 Open Journal of Endocrine and Metabolic Diseases, 2013, 3, 9-17 doi:10.4236/ojemd.2013.34A1002 Published Online August 2013 (http://www.scirp.org/journal/ojemd) Intracellular Cholesterol Retention—New Target for Direct Anti-Atherosclerotic Therapy* Alexander N. Orekhov1,2 1Institute for Atherosclerosis Research, Skolkovo Innovative Center, Moscow, Russia 2Institute of General Pathology and Pathophysiology, Russian Academy of Medical Sciences, Moscow, Russia Email: a.h.opexob@gmail.com Received April 24, 2013; revised May 24, 2013; accepted June 24, 2013 Copyright © 2013 Alexander N. Orekhov. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Accumulation of cholesterol in arterial cells, intracellular cholesterol retention, may be responsible for all major mani- festations of atherosclerosis on a cellular level. Previously we have shown that intracellular cholesterol retention is the principal event in the genesis of atherosclerotic lesions. This allows us to consider cellular retention of cholesterol as a novel target for anti-atherosclerotic therapy. In this case the target is not the level of blood cholesterol but the level of cholesterol in vascular cells. This review describes our approach based on the use of cultured human arterial cells for the development of direct anti-atherosclerotic therapy. We use natural products as the basis of promising drugs for anti-atherosclerotic therapy. Using natural products, we have developed an approach to prevent intracellular cholesterol retention in cultured cells. Our knowledge of the mechanisms of atherosclerosis is the foundation on which we have developed drugs that have a direct anti-atherosclerotic effect, namely Allicor on the basis of garlic powder, anti-in- flammatory drug Inflaminat (calendula, elder, and violet) possessing anti-cytokine activity and phytoestrogen-rich drug Karinat (garlic powder, extract of grape seeds, green tea leaves, hop cones, β-carotene, α-tocopherol, and ascorbic acid). Treatment with allicor or inflaminat has a direct anti-atherosclerotic effect on carotid atherosclerosis in asymptomatic men. Karinat prevents the development of carotid atherosclerosis in postmenopausal women. Thus, the main findings of our basic research have been successfully translated into clinical practice. As a result, this translation, a novel approach to the development of anti-atherosclerotic therapy, has been established. Our clinical trials have confirmed the sui- tability of innovative approach and the efficacy of novel drugs developed on the basis our methodology. Keywords: Allicor; Anti-Atherosclerotic Therapy; Atherosclerosis; Cell Culture; Drugs; Imaging; Natural Products 1. Introduction The only hypothesis that has received confirmation in the clinic is the cholesterol hypothesis. This hypothesis was proposed more than 100 years ago by Nikolai Anitsch- kow. The Anitschkow’s hypothesis linked atherosclerosis with high levels of total cholesterol in the blood. The mo- dern paradigm only explains some aspects of this hypo- thesis, in particular, as atherosclerosis is not associated with the total level of cholesterol but with atherogenic low-density lipoprotein (LDL) cholesterol and anti-athe- rogenic high-density lipoprotein (HDL) cholesterol [1-3]. Furthermore, the role of different molecules involved in lipoprotein metabolism is discussed. Retention of intracellular lipids or lipidosis that is the accumulation of cholesterol and other lipids in the arte- rial cells is the most prominent manifestation of athero- sclerosis at the arterial cell level. Retention of intracellu- lar cholesterol is accompanied by increased proliferative activity of vascular cells and increased synthesis of ex- tracellular matrix [4,5]. Both proliferation and fibrosis are characteristic features of atherosclerosis at the arterial cell level, too. Thus, intracellular cholesterol retention, may be responsible for all major manifestations of athe- rosclerosis on a cellular level. This allows us to consider cellular retention of cholesterol as a novel target for anti- atherosclerotic therapy. In this case the target is not the level of blood cholesterol but the level of cholesterol in vascular cells. This review summarizes the mechanisms of intracel- lular retention of cholesterol. We describe our cellular models to search for anti-atherosclerotic agents, and de- monstrate our successful attempt to use these models to develop effective anti-atherosclerotic drugs. *Conflict of interest: The author confirms that this article presents no conflicts of interest. Copyright © 2013 SciRes. OJEMD  A. N. OREKHOV 10 2. Mechanisms of Intracellular Cholesterol Retention Intracellular retention of cholesterol can be induced by LDL. However, native lipoprotein usually does not in- crease the cholesterol content of the cell but the incuba- tion of cell cultures with chemically modified LDL re- sults in a massive accumulation of cholesterol in the cells [6]. Thus, modified, but not native, LDL is the source of lipids that accumulate in arterial cells. Cells that populate atherosclerotic lesions are overloaded with lipids, and their cytoplasms are almost completely filled with lipid inclusions [7]. These cells are referred to as foam cells because of foamy appearance under microscope. In the blood plasma of patients with coronary athero- sclerosis we have discovered modified desialylated LDL [8-11]. This naturally occurring LDL induces cholesterol retention in cultured arterial cells [8-11]. Circulating mo- dified LDL is multiple modified lipoprotein possessing lower sialic acid, triglyceride and cholesterol contents; smaller particle size; greater density and negative charge; higher aggregative activity; and some other specific fea- tures [12]. In patients’ blood we have discovered an en- zyme, trans-sialydase, that is responsible for the desialy- lation of LDL particles in the blood [13]. In addition to desialylated LDL, more electronegative LDL and small dense LDL have been discover in human blood by other researchers [14,15]. A cooperative com- parative study showed that the more electronegative LDL isolated by ion-exchange chromatography is desialylated LDL [16]. On the other hand, desialylated LDL isolated from patient blood [8-11] is more electronegative. De- sialylated LDL particle is smaller and denser than that of native LDL [17]. On the other hand, small dense LDL isolated from patients has a low content of sialic acid, i.e., it is desialylated [18]. Glycosylation is a type of in vivo LDL modification in the blood of patients with diabetes mellitus [19]. This LDL is also atherogenic causing in- tracellular cholesterol retention [20]. Oxidation is likely a type of atherogenic modification of LDL, however, there is no direct evidence of the presence of oxidized LDL in blood [21]. Modified LDL stimulates anti-LDL auto-antibodies production [22-24]. The interaction of anti-LDL auto- antibodies with modified LDL results in the formation of LDL-containing circulating immune complexes [25]. Mul- tiple modified LDL, which enters the cells as a compo- nent of immune complexes, possesses a higher athero- genic potential compared with free modified lipoprotein; that is, it induces a more intense intracellular cholesterol retention [25,26]. We have found LDL-containing circu- lating immune complexes and anti-LDL auto-antibodies in the blood of most atherosclerotic patients [27-29]. We have demonstrated a positive correlation between the le- vels of LDL-containing immune complexes and the se- verity of atherosclerosis [27-31]. LDL is able to form complexes with cellular debris, collagen, elastin, and pro- teoglycans of the human aortic intima [32-37]. These LDL-containing complexes stimulate intracellular chole- sterol retention causing increased uptake and decreased intracellular degradation of lipoproteins in complexes [36]. Naturally occurring multiple modified LDL forms self-associates under cell culture conditions, while native LDL does not associate [35]. We have found a positive correlation between the atherogenic activity of modified LDL and the degree of LDL self-association [35,36]. LDL-associates isolated by gel filtration were shown to induce a dramatic intracellular cholesterol retention. The removal of LDL-associates from the incubation medium by filtration through filters with a pore diameter of 0.1 µm completely prevented intracellular cholesterol reten- tion [36]. We can conclude that the formation of large LDL containing complexes (self-associates, immune com- plexes, and complexes with connective tissue matrix) is a necessary and sufficient condition for intracellular cho- lesterol retention. Our knowledge of mechanisms of intracellular choles- terol retention allowed us to identify possible targets for anti-atherosclerotic therapy. Target 1 is atherogenic mo- dification (desialylation) of the LDL particle in blood. Target 2 is selective removal of modified LDL from blood. Target 3 is the prevention of modified LDL ac- cumulation in arterial cells. Target 4 is the removal of excess lipids from foam cells. We have used all four ap- proaches to anti-atherosclerotic therapy. All of them were effective, however, the most suitable approach is the third, namely, the prevention of modified LDL accumu- lation in arterial cells. i.e. the initial step in intracellular cholesterol retention. We use this approach for the deve- lopment of anti-atherosclerotic therapy. 3. Development of Anti-Atherosclerotic Drugs Preventing Intracellular Cholesterol Retention Using Cellular Models We use primary culture of human aortic cells for the screening of potential drugs, the investigation of their mechanisms of action, and the optimization of anti-athe- rosclerotic drug therapy. Cells are isolated from the subendothelial part of the human aortic intima between the endothelial lining and the media [38]. Using collagenase and elastase, viable cells are isolated from the subendothelial layer of the in- tima [39-41]. Isolated cells can be classified as the mix- ture of smooth muscle cells, pericyte-like cells, and ma- crophages [38-42]. The culture on which our experiments are performed is represented by this mixed population Copyright © 2013 SciRes. OJEMD  A. N. OREKHOV 11 [38]. Cells isolated from atherosclerotic lesions retain all major characteristics of atherosclerotic cells when cul- tured. They are capable of synthesizing collagen, prote- oglycans and other components of the extracellular ma- trix [4]. Cell cultured from fatty lesions have an enhan- ced proliferative activity [43], higher than that of cells cultured from unaffected intima [43,44]. Considerable part of cells cultured from atherosclerotic lesions are foam cells, which contain numerous inclusions, likely li- pid droplets, that fill the entirety of the cytoplasm [40]. Excess lipids in foam cells are mainly free cholesterol and cholesteryl esters [40]. It is important that the con- tent and composition of lipids in cultured cells within the first 10 - 12 days in culture remain unchanged and corre- spond to the respective indices of freshly isolated cells [40,44]. Thus, our investigations are carried out directly on exactly those cells that require a therapeutic action in vivo. Using this model, we have examined the effects of different drugs and chemicals. The prevention of intracellular cholesterol retention may be regarded as anti-atherosclerotic effect. In terms of arterial cells, any drug effect that does not directly prevent intracellular cholesterol retention is regarded as an indirect anti-atherosclerotic action. Only a drug that exhibits its preventive activity at the arterial level is a di- rect anti-atherosclerotic drug. Naturally, the question arises whether the anti-athero- sclerotic effects revealed in in vitro cellular model can be manifested in vivo. To answer this question, an ex vivo model was developed. In the ex vivo model, instead of agents, blood sera taken from patients after oral drug ad- ministration is added to cultured cells. 4. Natural Products Ex vivo cellular model can be used to test foodstuffs. We have investigated prevention of intracellular cholesterol retention caused by certain mushroom species and sea products. Extracts from 20 Korean mushroom species exhibit intracellular cholesterol retention revealed by cell culture test [45]. Among sea products, mollusk and krill meat were investigated. Two hours after a single dietary load with canned meat of a mollusk belonging to the genus Buccinum, the patient’s blood serum acquired marked anti-atherosclerotic properties (Table 1). Incubation of this serum with cultured atherosclerotic cells led to a fall in intracellular cholesterol retention. Patients of another group received a single dietary dose of Antarctic krill meat. Two hours later, the retention of cellular choles- terol induced by blood sera decreased, and four hours la- ter, it was practically absent (Table 1). To develop a dietary therapy based on the krill meat, Table 1. Effects of sea products on atherosclerosis-related properties of patients’ plasma. Intracellular cholesterol content, µg/mg cell protein Product Control 0 hours 2 hours 4 hours Buccinum196 + 17204 + 16 181 + 19 150 + 16* Krill 37 + 5 86 + 4 71 + 7 49 + 4* *Significant difference from 0 hr (p < 0.05). From [46]. the effective dose and proper regimen have been es- tablished. The anti-atherosclerotic activity of krill meat was evaluated by the ability to reduce intracellular cho- lesterol retention. The dose-effect dependence was re- vealed by comparing the efficacy of the two doses, and we found that krill meat possesses anti-atherosclerotic effects at a dose of 10 - 20 g, half-maximum effect was reached at a dose of 30 g, and the maximum effect was achieved at a dose of 50 g. We believe that this approach will be useful in the development and optimization of anti-aterhosclerotic dietary therapies. 4.1. Botanicals The anti-atherogenic effects of dietary products promote the development of anti-atherosclerotic therapies based on natural products. Atherosclerosis develops over many years, so anti-atherosclerotic therapies should be long- term or even lifelong. For such long-term therapies, con- ventional medicine will not work. Drugs based on natural products can be a good alternative. We have tested numerous natural products’ extracts to reveal their effects on blood atherogenicity or their capa- city to prevent intracellular cholesterol accumulation caus- ed by atherogenic blood sera from patients. Table 2 pre- sents only the effective natural products. Naturally, the tested agents included anti-atherogenic, pro-atherogenic, and neutral products. Among the anti-atherogenic natural products, the most effective was garlic. We investigated the in vitro effect of garlic extract on lipids of cultured human aortic cells. Garlic prevented the serum-induced accumulation of free cholesterol and re- duced the accumulation of cholesteryl esters [47]. The effect of garlic on the enzymes responsible for the intra- cellular metabolism of cholesteryl esters was studied. We have shown that garlic inhibits acyl-CoA: cholesterol acyl- transferase, which participates in cholesteryl ester forma- tion, and stimulates cholesteryl ester hydrolase, which degrades cholesteryl esters [47]. Further investigations ex vivo and in vivo confirmed the in vitro effects of garlic [48]. These data stimulated us to develop a drug based on garlic powder and carried out a clinical study of the effects of this drug on athero- sclerosis regression. Copyright © 2013 SciRes. OJEMD 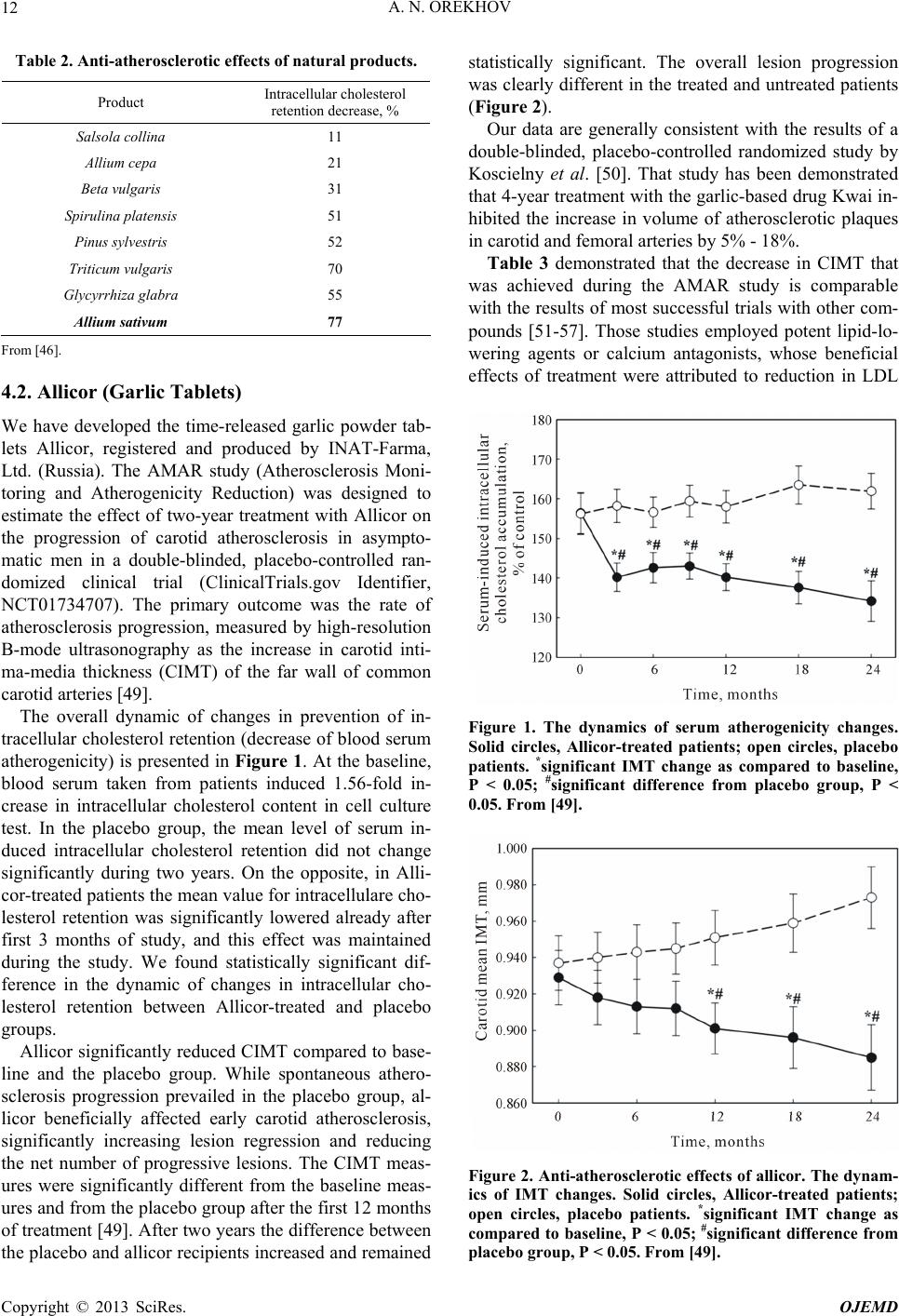 A. N. OREKHOV 12 Table 2. Anti-atherosclerotic effects of natural products. Product Intracellular cholesterol retention decrease, % Salsola collina 11 Allium cepa 21 Beta vulgaris 31 Spirulina platensis 51 Pinus sylvestris 52 Triticum vulgaris 70 Glycyrrhiza glabra 55 Allium sativum 77 From [46]. 4.2. Allicor (Garlic Tablets) We have developed the time-released garlic powder tab- lets Allicor, registered and produced by INAT-Farma, Ltd. (Russia). The AMAR study (Atherosclerosis Moni- toring and Atherogenicity Reduction) was designed to estimate the effect of two-year treatment with Allicor on the progression of carotid atherosclerosis in asympto- matic men in a double-blinded, placebo-controlled ran- domized clinical trial (ClinicalTrials.gov Identifier, NCT01734707). The primary outcome was the rate of atherosclerosis progression, measured by high-resolution B-mode ultrasonography as the increase in carotid inti- ma-media thickness (CIMT) of the far wall of common carotid arteries [49]. The overall dynamic of changes in prevention of in- tracellular cholesterol retention (decrease of blood serum atherogenicity) is presented in Figure 1. At the baseline, blood serum taken from patients induced 1.56-fold in- crease in intracellular cholesterol content in cell culture test. In the placebo group, the mean level of serum in- duced intracellular cholesterol retention did not change significantly during two years. On the opposite, in Alli- cor-treated patients the mean value for intracellulare cho- lesterol retention was significantly lowered already after first 3 months of study, and this effect was maintained during the study. We found statistically significant dif- ference in the dynamic of changes in intracellular cho- lesterol retention between Allicor-treated and placebo groups. Allicor significantly reduced CIMT compared to base- line and the placebo group. While spontaneous athero- sclerosis progression prevailed in the placebo group, al- licor beneficially affected early carotid atherosclerosis, significantly increasing lesion regression and reducing the net number of progressive lesions. The CIMT meas- ures were significantly different from the baseline meas- ures and from the placebo group after the first 12 months of treatment [49]. After two years the difference between the placebo and allicor recipients increased and remained statistically significant. The overall lesion progression was clearly different in the treated and untreated patients (Figure 2). Our data are generally consistent with the results of a double-blinded, placebo-controlled randomized study by Koscielny et al. [50]. That study has been demonstrated that 4-year treatment with the garlic-based drug Kwai in- hibited the increase in volume of atherosclerotic plaques in carotid and femoral arteries by 5% - 18%. Table 3 demonstrated that the decrease in CIMT that was achieved during the AMAR study is comparable with the results of most successful trials with other com- pounds [51-57]. Those studies employed potent lipid-lo- wering agents or calcium antagonists, whose beneficial effects of treatment were attributed to reduction in LDL Figure 1. The dynamics of serum atherogenicity changes. Solid circles, Allicor-treated patients; open circles, placebo patients. *significant IMT change as compared to baseline, P < 0.05; #significant difference from placebo group, P < 0.05. From [49]. Figure 2. Anti-atherosclerotic effects of allicor. The dynam- ics of IMT changes. Solid circles, Allicor-treated patients; open circles, placebo patients. *significant IMT change as compared to baseline, P < 0.05; #significant difference from placebo group, P < 0.05. From [49]. Copyright © 2013 SciRes. OJEMD 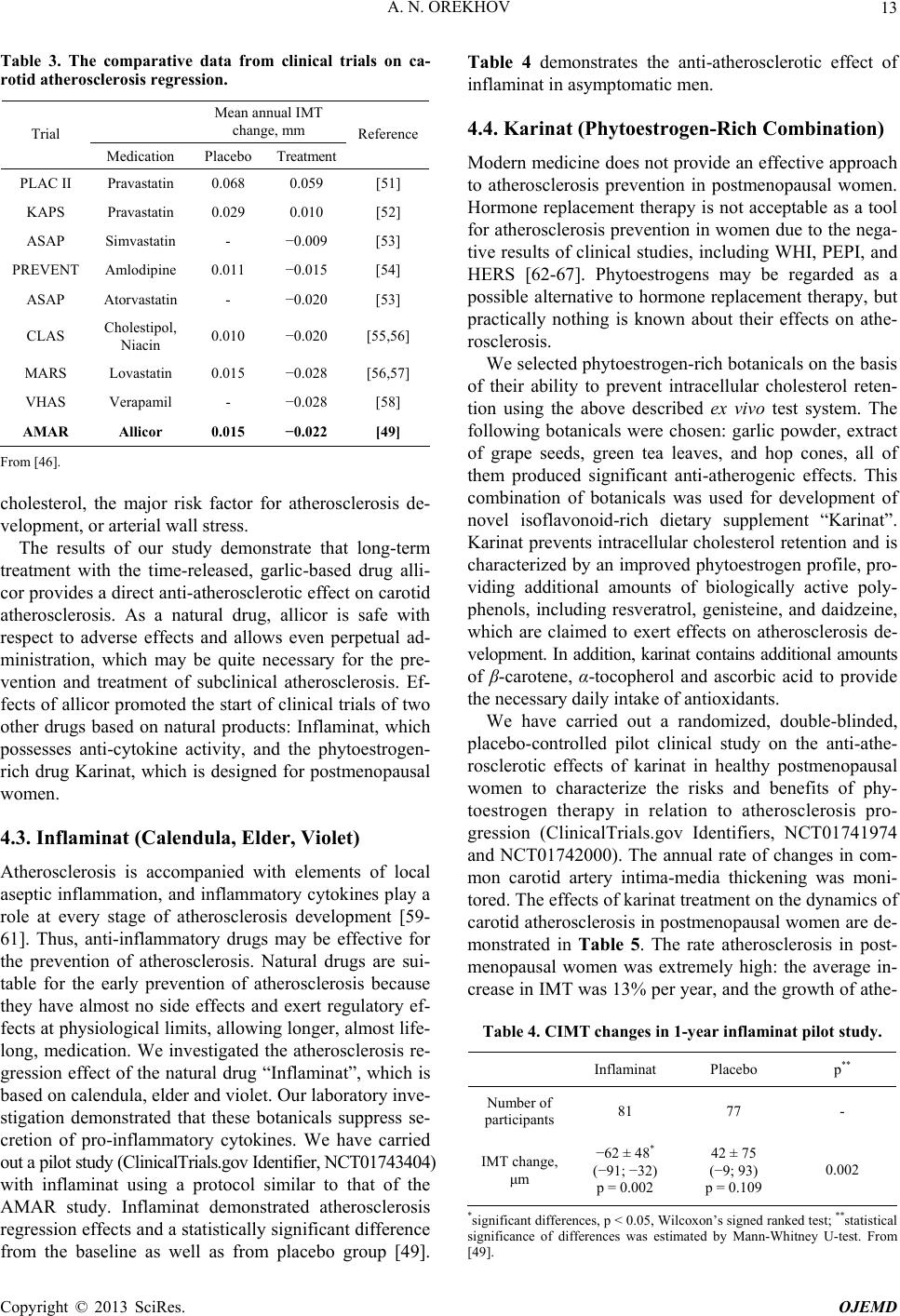 A. N. OREKHOV 13 Table 3. The comparative data from clinical trials on ca- rotid atherosclerosis regression. Mean annual IMT change, mm Trial Medication Placebo Treatment Reference PLAC II Pravastatin 0.068 0.059 [51] KAPS Pravastatin 0.029 0.010 [52] ASAP Simvastatin - −0.009 [53] PREVENT Amlodipine 0.011 −0.015 [54] ASAP Atorvastatin - −0.020 [53] CLAS Cholestipol, Niacin 0.010 −0.020 [55,56] MARS Lovastatin 0.015 −0.028 [56,57] VHAS Verapamil - −0.028 [58] AMAR Allicor 0.015 −0.022 [49] From [46]. cholesterol, the major risk factor for atherosclerosis de- velopment, or arterial wall stress. The results of our study demonstrate that long-term treatment with the time-released, garlic-based drug alli- cor provides a direct anti-atherosclerotic effect on carotid atherosclerosis. As a natural drug, allicor is safe with respect to adverse effects and allows even perpetual ad- ministration, which may be quite necessary for the pre- vention and treatment of subclinical atherosclerosis. Ef- fects of allicor promoted the start of clinical trials of two other drugs based on natural products: Inflaminat, which possesses anti-cytokine activity, and the phytoestrogen- rich drug Karinat, which is designed for postmenopausal women. 4.3. Inflaminat (Calendula, Elder, Violet) Atherosclerosis is accompanied with elements of local aseptic inflammation, and inflammatory cytokines play a role at every stage of atherosclerosis development [59- 61]. Thus, anti-inflammatory drugs may be effective for the prevention of atherosclerosis. Natural drugs are sui- table for the early prevention of atherosclerosis because they have almost no side effects and exert regulatory ef- fects at physiological limits, allowing longer, almost life- long, medication. We investigated the atherosclerosis re- gression effect of the natural drug “Inflaminat”, which is based on calendula, elder and violet. Our laboratory inve- stigation demonstrated that these botanicals suppress se- cretion of pro-inflammatory cytokines. We have carried out a pilot study (ClinicalTrials.gov Identifier, NCT01743404) with inflaminat using a protocol similar to that of the AMAR study. Inflaminat demonstrated atherosclerosis regression effects and a statistically significant difference from the baseline as well as from placebo group [49]. Table 4 demonstrates the anti-atherosclerotic effect of inflaminat in asymptomatic men. 4.4. Karinat (Phytoestrogen-Rich Combination) Modern medicine does not provide an effective approach to atherosclerosis prevention in postmenopausal women. Hormone replacement therapy is not acceptable as a tool for atherosclerosis prevention in women due to the nega- tive results of clinical studies, including WHI, PEPI, and HERS [62-67]. Phytoestrogens may be regarded as a possible alternative to hormone replacement therapy, but practically nothing is known about their effects on athe- rosclerosis. We selected phytoestrogen-rich botanicals on the basis of their ability to prevent intracellular cholesterol reten- tion using the above described ex vivo test system. The following botanicals were chosen: garlic powder, extract of grape seeds, green tea leaves, and hop cones, all of them produced significant anti-atherogenic effects. This combination of botanicals was used for development of novel isoflavonoid-rich dietary supplement “Karinat”. Karinat prevents intracellular cholesterol retention and is characterized by an improved phytoestrogen profile, pro- viding additional amounts of biologically active poly- phenols, including resveratrol, genisteine, and daidzeine, which are claimed to exert effects on atherosclerosis de- velopment. In addition, karinat contains additional amounts of β-carotene, α-tocopherol and ascorbic acid to provide the necessary daily intake of antioxidants. We have carried out a randomized, double-blinded, placebo-controlled pilot clinical study on the anti-athe- rosclerotic effects of karinat in healthy postmenopausal women to characterize the risks and benefits of phy- toestrogen therapy in relation to atherosclerosis pro- gression (ClinicalTrials.gov Identifiers, NCT01741974 and NCT01742000). The annual rate of changes in com- mon carotid artery intima-media thickening was moni- tored. The effects of karinat treatment on the dynamics of carotid atherosclerosis in postmenopausal women are de- monstrated in Table 5. The rate atherosclerosis in post- menopausal women was extremely high: the average in- crease in IMT was 13% per year, and the growth of athe- Table 4. CIMT changes in 1-year inflaminat pilot study. Inflaminat Placebo р** Number of participants 81 77 - IMT change, μm −62 ± 48* (−91; −32) р = 0.002 42 ± 75 (−9; 93) р = 0.109 0.002 *significant differences, p < 0.05, Wilcoxon’s signed ranked test; **statistical significance of differences was estimated by Mann-Whitney U-test. From [49]. Copyright © 2013 SciRes. OJEMD  A. N. OREKHOV 14 Table 5. CIMT changes in 1-year karinat pilot study on postmenopausal women. Karinat Р Placebo Р Number of participants 80 - 77 - IMT change, μm +6 (85) NS +111 (91) P < 0.02 Plaques, scores +0.21 (0.59) 0.009 +0.31 (0.55) <0.001 From [49]. rosclerotic plaques was 40% per year. In the karinat group the average CIMT was not changed (statistically insignificant increase of 6 μm per year, less than 1%). The progression of existing plaques was slower by 32% per year. Thus, the use of the phytoestrogen-rich combination in postmenopausal women almost completely suppresses the formation of new atherosclerotic lesions, and it slows the progression of existing lesions [49]. 5. Conclusion Our basic studies have shown that intracellular choles- terol retention is the principal event in the genesis of atherosclerotic lesions. On the basis of the results of our basic research we developed cellular models and an ap- proach to prevent intracellular cholesterol retention. Pre- vention of intracellular cholesterol retention led to the prevention of atherosclerosis progression and/or the re- gression in patients. We can conclude that our basic find- ings were successfully translated into clinical practice. Two-year treatment with allicor (garlic powder) has a direct anti-atherosclerotic effect on carotid atherosclero- sis. Anti-cytokine combination inflaminat (calendula, el- der and violet) caused the regression of carotid athero- sclerosis in a 1-year treatment of asymptomatic men. The phytoestrogen-rich combination karinat (garlic powder, extract of grape seeds, green tea leaves, hop cones, β-ca- rotene, α-tocopherol and ascorbic acid) prevented the de- velopment of carotid atherosclerosis in postmenopausal women. Unfortunately, natural products with anti-atheroscle- rotic therapeutic potential are not prescribed by medical practitioners as anti-atherosclerotic agents. However, our data allow us to consider botanicals as mainline addi- tional supplements or prescriptions [68]. 6. Acknowledgements This work was supported by the Russian Ministry of Education and Science. REFERENCES [1] S. S. Martin, R. S. Blumenthal and M. Miller, “LDL Cho- lesterol: The Lower the Better,” The Medical Clinics of North America, Vol. 96, No. 1, 2012, pp. 13-26. doi:10.1016/j.mcna.2012.01.009 [2] F. Sala, A. L. Catapano and G. D. Norata, “High Density Lipoproteins and Atherosclerosis: Emerging Aspects,” Journal of Geriatric Cardiology, Vol. 9, No. 4, 2012, pp. 401-407. doi:10.3724/SP.J.1263.2011.12282 [3] E. A. Fisher, J. E. Feig, B. Hewing, S. L. Hazen and J. D. Smith, “High-Density Lipoprotein Function, Dysfunction, and Reverse Cholesterol Transport,” Arteriosclerosis, Thrombosis, and Vascular Biology, Vol. 32, No. 12, 2012, pp. 2813-2820. doi:10.1161/ATVBAHA.112.300133 [4] A. N. Orekhov, V. V. Tertov, S. A. Kudryashov and V. N. Smirnov, “Triggerlike Stimulation of Cholesterol Accu- mulation and DNA and Extracellular Matrix Synthesis In- duced by Atherogenic Serum or Low Density Lipoprotein in Cultured Cells,” Circulation Research, Vol. 66, No. 2, 1990, pp. 311-320. doi:10.1161/01.RES.66.2.311 [5] A. N. Orekhov, V. V. Tertov, S. N. Pokrovsky, I. Yu. Ada- mova, O. N. Martsenyuk, A. A. Lyakishev and V. N. Smirnov, “Blood Serum Atherogenicity Associated with Coronary Atherosclerosis. Evidence for Nonlipid Factor Providing Atherogenicity of Low-Density Lipoproteins and an Approach to Its Elimination,” Circulation Re- search, Vol. 62, No. 3, 1988, pp. 421-429. doi:10.1161/01.RES.62.3.421 [6] H. S. Kruth, “Receptor-Independent Fluid-Phase Pinocy- tosis Mechanisms for Induction of Foam Cell Formation with Native Low-Density Lipoprotein Particles,” Current Opinion in Lipidology, Vol. 22, No. 5, 2011, pp. 386-393. doi:10.1097/MOL.0b013e32834adadb [7] Y. Yuan, P. Li and J. Ye, “Lipid Homeostasis and the Formation of Macrophage-Derived Foam Cells in Athero- sclerosis,” Protein & Cell, Vol. 3, No. 3, 2012, pp. 173- 181. doi:10.1007/s13238-012-2025-6 [8] A. N. Orekhov, V. V. Tertov, D. N. Mukhin and I. A. Mik- hailenko, “Modification of Low Density Lipoprotein by Desialylation Causes Lipid Accumulation in Cultured Cells. Discovery of Desialylated Lipoprotein with Altered Cel- lular Metabolism in the Blood of Atherosclerotic Pa- tients,” Biochemical and Biophysical Research Commu- nications, Vol. 162, No. 1, 1989, pp. 206-211. doi:10.1016/0006-291X(89)91982-7 [9] A. N. Orekhov, V. V. Tertov and D. N. Mukhin, “Desia- lylated Low Density Lipoprotein—Naturally Occurring Modified Lipoprotein with Atherogenic Potency,” Athe- rosclerosis, Vol. 86, No. 2-3, 1991, pp. 153-161. doi:10.1016/0021-9150(91)90211-K [10] V. V. Tertov, I. A. Sobenin, Z. A. Gabbasov, E. G. Popov and A. N. Orekhov, “Lipoprotein Aggregation as an Es- sential Condition of Intracellular Lipid Accumulation Caused by Modified Low Density Lipoproteins,” Bioche- mical and Biophysical Research Communications, Vol. 163, No. 1, 1989, pp. 489-494. doi:10.1016/0006-291X(89)92163-3 [11] V. V. Tertov, I. A. Sobenin, A. G. Tonevitsky, A. N. Ore- khov and V. N. Smirnov, “Isolation of Atherogenic Mo- Copyright © 2013 SciRes. OJEMD  A. N. OREKHOV 15 dified (Desialylated) Low Density Lipoprotein from Blood of Atherosclerotic Patients: Separation from Native Li- poprotein by Affinity Chromatography,” Biochemical and Biophysical Research Communications, Vol. 167, No. 3, 1990, pp. 1122-1127. doi:10.1016/0006-291X(90)90639-5 [12] V. V. Tertov, I. A. Sobenin, A. N. Orekhov, O. Jaakkola, T. Solakivi and T. Nikkari, “Characteristics of Low Den- sity Lipoprotein Isolated from Circulating Immune Com- plexes,” Atherosclerosis, Vol. 122, No. 2, 1996, pp. 191- 199. doi:10.1016/0021-9150(95)05737-4 [13] V. V. Tertov, V. V. Kaplun, I. A. Sobenin, E. Y. Boytso- va, N. V. Bovin and A. N. Orekhov, “Human Plasma Trans-Sialidase Causes Atherogenic Modification of Low Density Lipoprotein,” Atherosclerosis, Vol. 159, No. 1, 2001, pp. 103-115. doi:10.1016/S0021-9150(01)00498-1 [14] P. Avogaro, G. Bittolo-Bon and G. Cazzolato, “Presence of a Modified Low Density Lipoprotein in Humans,” Ar- teriosclerosis, Vol. 8, No. 6, 1988, pp. 79-87. doi:10.1161/01.ATV.8.1.79 [15] R. M. Krauss and D. J. Burke, “Identification of Multiple Subclasses of Plasma Low Density Lipoproteins in Nor- mal Humans,” Journal of Lipid Research, Vol. 23, No. 1, 1982, pp. 97-104. [16] V. V. Tertov, G. Bittolo-Bon, I. A. Sobenin, G. Cazzolato, A. N. Orekhov and P. Avogaro, “Naturally Occurring Mo- dified Low Density Lipoproteins are Similar if Not Iden- tical: More Electronegative and Desialylated Lipoprotein Subfractions,” Experimental and Molecular Pathology, Vol. 62, No. 3, 1995, pp. 166-172. doi:10.1006/exmp.1995.1018 [17] V. V. Tertov, I. A. Sobenin and A. N. Orekhov, “Simila- rity between Naturally Occurring Modified Desialylated, Electronegative and Aortic Low Density Lipoprotein,” Free Radical Research, Vol. 25, No. 4, 1996, pp. 313-319. doi:10.3109/10715769609149054 [18] M. La Belle and R. M. Krauss, “Differences in Carbohy- drate Content of Low Density Lipoproteins Associated with Low Density Lipoprotein Subclass Patterns,” Jour- nal of Lipid Research, Vol. 31, No. 9, 1990, pp. 1577- 1588. [19] J. K. Kirk, S. W. Davis, C. A. Hildebrandt, E. N. Strachan, M. L. Peechara and R. Lord, “Characteristics Associated with Glycemic Control among Family Medicine Patients with Type 2 Diabetes,” North Carolina Medical Journal, Vol. 72, No. 5, 2011, pp. 345-350. [20] H. Soran and P. N. Durrington, “Susceptibility of LDL and Its Subfractions to Glycation,” Current Opinion in Lipi- dology, Vol. 22, No. 4, 2011, pp. 254-261. doi:10.1097/MOL.0b013e328348a43f [21] X. Jiang, Z. Yang, A. N. Chandrakala, D. Pressley and S. Parthasarathy, “Oxidized Low Density Lipoproteins—Do We Know Enough about Them?” Cardiovascular Drugs and Therapy, Vol. 25, No. 5, 2011, pp. 367-377. doi:10.1007/s10557-011-6326-4 [22] A. N. Orekhov, V. V. Tertov, A. E. Kabakov, I. Yu. Ada- mova, S. N. Pokrovsky and V. N. Smirnov, “Autoantibo- dies against Modified Low Density Lipoprotein. Nonlipid Factor of Blood Plasma that Stimulates Foam Cell For- mation,” Arteriosclerosis and Thrombosis, Vol. 11, No. 2, 1991, pp. 316-326. doi:10.1161/01.ATV.11.2.316 [23] A. N. Orekhov and V. V. Tertov, “Atherogenicity of Auto- antibodies against Low Density Lipoprotein,” Agents and Actions, Vol. 32, 1991, No. 1-2, pp. 128-129. doi:10.1007/BF01983338 [24] M. F. Lopes-Virella and G. Virella, “Clinical Significance of the Humoral Immune Response to Modified LDL,” Clinical Immunology, Vol. 134, No. 1, 2010, pp. 55-65. doi:10.1016/j.clim.2009.04.001 [25] V. V. Tertov, A. N. Orekhov, Kh. S. Sayadyan, S. G. Se- rebrennikov, A. G. Kacharava, A. A. Lyakishev and V. N. Smirnov, “Correlation between Cholesterol Content in Cir- culating Immune Complexes and Atherogenic Properties of CHD Patients’ Serum Manifested in Cell Culture,” Atherosclerosis, Vol. 81, No. 3, 1990, pp. 183-189. doi:10.1016/0021-9150(90)90065-Q [26] A. G. Kacharava, V. V. Tertov and A. N. Orekhov, “Auto- Antibodies against Low-Density Lipoprotein and Athe- rogenic Potential of Blood,” Annals of Medicine, Vol. 25, No. 6, 1993, pp. 551-555. [27] V. V. Tertov, A. N. Orekhov, A. G. Kacharava, I. A. So- benin, N. V. Perova and V. N. Smirnov, “Low Density Li- poprotein-Containing Circulating Immune Complexes and Coronary Atherosclerosis,” Experimental and Molecular Pathology, Vol. 52, No. 3, 1990, pp. 300-308. doi:10.1016/0014-4800(90)90071-K [28] A. N. Orekhov, O. S. Kalenich, V. V. Tertov and I. D. No- vikov, “Lipoprotein Immune Complexes as Markers of Atherosclerosis,” International Journal of Tissue Reac- tions, Vol. 13, No. 5, 1991, pp. 233-236. [29] A. N. Orekhov, O. S. Kalenich, V. V. Tertov, N. V. Pero- va, Iy. D. Novikov, A. A. Lyakishev, A. D. Deev and M. Ya. Ruda, “Diagnostic Value of Immune Cholesterol as a Marker for Atherosclerosis,” Journal of Cardiovascular Risk, Vol. 2, No. 5, 1995, pp. 459-466. doi:10.1097/00043798-199510000-00011 [30] I. A. Sobenin, V. P. Karagodin, A. A. Melnichenko, Y. V. Bobryshev and A. N. Orekhov, “Diagnostic and Progno- stic Value of Low Density Lipoprotein-Containing Circu- lating Immune Complexes in Atherosclerosis,” Journal of Clinical Immunology, Vol. 33, No. 2, 2013, pp. 489-495. doi:10.1007/s10875-012-9819-4 [31] I. A. Sobenin, V. A. Orekhova, A. Melnichenko, Y. V. Bo- bryshev and A. N. Orekhov, “Low Density Lipoprotein- Containing Circulating Immune Complexes Have Better Prognostic Value in Carotid Intima-Media Thickness Pro- gression than Other Lipid Parameters,” International Journal of Cardiology, Vol. 166, No. 3, 2012, pp. 747- 748. doi:10.1016/j.ijcard.2012.09.175 [32] A. N. Orekhov, V. V. Tertov, D. N. Mukhin, V. E. Kote- liansky, M. A. Glukhova, M. G. Frid, G. K. Sukhova, K. A. Khashimov and V. N. Smirnov, “Insolubilization of Low Density Lipoprotein Induces Cholesterol Accumulation in Cultured Subendothelial Cells of Human Aorta,” Athe- rosclerosis, Vol. 79, No. 1, 1989, pp. 59-70. doi:10.1016/0021-9150(89)90034-8 [33] M. A. Glukhova, A. E. Kabakov, M. G. Frid, O. I. Ornat- sky, A. M. Belkin, D. N. Mukhin, A. N. Orekhov, V. E. Copyright © 2013 SciRes. OJEMD  A. N. OREKHOV 16 Koteliansky and V. N. Smirnov, “Modulation of Human Aorta Smooth Muscle Cell Phenotype: a Study of Mu- scle-Specific Variants of Vinculin, Caldesmon, and Actin Expression,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 85, No. 24, 1988, pp. 9542-9546. doi:10.1073/pnas.85.24.9542 [34] A. N. Orekhov, V. V. Tertov, D. N. Mukhin, V. E. Kote- liansky, M. A. Glukhova, K. A. Khashimov and V. N. Smirnov, “Association of Low-Density Lipoprotein with Particulate Connective Tissue Matrix Components En- hances Cholesterol Accumulation in Cultured Subendo- thelial Cells of Human Aorta,” Biochimica et Biophysica Acta, Vol. 928, No. 3, 1987, pp. 251-258. doi:10.1016/0167-4889(87)90183-2 [35] A. A. Melnichenko, D. V. Aksenov, V. A. Myasoedova, O. M. Panasenko, A. A. Yaroslavov, I. A. Sobenin, Y. V. Bo- bryshev and A. N. Orekhov, “Pluronic Block Copolymers Inhibit Low Density Lipoprotein Self-Association,” Li- pids, Vol. 47, No. 10, 2012, pp. 995-1000. doi:10.1007/s11745-012-3699-5 [36] V. V. Tertov, A. N. Orekhov, I. A. Sobenin, Z. A. Gabbasov, E. G. Popov, A. A. Yaroslavov and V. N. Smirnov, “Three Types of Naturally Occurring Modified Lipoproteins Induce Intracellular Lipid Accumulation Due to Lipoprotein Aggregation,” Circulation Research, Vol. 71, No. 1, 1992, pp. 218-228. doi:10.1161/01.RES.71.1.218 [37] D. V. Aksenov, L. A. Medvedeva, T. A. Skalbe, I. A. Sobenin, V. V. Tertov, Z. A. Gabbasov, E. V. Popov and A. N. Orekhov, “Deglycosylation of Apo B-Containing Lipoproteins Increase Their Ability to Aggregate and to Promote Intracellular Cholesterol Accumulation in Vi- tro,” Archives of Physiology and Biochemistry, Vol. 114, No. 5, 2008, pp. 349-356. doi:10.1080/13813450802227915 [38] M. D. Rekhter, E. R. Andreeva, A. A. Mironov and A. N. Orekhov, “Three-Dimensional Cytoarchitecture of Nomal and Atherosclerotic Intima of Human Aorta,” American Journal of Pathology, Vol. 138, No. 3, 1991, pp. 569-580. [39] A. N. Orekhov, E. R. Andreeva, A. V. Krushinsky and V. N. Smirnov, “Primary Cultures of Enzyme-Isolated Cells from Normal and Atherosclerotic Human Aorta,” Medical Biology, Vol. 62, No. 4, 1984, pp. 255-259. [40] A. N. Orekhov, V. V. Tertov, I. D. Novikov, A. V. Kru- shinsky, E. R. Andreeva, V. Z. Lankin and V. N. Smirnov, “Lipids in Cells of Atherosclerotic and Uninvolved Hu- man Aorta. I. Lipid Composition of Aortic Tissue and Enzyme Isolated and Cultured Cells,” Experimental and Molecular Pathology, Vol. 42, No. 1, 1985, pp. 117-137. doi:10.1016/0014-4800(85)90022-X [41] A. N Orekhov, A. V. Krushinsky, E. R. Andreeva, V. S. Repin and V. N. Smirnov, “Adult Human Aortic Cells in Primary Culture: Heterogeneity in Shape,” Heart and Vessels, Vol. 2, No. 4, 1986, pp. 193-201. doi:10.1007/BF02059968 [42] S. Yamada, X. Guo, M. Yoshizawa, Z. Li, A. Matsuyama, H. Hashimoto and Y. Sasaguri, “Primary Desmoplastic Cutaneous Leiomyosarcoma Associated with High MIB-1 Labeling Index: A Teaching Case Giving Rise to Diag- nostic Difficulties on a Small Biopsy Specimen,” Pa- thology, Research and Practice, Vol. 207, No. 11, 2011, pp. 728-732. doi:10.1016/j.prp.2011.08.008 [43] A. N. Orekhov, V. A. Kosykh, V. S. Repin and V. N. Smirnov, “Cell Proliferation in Normal and Atheroscle- rotic Human Aorta. II. Autoradiographic Observation on Deoxyribonucleic Acid Synthesis in Primary Cell Cul- ture,” Laboratory Investigation, Vol. 48, No. 6, 1983, pp. 749-754. [44] A. N. Orekhov, V. V. Tertov, S. A. Kudryashov, Kh. A. Khashimov and V. N. Smirnov, “Primary Culture of Hu- man Aortic Intima Cells as a Model for Testing Anti- Atherosclerotic Drugs. Effects of Cyclic AMP, Prostag- landins, Calcium Antagonists, Antioxidants, and Lipid- Lowering Agents,” Atherosclerosis, Vol. 60, No. 2, 1986, pp. 101-110. doi:10.1016/0021-9150(86)90002-X [45] H. R. Li, V. V. Tertov, A. V. Vasil’ev, V. A. Tutel’yan and A. N. Orekhov, “Anti-Atherogenic and Anti-Athe- rosclerotic Effects of Mushroom Extracts Revealed in Human Aortic Intima Cell Culture,” Drug Development Research, Vol. 17, No. 1, 1989, pp. 109-117. doi:10.1002/ddr.430170203 [46] A. N. Orekhov, “Direct Anti-Atherosclerotic Therapy; Development of Natural Anti-Atherosclerotic Drugs Pre- venting Cellular Cholesterol Retention,” Current Phar- maceutical Design, 2013. [47] A. N. Orekhov and V. V. Tertov, “In Vitro Effect of Gar- lic Powder Extract on Lipid Content in Normal and Atherosclerotic Human Aortic Cells,” Lipids, Vol. 32, No. 10, 1997, pp. 1055-1060. doi:10.1007/s11745-997-0136-7 [48] A. N. Orekhov and J. Grünwald, “Effects of Garlic on Atherosclerosis,” Nutrition, Vol. 13, No. 7-8, 1997, pp. 656-663. doi:10.1016/S0899-9007(97)83010-9 [49] A. N. Orekhov, I. A. Sobenin, N. V. Korneev, T. V. Kirichenko, V. A. Myasoedova, A. A. Melnichenko, M. Balcells, E. R. Edelman and Y. V. Bobryshev, “Anti- Atherosclerotic Therapy Based on Botanicals,” Recent Patents on Cardiovascular Drug Discovery, Vol. 8, No. 1, 2013, pp. 56-66. [50] J. Koscielny, D. Klüssendorf, R. Latza, R. Schmitt, H. Radtke, G. Siegel and H. Kiesewetter, “The Antiathero- sclerotic Effect of Allium sativum,” Atherosclerosis, Vol. 144, No. 1, 1999, pp. 237-249. doi:10.1016/S0021-9150(99)00060-X [51] J. R. Crouse 3rd, R. P. Byington, M. G. Bond, M. A. Espeland, T. E. Craven, J. W. Sprinkle, M. E. McGovern and C. D. Furberg, “Pravastatin, Lipids, and Atheroscle- rosis in the Carotid Arteries (PLAC-II),” American Jour- nal of Cardiology, Vol. 75, No. 7, 1995, pp. 455-459. doi:10.1016/S0002-9149(99)80580-3 [52] R. Salonen, K. Nyyssonen, E. Porkkala, J. Rummukainen, R. Belder, J. S. Park and J. T. Salonen, “Kuopio Athero- sclerosis Prevention Study (KAPS). A Population-Based Primary Preventive Trial of the Effect of LDL Lowering on Atherosclerotic Progression in Carotid and Femoral Arteries,” Circulation, Vol. 92, No. 7, 1995, pp. 1758- 1764. doi:10.1161/01.CIR.92.7.1758 [53] T. J. Smilde, S. van Wissen, H. Wollersheim, M. D. Trip, J. J. Kastelein and A. F. Stalenhoef, “Effect of Aggressive versus Conventional Lipid Lowering on Atherosclerosis Copyright © 2013 SciRes. OJEMD  A. N. OREKHOV Copyright © 2013 SciRes. OJEMD 17 Progression in Familial Hypercholesterolaemia (ASAP): A Prospective, Randomised, Double-Blind Trial,” Lancet, Vol. 357, No. 9256, 2001, pp. 577-581. doi:10.1016/S0140-6736(00)04053-8 [54] B. Pitt, R. P. Byington, C. D. Furberg, D. B. Hunning- hake, G. B. Mancini, M. E. Miller and W. Riley, “Effect of Amlodipine on the Progression of Atherosclerosis and the Occurrence of Clinical Events. PREVENT Investiga- tors,” Circulation, Vol. 102, No. 13, 2000, pp. 1503-1510. doi:10.1161/01.CIR.102.13.1503 [55] D. H. Blankenhorn, R. H. Selzer, D. W. Crawford, J. D. Barth, C. R. Liu, C. H. Liu, W. J. Mack and P. Alaupovic, “Beneficial Effects of Colestipol-Niacin Therapy on the Common Carotid Artery. Two- and Four-Year Reduction of Intima-Media Thickness Measured by Ultrasound,” Circulation, Vol. 88, No. 1, 1993, pp. 20-28. doi:10.1161/01.CIR.88.1.20 [56] H. N. Hodis, “Reversibility of Atherosclerosis—Evolving Perspectives from Two Arterial Imaging Clinical Trials: The Cholesterol Lowering Atherosclerosis Regression Study and the Monitored Atherosclerosis Regression Stu- dy,” Journal of Cardiovascular Pharmacology, Vol. 25, Suppl. 4, 1995, pp. S25-S31. [57] D. H. Blankenhorn, S. P. Azen, D. M. Kramsch, W. J. Mack, L. Cashin-Hemphill, H. N. Hodis, L. W. DeBoer, P. R. Mahrer, M. J. Masteller, L. I. Vailas, P. Alaupovic, and L. J. Hirsch, “Coronary Angiographic Changes with Lovastatin Therapy. The Monitored Atherosclerosis Re- gression Study (MARS). The MARS Research Group,” Annals of Internal Medicine, Vol. 119, No. 10, 1993, pp. 969-976. doi:10.7326/0003-4819-119-10-199311150-00002 [58] A. Zanchetti, E. A. Rosei, C. Dal Palù, G. Leonetti, B. Magnani and A. Pessina, “The Verapamil in Hyperten- sion and Atherosclerosis Study (VHAS): Results of Long-Term Randomized Treatment with Either Verapa- mil or Chlorthalidone on Carotid Intima-Media Thick- ness,” Journal of Hypertension, Vol. 16, No. 11, 1998, pp. 1667-1676. doi:10.1097/00004872-199816110-00014 [59] P. Libby, “Inflammation and Cardiovascular Disease Mechanisms,” The American Journal of Clinical Nutri- tion, Vol. 83, No. 2, 2006, pp. 456S-460S. [60] G. Aidinian, J. M. Weiswasser and S. Arora, “Carotid Pla- que Morphologic Characteristics,” Perspectives in Vas- cular Surgery and Endovascular Therapy, Vol. 18, No. 1, 2006, pp. 63-70. doi:10.1177/153100350601800124 [61] A. Daugherty, N. R. Webb, D. L. Rateri and V. L. King, “The Immune System and Atherogenesis. Cytokine Regu- lation of Macrophage Functions in Atherogenesis,” Jour- nal of Lipid Research, Vol. 46, No. 9, 2005, pp. 1812- 1822. doi:10.1194/jlr.R500009-JLR200 [62] H. G. Burger, A. H. Maclennan, K. E. Huang and C. Cas- telo-Branco, “Evidence-Based Assessment of the Impact of the WHI on Women’s Health,” Climacteric, Vol. 15, No. 3, 2012, pp. 281-287. doi:10.3109/13697137.2012.655564 [63] T. J. de Villiers and J. C. Stevenson, “The WHI: The Effect of Hormone Replacement Therapy on Fracture Prevention,” Climacteric, Vol. 15, No. 3, 2012, pp. 263- 266. doi:10.3109/13697137.2012.659975 [64] M. J. Ellis, V. J. Suman, J. Hoog, L. Lin, J. Snider, A. Prat, J. S. Parker, J. Luo, K. DeSchryver, D. C. Allred, L. J. Esserman, G. W. Unzeitig, J. Margenthaler, G. V. Ba- biera, P. K. Marcom, J. M. Guenther, M. A. Watson, M. Leitch, K. Hunt and J. A. Olson, “Randomized Phase II Neoadjuvant Comparison between Letrozole, Anastrozole, and Exemestane for Postmenopausal Women with Estro- gen Receptor-Rich Stage 2 to 3 Breast Cancer: Clinical and Biomarker Outcomes and Predictive Value of the Baseline PAM50-Based Intrinsic Subtype—ACOSOG Z1031,” Journal of Clinical Oncology, Vol. 29, No. 17, 2011, pp. 2342-2349. doi:10.1200/JCO.2010.31.6950 [65] N. L. Smith, J. R. Wiley, C. Legault, K. M. Rice, S. R. Heckbert, B. M. Psaty, R. P. Tracy and M. Cushman, “Effect of Progestogen and Progestogen Type on Hemo- stasis Measures in Postmenopausal Women: The Post- Menopausal Estrogen/Progestin Intervention (PEPI) Stu- dy,” Menopause, Vol. 15, No. 6, 2008, pp. 1145-1150. doi:10.1097/gme.0b013e3181775eca [66] D. E. Masood, E. C. Roach, K. G. Beauregard and R. A. Khalil, “Impact of Sex Hormone Metabolism on the Vas- cular Effects of Menopausal Hormone Therapy in Car- diovascular Disease,” Current Drug Metabolism, Vol. 11, No. 8, 2010, pp. 693-714. doi:10.2174/138920010794233477 [67] C. N. Pellegrini, E. Vittinghoff, F. Lin, S. B. Hulley and G. M. Marcus, “Statin Use is Associated with Lower Risk of Atrial Fibrillation in Women with Coronary Disease: The HERS Trial,” Heart, Vol. 95, No. 9, 2009, pp. 704- 708. doi:10.1136/hrt.2008.154054 [68] M. Slevin, N. Ahmed, Q. Wang, G. McDowell and L. Badimon, “Unique Vascular Protective Properties of Na- tural Products: Supplements or Future Main-Line Drugs with Significant Anti-Atherosclerotic Potential?” Vascu- lar Cell, Vol. 4, No. 1, 2012, p. 9. doi:10.1186/2045-824X-4-9
|