 Journal of Sustainable Bioenergy Systems, 2013, 3, 163-170 http://dx.doi.org/10.4236/jsbs.2013.33023 Published Online September 2013 (http://www.scirp.org/journal/jsbs) Biodiesel from Plant Resources—Sustainable Solution to Ever Increasing Fuel Oil Demands Md Enamul Hoque*, Lu Pui Gee University of Nottingham Malaysia Campus, Jalan Broga, Semenyih, Selangor, Malaysia Email: *enamul.hoque@nottingham.edu.my Received December 27, 2012; revised January 28, 2013; accepted February 15, 2013 Copyright © 2013 Md Enamul Hoque, Lu Pui Gee. This is an open access article distributed under the Creative Commons Attribu- tion License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The demand for fuel oil is ever increasing with the advance of the modern world, whereas worldwide reserves of fossil oils are diminishing at an alarming rate. However, there exist large stockpiles of vegetable oil feedstocks that could be exploited to produce fuel oil, called biodiesel with the aid of biotechnology. Initially, the biodiesel produced from vegetable oil did not attract much attention because of its high cost. However, the recent increase in petroleum prices and the uncertainties of petroleum availability led to the renewal of interest in biodiesel production from such sustain- able resources (i.e., vegetable oil feedstocks). This research focuses on the production of biodiesel from plant resources, and further investigates the influences of key process parameters, such as the molar ratio of methanol to oil, catalyst concentration, reaction temperature, reaction period and stirring speed on the biodiesel yield. This investigation is to determine the optimum process parameters for maximum biodiesel yield. The biodiesel was produced from three vege- table oil feedstocks, namely palm, soybean and sunflower oil via a transesterification process. It was observed that all the process parameters significantly influenced the biodiesel yield. The maximum biodiesel yields for palm, sunflower and soybean oil feedstocks were found to be 87.5%, 83.6% and 80.2%, respectively at optimum condition. The results suggest that through proper optimization of the process parameters the biodiesel yields could be maximized. In conclu- sion, the production of biodiesel from plant resources would be regarded as a sustainable solution to the ever increasing demand of fuel oils. Keywords: Sustainable Solution; Fuel Oil; Biodiesel; Plant Reso urce; Biotechn ology; Process Parameter 1. Introduction Biodiesel (known as fatty acid methyl ester) is a mono- alkyl ester of long chain fatty acids produced from re- newable resources such as virgin vegetable oils, waste vegetable oils, animal fats, algae etc. [1-3]. It is an alter- native fuel that is used in compression-ignition diesel engine with slight or no modification, and has attracted great attention because of its renewability, better gas emissivity and biodegradability [4]. Biodiesel has a lower volumetric heating values and higher cetane and flash points [5]. Biodiesel can be used in the pure form noted as B100 or as a blend (e.g. 20% biodiesel and 80% petroleum diesel). The biodiesel has been regarded as the potential substitute for petroleum diesel as it offers a number of attractive beneficial properties compared to conventional petroleum diesel [6]. For example, the use of biodiesel maintains a balanced carbon dioxide cycle as it is based on renewable biological materials. Besides, environmentally friendliness due to reduced emissions (carbon monoxide, sulphur, aromatic hydrocarbons and soot particles) during combustion is of another benefit, while it offers non-toxic and complete biodegradabile properties. The production of biodiesel from various sources has been explored by some researchers [7-14]. Direct use of vegetable oil containing high amount of free fatty acids in diesel engines, can cause oil ring sticking, lubricating problems, poor fuel atomization or even prevention of atomization as a result of plugged orifices, poor cold engine start up, and the creation of gum and other deposits [15]. Therefore, a special proce- dure is required to diminish the free fatty acids in the vegetable oil and thus converts the vegetable oil to bio- diesel which has similar properties and performances to that of petroleum diesel. Potential processes for the pro- duction of biodiesel include microemulsion, pyrolysis (thermal cracking) and transesterification. Among all *Corresponding a uthor. C opyright © 2013 SciRes. JSBS  M. E. HOQUE, L. P. GEE 164 these, transesterification is considered to be the most practiced process as it is relatively simple and cost-ef- fective. Besides, it also tends to lower the viscosity of the biodiesel and produces glycerol as a by-product which also has commercial value. The transesterification is a chemical process that in- volves a number of consecutive reversible reactions be- tween the triglyceride segment of vegetable oil and an alcohol in the presence of a catalyst to produce ester (i.e. biodiesel) and by-product (i.e . glycerin) [16]. The tri- glyceride is converted stepwise to diglyceride, mono- glyceride and finally glycerol, whereby 1 mol of alkyl ester is removed at each step. The formation of alkyl ester from monoglyceride is believed to be the step that determines the reaction rate since monoglyceride is the most stable intermediate compound [17]. Generally, the alcohols used in the transesterification process include methanol, ethanol, propanol and butanol. However, methanol is widely used because of its cost effectiveness, and favorable physical and chemical nature (shortest chain alcohol) [18]. A catalyst is usually used to enhance the reaction rate and biodiesel conversion. Since the re- action is reversible, excess alco hol is required to shift the equilibrium to the product side. Various types of cata- lysts such as alkali-based, acid-based or enzymatic are used depending upon the nature of the oil exploited for the biodiesel production. Alkali-based catalysts include sodium hydroxide (NaOH), potassium hydroxide (KOH) etc. Sulfuric acid (H2SO4) and hydrochloric acid (HCI) are usually used to as acid catalysts, while lipases and algaes have been tested as enzymatic catalysts [16]. However, the choice of catalyst depends on the free fatty acids (FFA) content in the feedstock oil. Alkaline-cata- lyzed transesterification is much faster than acid-cata- lyzed transesterification and thus most often used [17]. Overall, this study concentrates on the production of biodiesel from various plant (palm, soybean and sun- flower oil) feedstocks, and further investigates the influ- ences of key process parameters, such as the molar ratio of methanol to oil, catalyst concentration, reaction tem- perature, reaction period and stirring speed on the bio- diesel yields. 2. Materials and Methods 2.1. Feedstocks and Reactants Three types of locally produced (in Malaysia) vegetable oils were used in the experiments as fundamental feed- stocks for the production of biodiesel. They were palm oil (obtained from Yee Lee Edible Oils Private Limited), soybean oil (obtained from Yee Lee Edible Oils Private Limited) and sunflower oil (obtained from Econfood Manufacturing (M) Sdn. Bhd). The analytical-grade methanol and potassium hydroxide (KOH) provided by the Chemical Laboratory, University of Nottingham Ma- laysia Campus were used as the prime reactants. 2.2. Preparation of Potassium Methoxide The measured KOH was dissolved in methanol within a conical flask. The conical flask had been swirled for few minutes until the KOH was fully dissolved in methanol and hence, the final so lution of potassiu m methox ide was produced. To prevent the potassium methoxide to be ex- posed to the air the opening of the conical flask was wrapped with aluminum foil. Otherwise, the potassium methoxide may react with air which can lead to the com- promise of its reaction with methanol and catalyst. 2.3. Transesterification Reaction The feedstock oil was firstly preheated in a conical flask for 25 minutes to a preheating temperature (e.g. 55˚C) by means of a water bath shaker. Potassium methoxid e solu- tion was then added to the preheated oil in the flask. The overall solution was heated to the specified temperature for specified time period with continuous stirring at the specified speed for the transesterification process to oc- cur. The transesterification is a chemical process that involves a series of reversible reactions (Figure 1) be- tween a triglyceride (fat/oil) and an alcohol thus produc- ing esters and glycerol [15]. Generally a catalyst is em- ployed to enhance the reaction rate and ultimately the biodiesel yield. Because of the reversible nature of the transesterification reaction an excess amount of alcohol is required to shift the equilibrium to the product side. For this experiment, the transesterification reaction (i.e. biodiesel production) was started with the primarily set process parameters such as methanol to feedstock molar ratio of 6:1, catalyst (KOH) concentration of 1.0%, reac- tion temperature of 65˚C, reaction period of 3 hours and stirring speed of 130 rpm based on another study [19]. The process parameters were then varied over a range (methanol to feedstock molar ratios of 3:1, 6:1, 9:1, 12:1 & 15:1; catalyst (KOH) concentrations of 0.50 wt%, 0.75 wt%, 1.0 wt%, 1.25 wt% & 1.50 wt%; reaction tempera- tures of 55˚C, 60˚C & 65˚C, reaction periods of 1 hr, 2 hrs, 3 hrs & 4 hrs, and stirring speeds of 80 rpm, 100 rpm & 130 rpm) to investigate the influences of process pa- Figure 1. Transesterification process that involves a series of reversible reactions between a triglyceride (fat/oil) and an alcohol which produces esters and glycerol [15]. Copyright © 2013 SciRes. JSBS 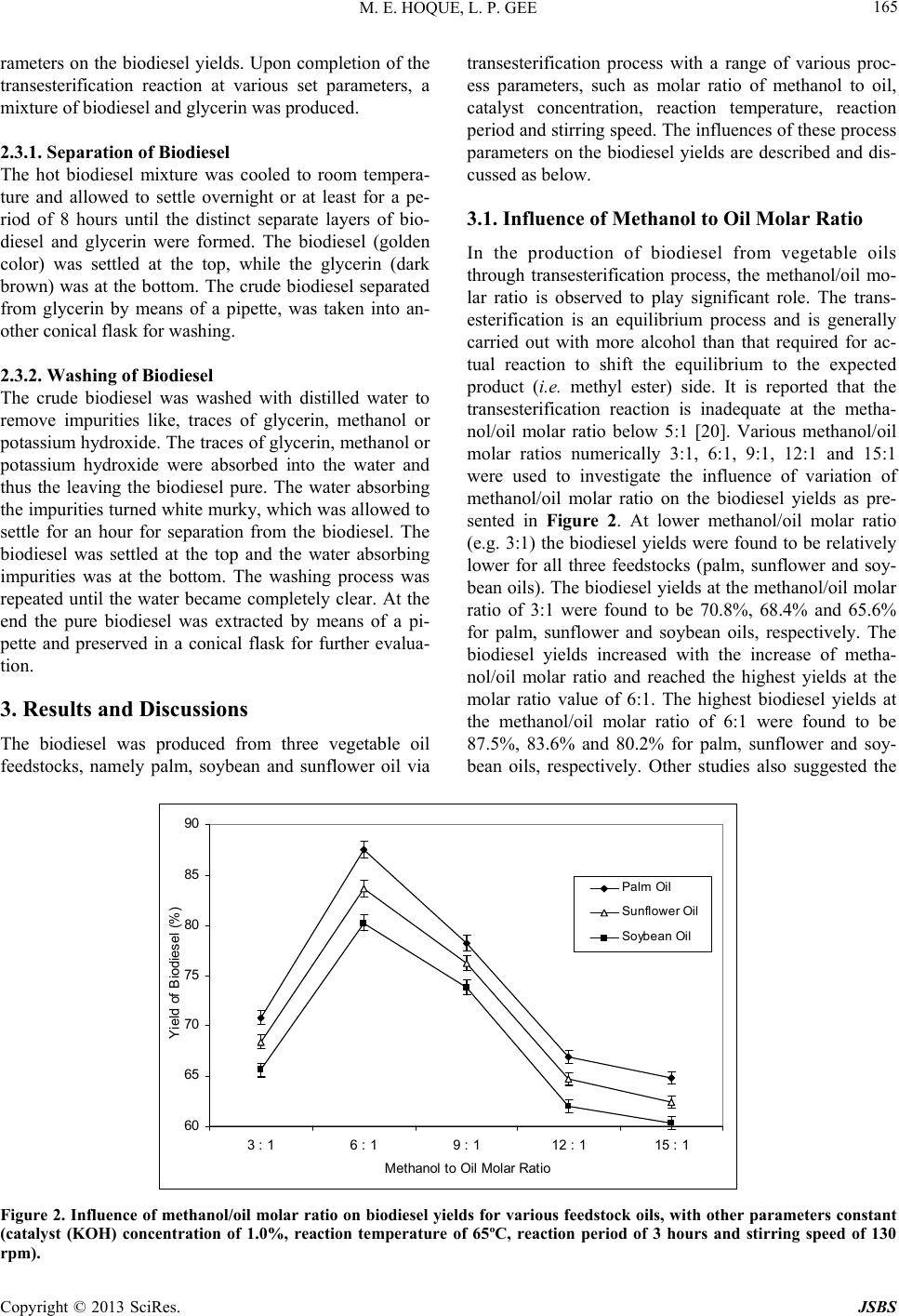 M. E. HOQUE, L. P. GEE Copyright © 2013 SciRes. JSBS 165 rameters on the biodiesel yields. Upon completion of the transesterification reaction at various set parameters, a mixtur e of biodie se l and glyce rin was pro duced. 2.3.1. Separati o n o f Biodiesel The hot biodiesel mixture was cooled to room tempera- ture and allowed to settle overnight or at least for a pe- riod of 8 hours until the distinct separate layers of bio- diesel and glycerin were formed. The biodiesel (golden color) was settled at the top, while the glycerin (dark brown) was at the bottom. The crude biodiesel separated from glycerin by means of a pipette, was taken into an- other con i c al flask for was hing. 2.3.2. Wa s hing of Bi o di esel The crude biodiesel was washed with distilled water to remove impurities like, traces of glycerin, methanol or potassium hydroxide. The traces of glycerin, methanol or potassium hydroxide were absorbed into the water and thus the leaving the biodiesel pure. The water absorbing the impurities turned white murky, which was allowed to settle for an hour for separation from the biodiesel. The biodiesel was settled at the top and the water absorbing impurities was at the bottom. The washing process was repeated until the water became completely clear. At the end the pure biodiesel was extracted by means of a pi- pette and preserved in a conical flask for further evalua- tion. 3. Results and Discussions The biodiesel was produced from three vegetable oil feedstocks, namely palm, soybean and sunflower oil via transesterification process with a range of various proc- ess parameters, such as molar ratio of methanol to oil, catalyst concentration, reaction temperature, reaction period and stirring speed. The influences of these process parameters on the biodiesel yields are described and dis- cussed as below. 3.1. Influence of Methanol to Oil Molar Ratio In the production of biodiesel from vegetable oils through transesterification process, the methanol/oil mo- lar ratio is observed to play significant role. The trans- esterification is an equilibrium process and is generally carried out with more alcohol than that required for ac- tual reaction to shift the equilibrium to the expected product (i.e. methyl ester) side. It is reported that the transesterification reaction is inadequate at the metha- nol/oil molar ratio below 5:1 [20]. Various methanol/oil molar ratios numerically 3:1, 6:1, 9:1, 12:1 and 15:1 were used to investigate the influence of variation of methanol/oil molar ratio on the biodiesel yields as pre- sented in Figure 2. At lower methanol/oil molar ratio (e.g. 3:1) the biodiesel yields were found to be relatively lower for all three feedstocks (palm, sunflower and soy- bean oils). The biod iesel yields at the methanol/oil molar ratio of 3:1 were found to be 70.8%, 68.4% and 65.6% for palm, sunflower and soybean oils, respectively. The biodiesel yields increased with the increase of metha- nol/oil molar ratio and reached the highest yields at the molar ratio value of 6:1. The highest biodiesel yields at the methanol/oil molar ratio of 6:1 were found to be 87.5%, 83.6% and 80.2% for palm, sunflower and soy- bean oils, respectively. Other studies also suggested the 60 65 70 75 80 85 90 3 : 16 : 19 : 112 : 115 : 1 Methanol t o O il M ol ar Rati o Y i eld of Bi odi esel (% ) Palm Oil Sunflower Oi l Soybean O i l Figure 2. Influence of methanol/oil molar ratio on biodiesel yields for various feedstock oils, with other parameters constant (catalyst (KOH) concentration of 1.0%, reaction temperature of 65ºC, reaction period of 3 hours and stirring speed of 130 rpm). 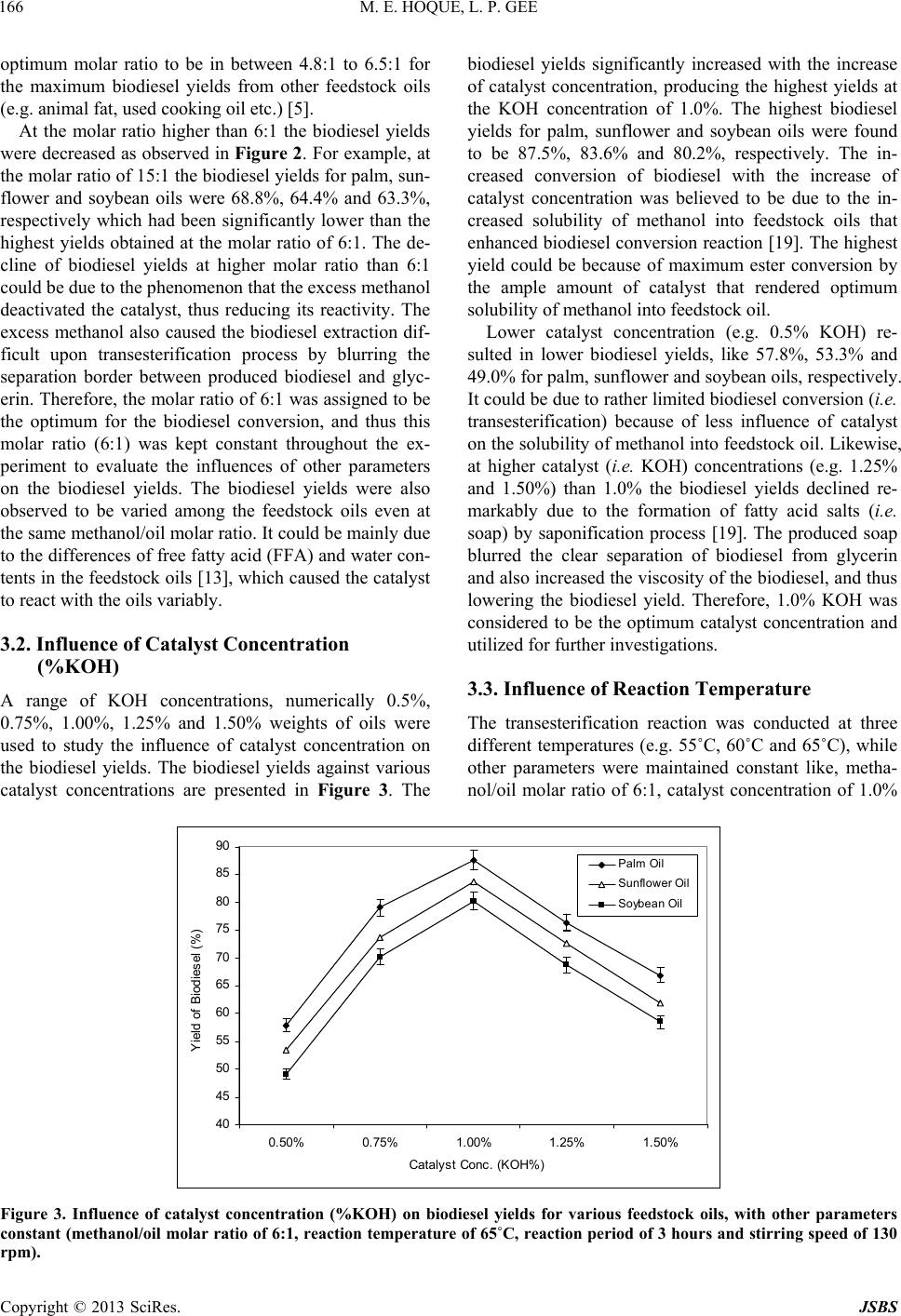 M. E. HOQUE, L. P. GEE 166 optimum molar ratio to be in between 4.8:1 to 6.5:1 for the maximum biodiesel yields from other feedstock oils (e.g. animal fat, used cooking oil etc.) [5]. At the molar ratio higher than 6:1 the biodiesel yields were decreased as observed in Figure 2. For example, at the molar ratio of 15:1 the biodiesel yields for palm, sun- flower and soybean oils were 68.8%, 64.4% and 63.3%, respectively which had been significantly lower than the highest yields obtained at the molar ratio of 6:1. The de- cline of biodiesel yields at higher molar ratio than 6:1 could be due to the phenomenon that the excess methanol deactivated the catalyst, thus reducing its reactivity. The excess methanol also caused the biodiesel extraction dif- ficult upon transesterification process by blurring the separation border between produced biodiesel and glyc- erin. Therefore, the molar ratio of 6:1 was assigned to be the optimum for the biodiesel conversion, and thus this molar ratio (6:1) was kept constant throughout the ex- periment to evaluate the influences of other parameters on the biodiesel yields. The biodiesel yields were also observed to be varied among the feedstock oils even at the same methanol/oil molar ratio. It could be mainly due to the differences of free fatty acid (FFA) and water con- tents in the feedstock oils [13], which caused the catalyst to react with the oils variably. 3.2. Influence of Catalyst Concentration (%KOH) A range of KOH concentrations, numerically 0.5%, 0.75%, 1.00%, 1.25% and 1.50% weights of oils were used to study the influence of catalyst concentration on the biodiesel yields. The biodiesel yields against various catalyst concentrations are presented in Figure 3. The biodiesel yields significantly increased with the increase of catalyst concentration, producing the highest yields at the KOH concentration of 1.0%. The highest biodiesel yields for palm, sunflower and soybean oils were found to be 87.5%, 83.6% and 80.2%, respectively. The in- creased conversion of biodiesel with the increase of catalyst concentration was believed to be due to the in- creased solubility of methanol into feedstock oils that enhanced biodiesel conversion reaction [19]. The highest yield could be because of maximum ester conversion by the ample amount of catalyst that rendered optimum solubility of methanol into feedstock oil. Lower catalyst concentration (e.g. 0.5% KOH) re- sulted in lower biodiesel yields, like 57.8%, 53.3% and 49.0% for palm, sunflower and soybean oils, respectively. It could be due to rather limited biodiesel conversion (i.e. transesterification) because of less influence of catalyst on the solubilit y of methanol into feedstock oil. Likewise, at higher catalyst (i.e. KOH) concentrations (e.g. 1.25% and 1.50%) than 1.0% the biodiesel yields declined re- markably due to the formation of fatty acid salts (i.e. soap) by saponification process [19]. The produced soap blurred the clear separation of biodiesel from glycerin and also increased the viscosity of the biodiesel, and thus lowering the biodiesel yield. Therefore, 1.0% KOH was considered to be the optimum catalyst concentration and utilized for further inve stigations. 3.3. Influence of Reaction Temperature The transesterification reaction was conducted at three different temperatures (e.g. 55˚C, 60˚C and 65˚C), while other parameters were maintained constant like, metha- nol/oil molar ratio of 6:1, catalyst concentration of 1.0% 40 45 50 55 60 65 70 75 80 85 90 0.50% 0.75% 1.00%1.25% 1.50% Catalyst Conc. (KOH%) Y i el d of Biodi esel (% ) Palm Oil Sunflower Oil Soy bean Oil Figure 3. Influence of catalyst concentration (%KOH) on biodiesel yields for various feedstock oils, with other parameters constant (methanol/oil molar ratio of 6:1, reaction temperature of 65˚C, reaction period of 3 hours and stirring speed of 130 pm). r Copyright © 2013 SciRes. JSBS  M. E. HOQUE, L. P. GEE 167 KOH, reaction period of 3 hours and stirring speed of 130 rpm. Although the biodiesel conversion (i.e. trans- esterification) process can happen at a range of tempera- tures (e.g. from ambient to a temperature close to the boiling point of methanol) usually higher temperature speeds up the reaction rate and shortens the reaction pe- riod. The maximum reaction temperature was set at 65˚C with the consideration that the temperature higher than 65˚C might cause burning of methanol. On the other hand, the minimum temperature of 55˚C was taken into consideration with the assumption that too low tempera- ture would slow down the reaction rate and thus increase the reaction period too long, which might not be techni- cally favorable. The overall biodiesel yields for different feedstock oils at different reaction temperatures are presented in Table 1. The results show that the biodiesel yields for all feed- stocks significantly increase with the increase of tem- perature as demonstrated in Figure 4. For example, the biodiesel yields at the temperatures of 55˚C, 60˚C and 65˚C were found to be 81.38%, 83.24% and 87.5%, re- spectively for palm oil, 78.31%, 80.17% and 83.6%, re- spectively for sunflower oil, and 76.07%, 77.93% and 80.2%, respectively for soybean oil. The highest yields of 87.5%, 83.6% and 80.2% were obtained for the palm, sunflower and soybean oil feedstocks, respectively at highest reaction temperature of 65˚C. This could be at- tributed to the phenomenon that the higher temperature (means higher energy input) increased the collision among the reacting molecules, which accelerated the chemical reaction (i.e. transesterification process) and thus increased the biodiesel yields. However, there had been variation in highest yield for different feedstock oils that could be because of the variation in their fatty acid compositions. Therefore, the reaction temperature of 65˚C was considered as optimal and was maintained for evaluating other p ar ameter s. Some other studies also reported the similar findings that the biodiesel yield was influenced by the reaction temperature [19,21]. In the investigation of the influence of temperature on the biodiesel conversion, National Biodiesel Board [20] observed that the increase of tem- perature from 30˚C to 50˚C increased the biodiesel yield by 10%. Canakci and Gerpen reported that the biodiesel yield had declined at the reaction temperature as high as 70˚C [22]. They argued that too high temperature pro- moted the saponification reaction that negatively affected the transesterification pro cess (i.e. biodiesel conversion). 3.4. Influence of Reaction Period Besides the temperature, reaction period also plays sig- nificant role in the optimization of biodiesel yield that allows completion of the biodiesel conversion (i.e. trans- esterification) reaction. Therefore, the transesterification reaction was conducted for the periods of 1, 2, 3 and 4 hours maintaining other parameters constant (oil/metha- nol molar ratio of 6:1, catalyst concentration of 1.0% weight of oil, reaction temperature of 65˚C and stirring speed of 130 rpm) to evaluate the influence of reaction period on the biodiesel yields. The overall biodiesel yields for different feedstock oils at different reaction periods are presented in Table 1. The yields were found to be increased significantly with the increase of reaction period reaching maximum values at the reaction period of 3 hours above which the yield values declined as demonstrated in Figure 5. For example, the reaction pe- riods of 1, 2, 3 and 4 hrs resulted in the biodiesel yields of 79.709%, 83.98%, 87.5% and 87.5%, respectively for palm oil, 76.1%, 80.56%, 83.6% and 81.7%, respectively for sunflower oil, and 72.61%, 76.03%, 80.2% and 77.7%, respectively for soybean oil. At the reaction pe- 65 70 75 80 85 90 5560 65 Reac t i on Temperat ure (oC) Biodies el Yields (%) Palm Sunflower Soybean (˚C) Figure 4. Influence of reaction temperature on biodiesel yields for various feedstock oils, with other parameters constant (methanol/oil molar ratio of 6:1, catalyst (KOH) concentration of 1.0%, reaction period of 3 hours and stirring speed of 130 rpm). Copyright © 2013 SciRes. JSBS 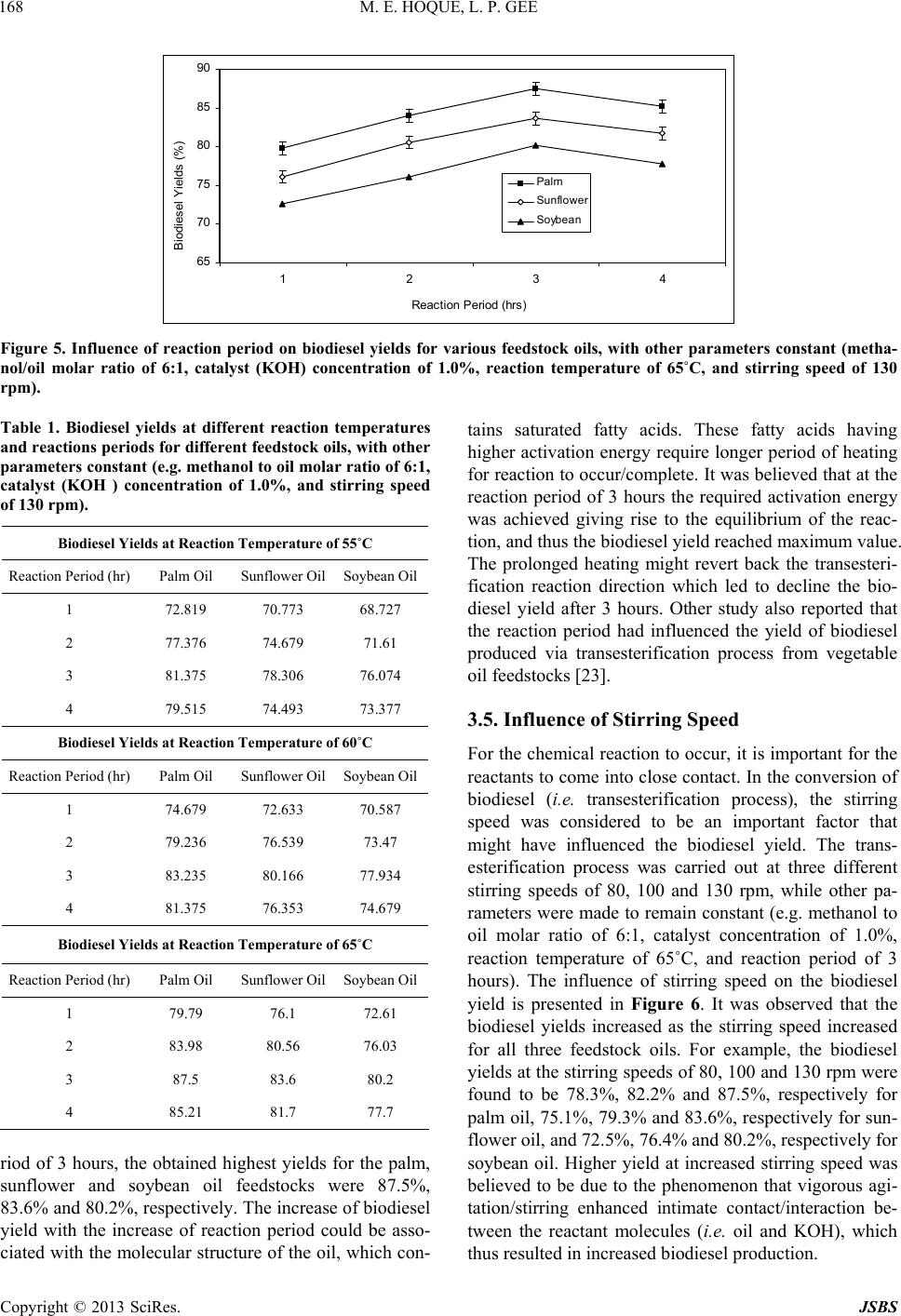 M. E. HOQUE, L. P. GEE 168 65 70 75 80 85 90 1234 Reac ti on P eri od (hrs) B i odi esel Y i el ds (% ) Palm Sunflower Soybean Figure 5. Influence of reaction period on biodiesel yields for various feedstock oils, with other parameters constant (metha- nol/oil molar ratio of 6:1, catalyst (KOH) concentration of 1.0%, reaction temperature of 65˚C, and stirring speed of 130 rpm). Table 1. Biodiesel yields at different reaction temperatures and reactions periods for different fee dstock oils, with other parameters constant (e.g. methanol to oil molar ratio of 6:1, catalyst (KOH ) concentration of 1.0%, and stirring speed of 130 rpm). Biodiesel Yields at Reaction Temperature of 55˚C Reaction Period (hr) Palm Oil Sunfl owe r Oil Soybean Oil 1 72.819 70.773 68.727 2 77.376 74.679 71.61 3 81.375 78.306 76.074 4 79.515 74.493 73.377 Biodiesel Yields at Reaction Temperature of 60˚C Reaction Period (hr) Palm Oil Sunfl owe r Oil Soybean Oil 1 74.679 72.633 70.587 2 79.236 76.539 73.47 3 83.235 80.166 77.934 4 81.375 76.353 74.679 Biodiesel Yields at Reaction Temperature of 65˚C Reaction Period (hr) Palm Oil Sunfl owe r Oil Soybean Oil 1 79.79 76.1 72.61 2 83.98 80.56 76.03 3 87.5 83.6 80.2 4 85.21 81.7 77.7 riod of 3 hours, the obtained highest yields for the palm, sunflower and soybean oil feedstocks were 87.5%, 83.6% and 80.2%, respectively. The increase of biodiesel yield with the increase of reaction period could be asso- ciated with the molecular structure of the oil, which con- tains saturated fatty acids. These fatty acids having higher activation energy require longer period of heating for reaction to occur/complete. It was believed that at the reaction period of 3 hours the required activation energy was achieved giving rise to the equilibrium of the reac- tion, and thus the biodiesel yield reached maximum value. The prolonged heating might revert back the transesteri- fication reaction direction which led to decline the bio- diesel yield after 3 hours. Other study also reported that the reaction period had influenced the yield of biodiesel produced via transesterification process from vegetable oil feedstocks [23]. 3.5. Influence of Stirring Speed For the chemical reaction to occur, it is important for the reactants to come into close contact. In the conversion of biodiesel (i.e. transesterification process), the stirring speed was considered to be an important factor that might have influenced the biodiesel yield. The trans- esterification process was carried out at three different stirring speeds of 80, 100 and 130 rpm, while other pa- rameters were made to remain constant (e.g. methanol to oil molar ratio of 6:1, catalyst concentration of 1.0%, reaction temperature of 65˚C, and reaction period of 3 hours). The influence of stirring speed on the biodiesel yield is presented in Figure 6. It was observed that the biodiesel yields increased as the stirring speed increased for all three feedstock oils. For example, the biodiesel yields at the stirr ing speeds of 80, 100 and 130 rpm were found to be 78.3%, 82.2% and 87.5%, respectively for palm oil, 75.1%, 79.3% and 83.6%, respectively for sun- flower oil, and 72.5%, 76.4% and 80.2%, respectively for soybean oil. Higher yield at increased stirring speed was believed to be due to the phenomenon that vigorous agi- tation/stirring enhanced intimate contact/interaction be- tween the reactant molecules (i.e. oil and KOH), which thus resulted in increased biodiesel production. Copyright © 2013 SciRes. JSBS  M. E. HOQUE, L. P. GEE 169 65 70 75 80 85 90 80100 130 S t irring Speed (rpm) Biodiesel Yields (%) Palm Sunflower Soybean Figure 6. Influence of stirring speed on biodiesel yields for various feedstock oils, with other parameters constant (metha- nol/oil molar ratio of 6:1, catalyst (KOH) concentration of 1.0%, reaction temperature of 65˚C and reaction period of 3 hrs). 4. Conclusion Various plant feedstocks (palm, soybean and sunflower oil) were exploited to successfully produce biodiesel via transesterification process which is considered to be more economic in compared to other processes (e.g. su- percritical methanol, thermal cracking, microemulsion etc.). Besides the cost effectiveness, transesterification process allows a wide range of parameters to play with for the maximization of biodiesel yield and easy recovery of byproduct glycerin. The process parameters had direct influence on the biodiesel yields for all three types of feedstocks. For maximum biodiesel yields, the optimum parameters were determined to be methanol/oil molar ratio of 6:1, catalyst (KOH) concentration of 1.0%, reac- tion temperature of 65˚C, a reaction period of 3 hours and stirring speed of 130 rpm. At op timum condition, the maximum biodiesel yields were measured to/be 87.5%, 83.6% and 80.2% of the palm, sunflower and soybean oil feedstocks, respectively. In conclusion, the vegetable oil feedstocks hold high potential as renewable plant re- sources for the production of biodiesel that is to contrib- ute as a sustainable solution to the ever increasing fuel oil demands. However, the exploitation of vegetable oil feedstocks in producing biodiesel may give arise to an- other concern (e.g. extensive acreage required for suffi- cient production of oilseed crops etc.), which should be dealt accordingly. 5. Acknowledgements The authors would like to thank the Faculty of Engineer- ing, University of Notting ham Malaysia Campus for pro- viding the fund to carry out this highly prospective re- search. REFERENCES [1] R Kalscheuer, T Stolting and A Steinbuchel, “Micro- diesel: Escherichia coli Engineered for Fuel Production,” Microbiology, Vol. 152, No. 9, 2006, pp. 2529-2536. doi:10.1099/mic.0.29028-0 [2] P. M. Schenk, S. R. Thomas-Hall, E. Stephens, U. C. Marx, J. H. Mussgnug, C. Posten, O. Kruse and B. Hankamer, “Second Generation Biofuels: High-Efficiency Microal- gae for Biodiesel Production,” Bioenergy Research, Vol. 1, No. 1, 2008, pp. 20-43. doi:10.1007/s12155-008-9008-8 [3] K. M. Weyer, D. R. Bush, A. Darzins and B. D. Willson, “Theoretical Maximum Algal Oil Production,” Bioenergy Research, Vol. 3, No. 2, 2010, pp. 204-213. doi:10.1007/s12155-009-9046-x [4] T. Prokop, “Imperial Western Products,” In: Communica- tion, Chandler: St., Coachella, 2002, p. 14970. [5] J. Jitputti, B. Kitiya nan, P. Rangsunvigit, K. Bunyakiat, L. Attanatho and P. Jenvanitpanjakul, “Transesterification of Crude Palm Kernel Oil and Crude Coconut Oil by Dif- ferent Solid Catalyst,” Chemical Engineering Journal, Vol. 116, No. 1, 2006, pp. 61-66. doi:10.1016/j.cej.2005.09.025 [6] T. Krawczyk, “Biodiesel-Alternative Fuel Makes Inroads but Hurdles Remain,” INFORM, Vol. 7, No. 8, 1996, pp. 801-829. [7] B. Freedman, E. H. Pryde and T. L. Mounts, “Variables Affecting the Yields of Fatty Esters from Transesterified Vegetable Oils,” Journal of the American Oil Chemists’ Society, Vol. 61, No. 10, 1984, pp. 1638-1643. doi:10.1007/BF02541649 [8] B. Freedman, R. O. Butterfield and E. H. Pryde, “Trans- esterification Kinetics of Soybean Oil,” Journal of the American Oil Chemists’ Society, Vol. 63, No. 10, 1986, pp. 1375-1380. doi:10.1007/BF02679606 [9] F. Ma, L. D. Clements and M. A. Hana, “The Effects of Catalyst, Free Fatty Acids and Water on transesterifica- tion of Beef Tallow,” Transactions of the ASAE (Ameri- can Society of Agricultural Engineers), Vol. 41, No. 5, 1998, pp. 1261-1264. [10] M. Canakci and J. V. Gerpen, “Biodiesel Production via Acid Ca talysi s,” Transactions of the ASAE (American So- ciety of Agricultural Engineers), Vol. 42, No. 5, 1999, pp. 1203-1210. Copyright © 2013 SciRes. JSBS  M. E. HOQUE, L. P. GEE 170 [11] M. Iso, B. Chen, M. Eguchi, T. Kudo and S. Shrestha, “Production of Biodiesel Fuel from Triglycerides and Alcohol Using Immobilized Lipase,” Journal of Molecu- lar Catalysis B: Enzymatic, Vol. 16, No. 1, 2001, pp. 53- 58. doi:10.1016/S1381-1177(01)00045-5 [12] G. Antonlin, F. V. Tinaut, Y. Brceno, V. Castaño, C. Póez and A. I. Ramírez, “Optimisation of Biodiesel Pro- duction by Sunflower Oil Transesterification,” Biore- source Technology, Vol. 83, No. 2, 2002, pp. 1111-1114. [13] A. Demirbas, “Biodiesel Fuels from Vegetable Oils via Catalytic and Non-Catalytic Supercritical Alcohol Trans- esterifications and Other Methods: A Survey,” Energy Conversion and Management, Vol. 44, No. 13, 2003, pp. 2093-2109. doi:10.1016/S0196-8904(02)00234-0 [14] U. Rashid and F. Anwar, “Production of Biodiesel through Optimized Alkaline Catalysed Transesterification of Ra- peseed Oil,” Fuel, Vol. 87, No. 3, 2008, pp. 265-273. doi:10.1016/j.fuel.2007.05.003 [15] F. Ma and M. A. Hanna, “Biodiesel Production: A Re- view,” Bioresource Technology, Vol. 70, No. 1, 1999, pp. 1-15. doi:10.1016/S0960-8524(99)00025-5 [16] J. Marchetti, V. Miguel and A. Errazu, “Possible Methods for Biodiesel Production,” Renewable & Sustainable En- ergy Reviews, Vol. 11, No. 6, 2007, pp. 1300-1311. doi:10.1016/j.rser.2005.08.006 [17] M. Cetinkaya and F . Karaosmanoglu, “Op tim is ation of Base- Catalysed Transesterification Reaction of Used Cooking Oil,” Energy & Fuels, Vol. 18, No. 6, 2004, pp. 1888- 1895. doi:10.1021/ef049891c [18] A. Khan, “Research into biodiesel, “Kinetics and Catalyst Development. Individual Inquiry,” University of Queen- sland, Brisbane, 2002. [19] M. E. Hoque, A. Singh and Y. L. Chuan, “Biodiesel from Low Cost Feedstocks: The Effects of Process Parameters on the Biodiesel Yield,” Biomass and Bioenergy, Vol. 35, No. 4, 2011, pp. 1582-1587. [20] National Biodiesel Board, 2009. www.biodieselorg [21] K. G. Georgogianni, M. G. Kontominas, E. Tegou, D. Avlonitis and V. Vergis, “Biodiesel Production: Reaction and Process Parameters of Alkali-Catalysed Transesteri- fication of Waste Frying-Oils,” Energy & Fuels, Vol. 21, No. 5, 2007, pp. 3023-3027. doi:10.1021/ef070102b [22] M. Canakci and J. V. Gerpen, “Biodiesel Production from Oils and Fats with High Free Fatty Acids,” Transactions of the ASAE, Vol. 44, No. 6, 2001, pp. 1429-1436. [23] A. Demirbas, “Biodiesel from Vegetable Oils via Trans- esterification in Supercritical Methanol,” Energy Conver- sion and Management, Vol. 43, No. 17, 2002, pp. 2349- 2356. doi:10.1016/S0196-8904(01)00170-4 Copyright © 2013 SciRes. JSBS
|