Paper Menu >>
Journal Menu >>
 Chinese Medicine, 2010, 1, 69-74 doi:10.4236/cm.2010.13014 Published Online December 2010 (http://www.SciRP.org/journal/cm) Copyright © 2010 SciRes. CM Myocardial Infarction and Intracerebral Hemorrhage in a Chinese Population: Relationship with Lipoproteins and Adipokines Jessica Smith1, Zhenjun Liu2, Huiling Lu1,2, Daowen Wang2, Katherine Cianflone1 1Centre de Recherche Institu t Universitaire de Cardiologie et de Pneumologie de Québec, Québec, Canada 2Tongji Medical Cen tre, HuaZhong University of Science and Tech nology, Wuhan, China Email: katherine.cianflone@crhl.ulaval.ca Received June 15, 2010; revised August 23, 2010; accepted September 30, 2010 Abstract BACKGROUND: Adipokines and inflammatory factors play an important role in disease progression. Two cardiovascular diseases which have important contributions to mortality and morbidity in China are in- tracerebral hemorrhage (ICH) and myocardial infarction (MI). Acylation stimulating protein has been shown in North American populations to have strong associations with risk factors for MI. Complement C3 (C3) a component of the innate complement immune system is the precursor protein to ASP; C3 has been impli- cated in the pathogenesis of ICH. OBJECTIVE: In this case-control study we examined the association be- tween BMI, lipoproteins adiponectin, C3 and ASP) in a Chinese population. METHODS AND RESULTS: Three groups of subjects were studied: ICH group (N = 41), MI group (N = 60) and a control group (N = 44). There was no difference in BMI for either ICH or MI compared to controls (Control: 22.3 ± 0.3 kg/m2; ICH: 21.3 ± 0.4 vs MI: 22.5 ± 0.2, ICH and MI versus control pNS). The ICH group had lower LDL-C (Control: 3.21 ± 0.13 mmol/L; ICH: 2.54 ± 0.13; MI: 2.99 ± 0.13; ICH vs control p < 0.05), total cholesterol (Control: 5.06 ± 0.16 mmol/L; ICH: 4.40 ± 0.15 ; MI: 4.51 ± 0.14 ; ICH and MI vs control p < 0.05),, HDL-C (Control: 1.34 ± 0.05 mmol/L; ICH: 1.22 ± 0.06; MI: 0.95 ± 0.04; ICH and MI vs control p < 0.05), and C3 (Control: 2.58 0.21 g/L; ICH: 1.85 0.19; MI: 2.87 0.16; ICH vs control p < 0.05), and higher TG (Control: 1.10 ± 0.07 mmol/L; ICH: 1.77 ± 0.17; MI: 1.61 ± 0.10, ICH and MI vs control p < 0.05), compared to the controls. The MI group had lower total cholesterol and HDL-C and higher TG and ASP (Control: 33.70 2.07 nM; ICH: 35.10 2.33; MI: 41.50 1.81; MI vs control p < 0.05) compared to control. CONCLUSION: Chinese men and women who had an MI displayed elevated ASP unrelated to an increase in the precursor protein, C3. Chinese men and women with ICH had ASP levels similar to controls yet lower C3 suggesting that C3, and the regulation of C3 conversion to ASP may be important in ICH disease pathology. Keywords: Myocardial Infarction, Intracerebral Hemorrhage, Acylation Stimulating Protein, Complement C3, Adiponectin 1. Introduction Hemorrhagic stroke, specifically intracerebral hemor- rhage (ICH), has a high rate of morbidity and mortality yet it has not received the same intense research interest as ischemic vascular diseases [1]. In fact, in the past 20 years, little progress has been made to improve disease outcomes [2]. In Europe and North America there is a very low incidence of this type of stroke, however, in China, ICH occurs more frequently [3,4]. Economic and social changes that are taking place in many Asian coun- tries, including China, have also led to increasing inci- dence of ischemic heart diseases, such as myocardial infarction (MI) [5]. The risk factors for MI are well known in Caucasian populations and include obesity, hypertension, and high apolipoprotein B to apolipoprotein A1 (apoB/apoA1) ratio, as well as other factors [6-8]. While these may be widespread risk factors, as suggested by the INTER- HEART study, it is unknown if these same risk factors will apply to a Chinese population [6,7]. Recently, in- creasing rates of obesity, as well as high rates of smoking  70 J. SMITH ET AL. and increasing incidence of insulin resistance and diabe- tes have begun to coincide with increasing rates of MI in China [9]. By contrast, Chen et al found that a BMI be- low 20 was also a risk for factor ischemic heart disease in the Chinese population [2]. Hypertension, which is prevalent in the Chinese population, is another major risk factor for both ICH and MI [10,8]. Taken together, these risk factors may be responsible for the higher rates of cardiovascular diseases (CVD) and the development of CVD at a young age in Chinese individuals [11]. In Caucasian populations, obesity and CVD are asso- ciated with alterations in several adipose tissue derived hormones, including acylation stimulating protein (ASP), its precursor complement C3 (C3), and adi- ponectin [12]. It is believed that these hormones may play roles in mediating some co-morbidities of obesity [12] due to their effects on lipid metabolism. ASP in- creases the uptake of dietary triglyceride (TG) into adipose tissue for storage; however, it is believed that ASP resistance can develop since higher ASP levels are often associated with delays in TG clearance and the development of dyslipidemia. ICH and MI have different etiologies and ICH may not be associated with lipid disorders. However, the precur- sor protein to ASP, C3, is a key component of the com- plement innate immune system and has been implicated in ICH disease progression. In rodent models, C3 gene expression has been shown to increase immediately after the occurrence of an ICH [13] and mice lacking C3 are protected from further brain damage after ICH [14,10]. In humans who have had an ICH, it was reported that they had elevated C3 concentrations [14]. Whether ASP and adiponectin concentrations will be different in indi- viduals who have had an ICH is unknown. Therefore, our goal was to evaluate the concentrations of the hormones ASP, C3 and adiponectin, as well as blood pressure, an- thropometric measurements and blood lipids, in subjects who have had MI and ICH in China. 2. Materials and Methods 2.1. Subjects One hundred and forty-five participants were recruited at the Tongji Medical Centre, Tongji Hospital, HuaZhong University of Science and Technology, Wuhan, Hubei, China to participate in a case-control study. The subjects were a subset of men and women who had participated in a multicenter study for the assessment of risk factors of stroke sponsored by the Ministry of Science and Tech- nology of China and were included in this analysis based on the following inclusion criteria: non-diabetic (fasting plasma glucose less than 7.0 mmol/L or casual plasma glucose less than 11.1 mmol/L) and age between 45 and 75. Exclusion criteria for all groups included other types of stroke (cerebral thrombosis, lacunar infarction, tran- sient ischemic attack, subarachnoid hemorrhage, embolic brain infarction, brain tumors and cerebrovascular mal- formation), and severe systemic diseases (collagenosis, endocrine and metabolic diseases, inflammation, liver, neoplastic or renal diseases). Forty-four patients were classified as controls, 41 as having had ICH and 60 as having had MI. Control participants were in-patients at the hospital with minor illnesses from the departments of ophthalmology, gastroenterology, otorhinolaryngology, and orthopedics and were free of neurological diseases or CVD. Diagnosis of ICH was based on neurological ex- amination including CT scan, MRI or both and subjects were evaluated 1 to 12 months after ICH. Diagnosis of MI (ST-segment elevation myocardial infarction) was based on 12-lead ECG and data was collected 1 month after diagnosis. None of the subjects were taking medi- cation at the time of the data collection that affected lip- ids or blood pressure. Informed consent was obtained and the study was approved by the Tongji Hospital Eth- ics Committee. 2.2. Anthropometric Measurements Height and weight were measured using standard meth- ods. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Each participant completed a questionnaire providing health and lifestyle information including participation in regular exercise (yes or no), smoking status (non smoker, previous smoker and current smoker), and medical information on previous/current diseases and medications. Two meas- urements each for systolic (SBP) and diastolic blood pressure (DBP) were taken on two different days. Re- ported values for SBP and DBP are an average of these two measurements. 2.3. Lipoproteins Venous blood samples were collected after an overnight fast (12 hours) from an antecubital vein. The samples were centrifuged, aliquoted and frozen at −80 C. Plasma samples were analyzed for concentrations of nonesteri- fied fatty acids (NEFA), triglycerides (TG), total choles- terol (TC), high-density lipoprotein cholesterol (HDL-C), apolipoprotein B (apoB), apolipoprotein A1 (apoA1), adiponectin, C3 and ASP. Plasma NEFA was determined by colorimetric enzymatic assay (WAKO Chemicals, Tokyo, Japan) and TG was determined via GPO-PAP method. TC was analyzed by COD-PAP. HDL-C con- centration was determined using an enzymatic colori- Copyright © 2010 SciRes. CM 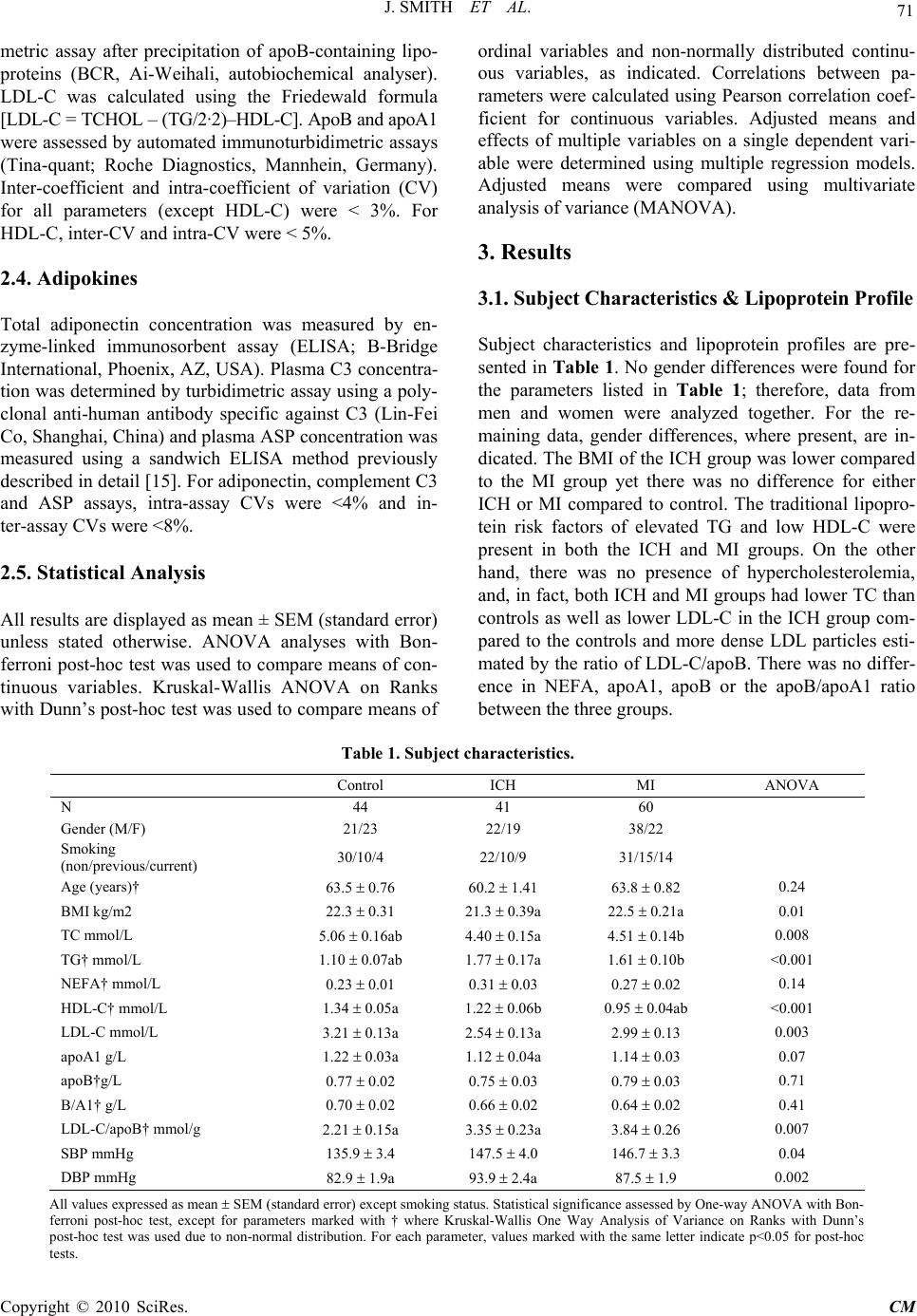 J. SMITH ET AL. Copyright © 2010 SciRes. CM 71 metric assay after precipitation of apoB-containing lipo- proteins (BCR, Ai-Weihali, autobiochemical analyser). LDL-C was calculated using the Friedewald formula [LDL-C = TCHOL – (TG/2·2)–HDL-C]. ApoB and apoA1 were assessed by automated immunoturbidimetric assays (Tina-quant; Roche Diagnostics, Mannhein, Germany). Inter-coefficient and intra-coefficient of variation (CV) for all parameters (except HDL-C) were < 3%. For HDL-C, inter-CV and intra-CV were < 5%. 2.4. Adipokines Total adiponectin concentration was measured by en- zyme-linked immunosorbent assay (ELISA; B-Bridge International, Phoenix, AZ, USA). Plasma C3 concentra- tion was determined by turbidimetric assay using a poly- clonal anti-human antibody specific against C3 (Lin-Fei Co, Shanghai, China) and plasma ASP concentration was measured using a sandwich ELISA method previously described in detail [15]. For adiponectin, complement C3 and ASP assays, intra-assay CVs were <4% and in- ter-assay CVs were <8%. 2.5. Statistical Analysis All results are displayed as mean ± SEM (standard error) unless stated otherwise. ANOVA analyses with Bon- ferroni post-hoc test was used to compare means of con- tinuous variables. Kruskal-Wallis ANOVA on Ranks with Dunn’s post-hoc test was used to compare means of ordinal variables and non-normally distributed continu- ous variables, as indicated. Correlations between pa- rameters were calculated using Pearson correlation coef- ficient for continuous variables. Adjusted means and effects of multiple variables on a single dependent vari- able were determined using multiple regression models. Adjusted means were compared using multivariate analysis of variance (MANOVA). 3. Results 3.1. Subject Characteristics & Lipoprotein Profile Subject characteristics and lipoprotein profiles are pre- sented in Table 1. No gender differences were found for the parameters listed in Table 1; therefore, data from men and women were analyzed together. For the re- maining data, gender differences, where present, are in- dicated. The BMI of the ICH group was lower compared to the MI group yet there was no difference for either ICH or MI compared to control. The traditional lipopro- tein risk factors of elevated TG and low HDL-C were present in both the ICH and MI groups. On the other hand, there was no presence of hypercholesterolemia, and, in fact, both ICH and MI groups had lower TC than controls as well as lower LDL-C in the ICH group com- pared to the controls and more dense LDL particles esti- mated by the ratio of LDL-C/apoB. There was no differ- ence in NEFA, apoA1, apoB or the apoB/apoA1 ratio between the three groups. Table 1. Subject characteristic s. Control ICH MI ANOVA N 44 41 60 Gender (M/F) 21/23 22/19 38/22 Smoking (non/previous/current) 30/10/4 22/10/9 31/15/14 Age (years)† 63.5 0.76 60.2 1.41 63.8 0.82 0.24 BMI kg/m2 22.3 0.31 21.3 0.39a 22.5 0.21a 0.01 TC mmol/L 5.06 0.16ab 4.40 0.15a 4.51 0.14b 0.008 TG† mmol/L 1.10 0.07ab 1.77 0.17a 1.61 0.10b <0.001 NEFA† mmol/L 0.23 0.01 0.31 0.03 0.27 0.02 0.14 HDL-C† mmol/L 1.34 0.05a 1.22 0.06b 0.95 0.04ab <0.001 LDL-C mmol/L 3.21 0.13a 2.54 0.13a 2.99 0.13 0.003 apoA1 g/L 1.22 0.03a 1.12 0.04a 1.14 0.03 0.07 apoB†g/L 0.77 0.02 0.75 0.03 0.79 0.03 0.71 B/A1† g/L 0.70 0.02 0.66 0.02 0.64 0.02 0.41 LDL-C/apoB† mmol/g 2.21 0.15a 3.35 0.23a 3.84 0.26 0.007 SBP mmHg 135.9 3.4 147.5 4.0 146.7 3.3 0.04 DBP mmHg 82.9 1.9a 93.9 2.4a 87.5 1.9 0.002 All values expressed as mean SEM (standard error) except smoking status. Statistical significance assessed by One-way ANOVA with Bon- ferroni post-hoc test, except for parameters marked with † where Kruskal-Wallis One Way Analysis of Variance on Ranks with Dunn’s post-hoc test was used due to non-normal distribution. For each parameter, values marked with the same letter indicate p<0.05 for post-hoc tests.  J. SMITH ET AL. Copyright © 2010 SciRes. CM 72 3.2. Hypertension Chi squared analysis of prevalence of hypertension (de- fined as SBP/DBP ≥ 140/90 mm Hg) revealed that there was a difference in the prevalence of hypertension, with the ICH having significantly higher incidence than con- trol (p = 0.01). SBP was significantly different by one- way ANOVA between the three groups. DBP was also significantly different with the ICH group having a sig- nificantly higher DBP than controls, even when cor- rected for BMI (adjusted means; control 82.6 1.87 mmHg; ICH 92.3 2.40; MI 86.9 1.89, p = 0.0003 MANOVA). Both SBP and DBP correlated with BMI (SBP, R = 0.21, p = 0.02; DBP, R = 0.18, p = 0.04) and TG (SBP, R = 0.19, p = 0.01; DBP, R = 0.26, p = 0.003) in a pooled analysis of data from all groups. In addition, adiponectin correlated with DBP (R = -0.24, p=0.006). In a multivariate model, with data from all groups pooled, including BMI, TG and group, only BMI (p = 0.02) and TG (p = 0.05) significantly contributed to predicting 12.0% (p = 0.002) of the SBP variance; while BMI (p = 0.007), TG (p = 0.03), and group (p = 0.004) all signifi- cantly and independently predicted 17.2% of DBP (P < 0.001). 3.3. Adipokines There were no differences in adiponectin as shown in Figure 1 (Control, 6.10 0.61 mg/mL; ICH, 4.81 0.54; MI, 6.05 0.45; pNS, Kruskal-Wallis One Way ANOVA on Ranks), but C3 was lower in the ICH group compared to both the control and the MI groups (control, 2.58 0.21 g/L; ICH, 1.85 0.19; MI, 2.87 0.16; p < 0.001 ANOVA) (Figure 1). When analyzed separately for gender, there was a significant difference in C3 con- centration between men and women with ICH (ICH men 2.42 0.28 g/L vs. ICH women 1.23 0.17 g/L, p<0.05, ANOVA with post-hoc test) and the differences seen between control and ICH and between ICH and MI were solely due to the lower C3 value in ICH women and not ICH men (data not shown). However, in the control group, there was no difference in C3 values between men and women. When data from all groups were pooled C3 significantly correlated with HDL-C (R = –0.23 p = 0.006) and adiponectin correlated with TG (R = –0.21 p = 0.01) and apoA1 (R = 0.22, p = 0.01). ASP was higher in the MI group compared to the con- trol (control, 33.70 2.07 nM; ICH, 35.10 2.33 nM; MI, 41.50 1.81 nM; p = 0.002, Kruskal-Wallis One Way ANOVA on Ranks). Exercise frequency moderately, yet significantly, correlated negatively with ASP, even when adjusted for BMI (adjusted R2 = 0.10, p = 0.003). In ad- dition, ASP correlated with NEFA (R = 0.21, p = 0.01), TG (R = 0.22 p = 0.01) and HDL-C (R = –0.26 p = 0 5 10 15 20 25 30 35 40 45 aa ASP Control MI ICH ASP nM 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 aab b Control MI ICH C3 C3 g/L 0.0 0.1 0.2 0.3 0.4 0.5 aa %ASP/C3 Control MI ICH %ASP/C3 0 1 2 3 4 5 6 7 Adiponectin Control MI ICH adiponectin g/ m L Figure 1. Acylation stimulating protein (ASP, panel A), complement component C3 (C3, panel B), the percentage of ASP to C3 (%ASP/C3, panel C), and adiponectin (panel D) in control, intracerebral hemorrhagic stroke (ICH) and myocardial infarction (MI). Results are presented as mean standard error of the mean (SEM). Statistical significance for C3 was assessed by one-way ANOVA with Bonferroni post-hoc test. Statistical significance for ASP, %ASP/C3 and adiponectin assessed by Kruskal-Wallis One Way ANOVA on Ranks with Dunn’s post-hoc test due to non- normal distribution. For each parameter, values marked with the same letter indicate p < 0.05 for post-hoc tests. 0.002). The %ASP/C3 ratio was significantly lower in the control group versus the ICH group, suggesting that the ICH group had either a higher rate of conversion of C3 to ASP, or decreased degradation or clearance of ASP (control, 0.32 0.03%; ICH, 0.45 0.05%; MI, 0.33 0.03%; p = 0.005, Kruskal-Wallis One Way ANOVA on Ranks). Overall, the ICH and MI group had altered lipoprotein profile, altered adipokines and were more hypertensive than controls. 4. Discussion The relationship between blood pressure and MI is well established and is a continuous, positive relationship even at levels below the cut-point for clinical hyperten- sion [8]. There are confounding and additive effects of obesity, hypertension and dyslipidemia on the risk for MI and the independent contribution of each of these factors is difficult to separate. However, in the current study all of the participants were lean and participants within the control and MI groups did not have hypertension. Therefore, dyslipidemia and/or adipokines may poten- tially be the most prevalent influences for this study. Several prospective studies on ICH have found a strong positive relationship between ICH and hyperten- sion [16,17] and we found supporting evidence of this: a greater proportion of participants had hypertension in the  J. SMITH ET AL. 73 ICH group compared to control or MI. In addition, DBP was higher in the ICH group compared to control, and BMI, TG, and group significantly contributed to predict- ing DBP. Previous studies have shown that compared to other stroke subtypes, ICH is more strongly associated with hypertension [17], possibly because the small ves- sels of the brain are vulnerable to damage [18]. The ICH group also had a lower BMI than the other groups and the effects of hypertension may be particularly relevant in the context of low BMI [19]. Hypocholesterolemia has also been suggested as a risk factor for ICH possibly because low cholesterol levels can adversely affect the integrity of the blood vessel in the brain, making them more prone to damage, especially under conditions of hypertension [20,21]. We did find that our ICH group had lower TC and lower LDL-C than the controls. Therefore, our ICH group displayed three potentially exacerbating risk factors for ICH: low BMI, hypocholesterolemia and hypertension. These three factors have not been looked at in combi- nation in predicting the risk of ICH yet all three are present in our ICH group. The MI group did not display many of the typical features of atherogenic dyslipidemia seen in Western populations: they had lower TC than controls and had comparable concentrations of NEFA, LDL-C, apoA1, apoB, and apoB/apoA1 to controls. However, the MI group did have elevated TG and low HDL-C. Also, the MI group did not have an elevated BMI, which is typi- cally seen in Western populations with this disease. The factors used to identify 10 year risk of CVD by the Third Report of the National (USA) Cholesterol Education Program Adult Treatment Panel III are age (> 45 years for men and > 55 years considered as high risk), gender (male at higher risk), elevated total cho- lesterol, low HDL-C, elevated SBP, and smoking status [8]. Much research has also indicated that high apoB and low apoA1 may be are better indicators of risk than cholesterol alone [6]. However, in this Chi- nese population positive for MI, only a low HDL-C was present. Therefore, many of the Western criteria used to calculate the risk for developing CVD may not be applicable in a lean Chinese population which is nonetheless at risk for developing CVD. Other, non-traditional, risk factors may need to be imple- mented to correctly identify those at risk, such as adi- pokines. And, in fact, ASP, which has been linked with CVD in several studies [22], was higher in the MI group compared to controls. Very few studies have evaluated adipokines in relation to ICH although C3 has been implicated in ICH patho- genesis [23,13,10]. We did see differences in the %ASP/C3, ASP, and C3 but not adiponectin, as has been shown with ICH and stroke in a previous study [24]. Söderberg and colleagues found no association of adi- ponectin with hemorrhagic stroke in an European popu- lation; however, there were very few cases of hemor- rhagic stroke included in their analysis [24]. Complement C3, as part of the complement immune cascade, has been studied post-ICH. C3-deficient mice are protected from further brain injury due to an inhibi- tion of the immune cascade [25]. In addition, individuals who have had ICH had increased complement C3 com- pared to controls [14], yet in our study individuals who have had an ICH had lower C3. While we do not know the concentration of C3 prior to the brain injury, poten- tially the lower C3 could be a survival bias protecting individuals who have had ICH. Our study, along with others, supports further research in the area of C3 in dis- ease prediction, prevention and treatment. We also found that, despite lower C3, ASP levels were maintained suggesting that there was increased conversion of C3 to ASP, or a decreased clearance or degradation of ASP in the ICH group. Normally, only a small proportion of C3 is converted to ASP, and the concentration of C3 may not be a limiting step in de- termining the concentration of ASP; however, other mechanisms may be playing a role. It is interesting to note that there were distinct patterns of C3 and ASP in the MI (normal C3, high ASP) and ICH (low C3, nor- mal ASP) compared to our controls. Thus far, little research has been conducted on the factors that influ- ence the rate of conversion of C3 to ASP, or the fac- tors that influence ASP degradation or clearance. Therefore, further research is need in this area, not only in relation to the pharmacokinetics of C3/ASP in healthy individuals but how these patterns change in diseases such as ICH and MI. This is the first study to our knowledge which demonstrates different patterns of C3 and ASP. This is the first study to our knowledge to measure ASP in an ICH group. In addition, we simultaneously examined several risk factors in both an MI and ICH population. Further, understanding the risk factors in a Chinese population, instead of extrapolating data from animal studies or studies from a North American or European population, may improve our ability to prevent and treat these cardiovascular diseases in China. 5. Acknowledgments JS is supported by a CIHR scholarship. KC is the recipi- ent of a senior Canada Research Chair position. This study was supported by grants from CIHR (KC) and Na- tional Basic Research Program of China (973 Program, #2006CB503801 to DWW). Copyright © 2010 SciRes. CM  J. SMITH ET AL. Copyright © 2010 SciRes. CM 74 6. References [1] M. E. Fewel, B. G. Jr Thompson and J. T. Hoff, “Spon- taneous Intracerebral Hemorrhage: A Review,” Neuro- surg Focus, Vol. 15, No. 4, 2003, p. E1. [2] Z. Chen, G. Yang, M. Zhou, M. Smith, A. Offer, J. Ma, L. Wang, H. Pan, G. Whitlock, R. Collins, S. Niu and R. Peto, “Body Mass Index and Mortality from Ischaemic Heart Disease in A Lean Population: 10 Year Prospective Study of 220,000 Adult Men,” International Journal of Epidemiology, Vol. 35, No. 1, 2006, pp. 141-150. [3] B. Jiang, W. Z. Wang, H. Chen, Z. Hong, Q. D. Yang, S. P. Wu, X. L. Du and Q. J. Bao, “Incidence and Trends of Stroke and Its Subtypes in China: Results from Three Large Cities,” Stroke, Vol. 37, No. 1, 2006, pp. 63-68. [4] L. F. Zhang, J. Yang, Z. Hong, G. G. Yuan, B. F. Zhou, L. C. Zhao, Y. N. Huang, J. Chen and Y. F. Wu, “Proportion of Different Subtypes of Stroke in China,” Stroke, Vol. 34, No. 9, 2003, pp. 2091-2096. [5] L. Wang, L. Kong, F. Wu, Y. Bai and R. Burton, “Pre- venting Chronic Diseases in China,” Lancet, Vol. 366, No. 9499, 2005, pp. 1821-1824. [6] M. J. McQueen, S. Hawken, X. Wang, S. Ounpuu, A. Sniderman, J. Probstfield, K. Steyn, J. E. Sanderson, M. Hasani, E. Volkova, K. Kazmi and S. Yusuf, “Lipids, Lipoproteins and Apolipoproteins as Risk Markers of Myocardial Infarction in 52 Countries (the INTER- HEART study): A Case-Control Study,” Lancet, Vol. 372, No. 9634, 2008, pp. 224-233. [7] X. F. Zhang, J. Attia, C. D'Este, X. H. Yu and X. G. Wu, “A Risk Score Predicted Coronary Heart Disease and Stroke in a Chinese Cohort,” Journal of Clinical Epide- miology, Vol. 58, No. 9, 2005, pp. 951-958. [8] National Cholesterol Education Program (NCEP) Expert Panel on Detection, “Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), Third report of the National Cholesterol Education Pro- gram (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Final report,” Circulation, Vol. 106, No. 25, 2002, pp. 3143-3421. [9] T. O. Cheng, “The Current State of Cardiology in China,” International Journal of Cardiology, Vol. 96, No. 3, 2004, pp. 425-439. [10] S. Yang, T. Nakamura, Y. Hua, R. F. Keep, J. G. Younger, Y. He, J. T. Hoff and G. Xia, “The Role of Complement C3 in Intracerebral Hemorrhage-Induced Brain Injury,” Journal of Cerebral Blood Flow Metabo- lism, Vol. 26, No. 12, 2006, pp. 1490-1495. [11] J. Parry, “China and Japan Face Epidemic of Heart Dis- ease,” British Medical Journal, Vol. 329, 2004, p. 643. [12] M. Faraj, H. L. Lu and K. Cianflone, “Diabetes, Lipids and Adipocytes Secretagogues,” Biochemistry Cell Biol- ogy, Vol. 82, No. 1, 2004, pp. 170-190. [13] X. Zhang, H. Li, S. Hu, L. Zhang, C. Lui, C. Zhu, R. Liu and C. Li, “Brain Edema after Intracerebral Hemorrhage in Rats: the Role of Inflammation,” Neurology India, Vol. 54, No. 4, 2006, pp. 402-407. [14] H. F. Chen, X. M. Wang, J. Q. Luo, Z. J. Zheng, R. D. Wu and R. L. Xu, “Change in Humoral Immunological Function and Their Clinical Significance in Patients with Cerebral Hemorrhage,” Chinese Critical Care Medicine, Vol. 17, No. 3, 2005, pp. 177-179. [15] M. Maslowska, H. Vu, S. Phelis, A. D. Sniderman, B. M. Rhode, D. Blank and K. Cianflone, “Plasma Acylation Stimulating Protein, Adipsin and Lipids in Non-Obese and Obese populations,” European Journal of Clinical Investigation, Vol. 29, No. 8, 1999, pp. 679-686. [16] X. F. Zhang, J. Attia, C. D'Este and X. Y. Ma, “The Relationship Between Higher Blood Pressure and Ischemic, Haemorrhagic Stroke among Chinese and Caucasians: Meta-Analysis,” European Journal of Cardio-vascular Prevention Rehabilitation, Vol. 13, No. 3, 2006, pp. 429-437. [17] H. C. Kim, C. M. Man, S. H. Jee and I. Suh, “Comparison of Blood Pressure — Associated Risk of Intracerebral Hemorrhage and Subarachnoid Hemorrhage: Korea Medi- cal Insurance Corporation Study,” Hyperten sion, Vol. 46, No. 2, 2005, pp. 393-397. [18] G. R. Sutherland and R. N. Auer, “Primary in-Tracerebral Hemorrhage,” Journal of Clinical Neuroscience, Vol. 13, No. 5, 2006, pp. 511- 517. [19] N. Miyamatsu, T. Kadowaki, T. Okamura, T. Ma-yakawa, Y. Kita, A. Okayama, Y. Nakamura, I. Oki and H. Ue- shima, “Different Effects of Blood Pressure on Mortality from Stroke Subtypes Depending on BMI Levels: A 19- Year Cohort Study in the Japanese General Population — NIPPON DATA80,” Journal of Human Hypertension, Vol. 19, 2005, pp. 285-291. [20] N. Badjatia and J. Rosand, “Intracerebral Hemorrhage,” Neurologist, Vol. 11, No. 6, 2005, pp. 311-324. [21] H. Iso, D. J. Jr Jacobs, D. Wentworth, I. D. Neaton and J. D. Cohen, “Serum Cholesterol Levels and Six Year Mor- tality from Stroke in 350,977 Men Screened for the Mul- tiple Risk Factor Intervention Trial,” The New England Journal of Medicine, Vol. 320, No. 14, 1989, pp. 904-910. [22] K. Cianflone, Z. Xia and L. Y. Chen, “Critical Review of Acylation-Stimulating Protein Physiology in Humans and Rodents,” Biochimica et Biophysica Acta, Vol. 1609, No. 2, 2003, pp. 127-143. [23] A. F. Ducruet, B. E. Zacharia, Z. L. Hickman, B. T. Gro-belny, M. L. Yeh, S. A. Sosunov and E. S. Jr Con- nolly, “The Complement Casade as a Therapeutic Target in Intracerebral Hemorrhage,” Experimental Neurology, Vol. 219, No. 2, 2009, pp. 398-403. [24] S. Söderberg, B. Stegmayr, H. Stenlund, L. G. Sjöström, Å. Ågren, L. Johansson, L. Weinehall and T. Olsson, “Leptin, But not Adiponectin, Predicts Stroke in Males,” Journal of Internal Medicine, Vol. 256, No. 2,2004, pp. 128-136. [25] S. Yang, T. Nakamura, Y. Hua, R. F. Keep, J. G. Younger, J. T. Hoff and G. Xi, “Intracerebral Hemor- rhage in Complement C3-Deficient Mice,” Acta Neuro- chirurgica Supplement, Vol. 96, 2006, pp. 227-231. |

