. ANP  Z. FAKHROUEIAN ET AL. 257 doi:10.1016/j.matlet.2009.02.009 [7] Y. Zeng, T. Zhang and L. Qiao, “Preparation and Gas Sensing Properties of the Nutlike ZnO Microcrystals via a Simple Hydrothermal Route,” Materials Letters, Vol. 63, No. 11, 2009, pp. 843-846. doi:10.1016/j.matlet.2009.01.012 [8] M. L. Singla, M. Shafeeq and M. Kumar, “Optical Char- acterization of ZnO Nanoparticles Capped with Various Surfactants,” Journal of Luminescence, Vol. 129, No. 12, 2009, pp. 434-438. doi:10.1016/j.jlumin.2008.11.021 [9] K. Senthilkumar, M. Tokunaga, H. Okamoto, O. Senthi- lkumar and Y. Fujita, “Hydrogen Related Defect Com- plexes in ZnO Nanoparticles,” Applied Physics Letters, Vol. 97, No. 9, 2010,Article ID: 091907. doi:10.1063/1.3485049 [10] X. Xu, C. Xu, J. Dai, J. Hu, F. Li and S. Zhang, “Size Dependence of Defect-Induced Room Temperature Ferro- magnetism in Undoped ZnO Nanoparticles,” The Journal of Physical Chemistry C, Vol. 116, No. 15, 2012, pp. 8813-8818. doi:10.1021/jp3014749 [11] H. Q. Shi, W. N. Li, L.W. Sun, Y. Liu, H. M. Xiao and S. Y. Fu, “Synthesis of Silane Surface Modified ZnO Quan- tum Dots with Ultrastable, Strong and Tunable Lumines- cence,” Chemical Communications, Vol. 47, No. 43, 2011, pp. 11921-11923. doi:10.1039/c1cc15411g [12] K. H. Tam, A. B. Djurisic, C. M. N. Chan, Y. Y. Xi, C. W. Tse, Y. H. Leung, W. K. Chan, F. C. C. Leung and D. W. T. Au, “Antibacterial Activity of ZnO Nanorods Pre- pared by a Hydrothermal Method,” Thin Solid Films, Vol. 516, No. 18, 2008, pp. 6167-6174. doi:10.1016/j.tsf.2007.11.081 [13] Y. Xie, Y. He, P. L. Irwin, T. Jin and X. Shi, “Antibacte- rial Activity and Mechanism of Action of Zinc Oxide Nanoparticles against Campylobacter jejuni,” Applied and Environmental Microbiology, Vol. 77, No. 7, 2011, pp. 2325-2331. doi:10.1128/AEM.02149-10 [14] N. Padmavathy and R. Vijayaraghavan, “Enhanced Bio- activity of ZnO Nanoparticles an Antimicrobial Study,” Science and Technology of Advanced Materials, Vol. 9, No. 3, 2008, Article ID: 035004. doi:10.1088/1468-6996/9/3/035004 [15] L. Zhang, Y. Ding and Y. L. D. Cang, “Antimicroorgan- ism Activities Comparison among ZnO, TiO2, MgO and SiO2 Nanoparticles Suspensions at Different pH Values,” The 2nd International Conference on Chemical, Biologi- cal and Environmental Engineering, 2010, pp. 257-260. [16] A. A. Tayel, W. F. EL-Tras, S. Moussa, A. F. EL-Baz, H. Mahrous, M. F. Salem and L. Brimer, “Antibacterial Ac- tion of Zinc Oxide Nanoparticles against Foodborne Pa- thogens,” Journal of Food Safety, Vol. 31, No. 2, 2011, pp. 211-218. doi:10.1111/j.1745-4565.2010.00287.x [17] R. O. Moussodia, L. Balan, C. Merlin, C. Mustin and R. Schneider, “Biocompatible and Stable ZnO Quantum Dots Generated by Functionalization with Siloxane-Core PAMAM Dendrons,” Journal of Materials Chemistry, Vol. 20, No. 6, 2010, pp. 1147-1155. doi:10.1039/b917629b [18] M. Premanathan, K. Karthikeyan, K. Jeyasubramanian and G. Manivannan, “Selective Toxicity of ZnO Nano- particles toward Gram-Positive Bacteria and Cancer Cells by Apoptosis through Lipid Peroxidation,” Nanomedicine: Nanotechnology, Biology and Medicine, Vol. 7, No. 2, 2011, pp. 184-192. doi:10.1016/j.nano.2010.10.001 [19] G. Singh, E. M. Joyce, J. Beddow and T. J. Mason, “Evo- lution Antibacterial Activity of ZnO Nanoparticles Coated Sonochemically onto Textile Fabrics,” Journal of Micro- biology, Biotechnology and Food Sciences, Vol. 2, No. 1, 2012, pp. 106-120. [20] Z. E. Karvani and P. Chehrazi, “Antibacterial Activity of ZnO Nanoparticle on Gram-Positive and Gram Negative Bacteria,” African Journal of Microbiology Research, Vol. 5, No. 12, 2011, pp. 1368-1373. doi:10.5897/AJMR10.159 [21] O. Yamamoto, “Influence of Particle Size on the Anti- bacterial Activity of Zinc Oxide,” International Journal of Inorganic Materials, Vol. 3, No. 7, 2001, pp. 643-646. [22] T. Phaechamud, J. Mahadlek, N. Aroonrerk, S. Choopun and J. Charoenteeraboon, “Antimicrobial Activity of ZnO- Doxycycline Hyclate Thermosensitive Gel,” Science Asia, Vol. 38, No. 1, 2012, pp. 64-74. doi:10.2306/scienceasia 1513-1874.2012.38.064 [23] S. Kumar and P. D. Sahare, “Effects of Annealing on the Surface Defects of Zinc Oxide Nanoparticles,” Nano: Brief Reports and Reviews, Vol. 7, No. 3, 2012, Article ID: 1250022. doi:10.1142/s1793292012500221 [24] M. H. Wong, A. Berenov, X. Qi, M. J. Kappers, Z. H. Barber, B. Illy, Z. Lockman, M. P. Ryan and J. L. M. Driscoll, “Electrochemical Growth of ZnO Nano-Rods on Polycrystalline Zn Foil,” Nanotechnology, Vol. 14, No. 9, 2003, pp. 968-973. [25] D. Bera, L. Qian, S. Sabui, S. Santra and P. H. Holloway, “Photoluminescence of ZnO Quantum Dots Produced by a Sol-Gel Process,” Optical Materials, Vol. 30, No. 8, 2008, pp. 1233-1239. doi:10.1016/j.optmat.2007.06.001 [26] A. Saric, S. Music and M. Ivanda, “Varying the Micro- structural Properties of ZnO Particles Using Different Synthesis Routes,” Journal of Molecular Structure, Vol. 993, No. 1-3, 2011, pp. 219-224. doi:10.1016/j.molstructruc.2010.10.018 [27] R. Sreeja, Jobina John, P. M. Aneesh and M. K. Jayaraj, “Linear and Nonlinear Optical Properties of Luminescent ZnO Nanoparticles Embedded in PMMA Matrix,” Optics Communications, Vol. 283, No. 14, 2010, pp. 2908-2913. [28] H. M. Cheng, H. C. Hsu, S. L. Chen, W. T. Wu, C. C. Kao, L. J. Lin and W. F. Hsieh, “Efficient UV Photolu- minescence from Monodispersed Secondary ZnO Colloi- dal Spheres Synthesized by Sol-Gel Method,” Journal of Crystal Growth, Vol. 277, No. 1-4, 2005, pp. 192-199. doi:10.1016/j.jcrysgro.2004.12.133 [29] B. X. Hu, J. Gong, L. Zhang and J. C. Yu, “Continuous Size Tuning of Monodisperse ZnO Colloidal Nanocrystal Clusters by a Microwave-Polyol Process and Their Ap- plication for Humidity Sensing,” Advanced Materials, Vol. 20, No. 24, 2008, pp. 4845-4850. doi:10.1002/adma.200801433 [30] S. Y. Kuo, F. I. Lai, W. C. Chen, C. P. Cheng, H. C. Kuo and S. C. Wang, “Ultraviolet Lasing of Sol-Gel-Derived Zinc Oxide Polycrystalline Films,” Japanese Journal of Applied Physics, Vol. 45, No. 4B, 2006, pp. 3662-3665. doi:10.1143/JJAP.45.3662 Copyright © 2013 SciRes. ANP  Z. FAKHROUEIAN ET AL. Copyright © 2013 SciRes. ANP 258 [31] K. F. Lin, H. M. Cheng, H. C. Hsu, L. J. Lin and W. F. Hsieh, “Band Gap Variation of Size-Controlled ZnO Quantum Dots Synthesized by Sol-Gel Method,” Chemi- cal Physics Letters, Vol. 409, No. 4-6, 2005, pp. 208-211. doi:10.1016/j.cp1ett.2005.05.027 [32] C. Valgas, S. M. de Souza, E. F. Smania and A. Smania Jr., “Screening Methods to Determine Antibacterial Ac- tivity of Natural Products,” Brazilian Journal of Microbi- ology, Vol. 38, No. 2, 2007, pp. 369-380. [33] European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), “De- termination of Minimum Inhibitory Concentrations (MICs) of antibacterial agents by agar dilution,” Clinical Microbiology and Infection, Vol. 6, No. 9, 2000, pp. 509- 515. [34] F. Arabi, M. Imandar, M. Negahdary, M. Imandar, M. Torkamani Noughabi, H. Akbari dastjerdi and M. Fazilati, “Investigation Anti-Bacterial Effect of Zinc Oxide Nano- particles upon Life of Listeria monocytogenes,” Annals of Biological Research, Vol. 3, No. 7, 2012, pp. 3679-3685. [35] K. Chitra and G. Annadurai, “Antimicrobial Activity of Wet Chemically Engineered Spherical Shaped ZnO Na- noparticles on Food Borne Pathogen,” International Food Research Journal, Vol. 20, No. 1, 2013, pp. 59-64. [36] J. M. Yousef and E. N. Dania “In Vitro Antibacterial Activity and Minimum Inhibitory Concentration of Zinc Oxide and Nano-Particle Zinc Oxide against Pathogenic Strains,” Journal of Health Science, Vol. 2, No. 4, 2012, pp. 38-42. doi:10.5923/j.health.20120204.04 |

