Paper Menu >>
Journal Menu >>
 American Journal of Plant Sciences, 2010, 1, 138-146 doi:10.4236/ajps.2010.12018 Published Online December 2010 (http://www.SciRP.org/journal/ajps) Copyright © 2010 SciRes. AJPS Maintenance of Ephedra alata Seeds Viability via Storage Containers Abdulaziz A. Al-Qarawi, Elsayed F. Abd_Allah* 1Plant Production Department, College of Food & Agriculture Sciences, King Saud University, Riyadh, Saudi Arabia. Email: *eabdallah@ksu.edu.sa Received October 22nd, 2010; revised November 19th, 2010; accepted November 22nd, 2010. ABSTRACT Sequential incubation of seed samples yielded 20 fungal species belonging to 13 genera. The prevalent genera were Aspergillus (A. flavus and A. parasiticus), Fusarium (F. moniliforme and F. oxysporum) and Penicillium (Penicillium sp.). Such seedborne fungi differ in their coloniza tion in different parts of seeds with most of them are colon ized in seed coat, endosperm, and embryo of the seed. The usage of different storage containers for storing seeds indicated that the cotton cloth bags were the most favorable ones as they maintain seed moisture content (SMC) below the critical level resulting in minimum seed deterioration compared with other seed storage containers. Keywords: Seedb orne Fungi, Ephedra alata, Sto ra ge C ontainers, Saudi Arabia 1. Introduction Ephedra alata Decne, a gymnosperm belong to family Ephedraceae, is one of the oldest range and medicinal herb known in Saudi Arabia as well as in different rangelands in the world [5]. It was noted as it is accom- panied with sand dunes formation in Saudi Arabia espe- cially the mobile ones and therefore it is a very effective sand-binder and resistant to desertification [3]. The foli- age of E. alata have acceptable aroma and used as food- stuff for animals especially camels, cattle and sheep. The deterioration of plant community had occurred in Saudi Desert due to abiotic factors such as soil salinity [7], ed- aphic factors of the soil [29] and soil drought [6]. On other hand, the biotic stresses such as seedborne fungi play an important and vital role in deterioration of seed quality [1,2]. Seedborne fungi use different mechanisms to deteriorate seeds such as production of both mycotox- ins [2] and enzymes [26] which have attracted much at- tention of our investigations. Application of prophylactic fungicides is not the pre- ferred choice in range seeds especially in the sheltered areas. This raised the need to study safe alternative strategies to control seedborne fungi that attack range plants and might be transmitted to the aerial parts of the plants [4]. Seed storage containers play an important and considerable role in the production of healthy and vital seeds [21, 25; 27]. The successfulness of the storage con- tainers restricted with surrounding factors such as seed nature, storage period, temperature, relative humidity, and seed moisture content. Consequently, the production of healthy and vital range seeds should managed through integrated approach. The present study was designed to investigate the seedborne fungal flora of E. alata with special reference to their incidence in different seed parts. Furthermore, the effect of different storage containers on seed moisture content (SMC), seed vigor (SV), aflatoxins accumulation, and nutritional value of storage seeds (E. alata) was studied. 2. Materials and Methods 2.1. Seed Samples Collection Seed samples of Ephedra alata Decne (approximately 100 g seeds per sample, each in replicates) were col- lected from King Khalid Center for Wildlife Research and Development at Thumama which belonging to Ri- yadh Region, Saudi Arabia during 2009. The samples were collected in sterile cellophane bags and held at 2℃ until analyzed according to the International Seed Test- ing Association [20]. 2.2. Enumeration of Seedborne Fungi From each seed sample, 400 seeds were surface-disin- fected in Na-hypochlorite for two minutes followed washing with several changes of sterile saline water (8.5 gm NaCl in 1000 ml distilled H2O). The disinfected  Maintenance of Ephedra alata Seeds Viability via Storage Containers Copyright © 2010 SciRes. AJPS 139 seeds were then incubated aseptically on potato dextrose agar (PDA, Difco Laboratories, Detriot MI). Rose Ben- gal (33 mg/ml, w/v) and Streptomycin (30 mg/ml, w/v) were added as bacteriostatic agents. Surface-disinfected seeds were spaced on Petri dishes (9 cm in diameter) and incubated at 28 ± 2℃ for 10 days. Similarly, sur- face-disinfected seeds were incubated on sterile moist filter paper with cellulose wadding as blotters and incu- bated as above. The fungal colonies developing around the seeds incubated on both agar plates and filter papers were examined and the fungi were identified micro- scopically [15] and the level of incidence were recorded. To enumerate seedborne fungi in different seed parts, surface disinfected seeds were soaked in sterile water for four hours and then dissected aseptically into different parts (coat, endosperm, and embryo). Each seed part aseptically used for investigation of seedborne fungi as described above. 2.3. Determination of Seed Moisture Content (SMC) Each seed sample (100 gm) was grounded in a blender and known weight of the resultant powder was dried in an oven for 24 hours at 105℃, cooled in a desiccators and reweighed. The moisture content (MC) is expressed as percentage of the wet weight. 2.4. Storage Experiment Seed samples (100 g each) were stored in four storage containers namely polyethylene bags, cotton cloth bags, tin cans and paper bags for six months at room tempera- ture (25 ± 1℃) in the dark. 2.5. Seed Analysis Vigor index (VI) of E. alata seeds were calculated Vigor index (VI) for each treatment was determined according to the following formula: VI = [mean of root length (cm) + mean of shoot length (cm)] X percent seed germination. Nutritional values (total lipids, total nitrogen, ash content, and fiber content) of stored seeds were determined ac- cording to AOAC [11]. Aflatoxins (B1, B2, and G1) were extracted and cleaned up from storage seeds using chloroforme and cleaned using column chromatography according to AOAC [10]. Quantitative estimation of aflatoxins were carried spectrophotometrically [24] using standard aflatoxins (Sigma) as reference. 2.6. Statistical Analysis For each experiment, the data were statistically analyzed using the analysis of variance procedure for completely randomized design. Treatment means were compared using the protected least significant difference (LSD) analysis according to Daniel [14]. 3. Results and Discussions In the present investigation, 31 seed samples of E. alata were analyzed to investigate their seedborne fungal flora by means of standard blotter and agar plate methods (Ta- ble 1 and Figure 1). As suggested by Abd_Allah and Hashem [2] the comprehensive outcome recommended Table 1. Incidence (%); case of isolations and occurrence remarks of seedborne fungal flora of E. alata following incubation on agar plate and blotter. Incidence (%) of fungal species Fungal species Blotter test Agar plate Cases of isolation (No.) OccurrenceZ Alternaria alternata 3.87 2.35 1 R Alternaria sp. 4.36 2.21 3 R Aspergillus flavus 14.65 16.37 42 H Aspergillus nidulans 2.75 0 4 L Aspergillus niger 8.36 12.75 13 M Aspergillus parasitcus 18.52 21.18 37 H Aspergillus sp. 16.32 12.16 7 L Aspergillus terreus 7.15 8.13 6 L Chaetomium globsum 0.95 1.15 1 R Cladosporium sp. 1.78 0 2 R Drechslera sp. 1.07 0 1 R Epicoccum sp. 0.54 0.63 1 R Fusarium moniliforme 3.27 3.85 7 L Fusarium oxysporum 2.96 2.56 6 L Penicillium sp. 7.36 8.81 7 L Pythium sp. 0.62 0.87 1 R Rhizoctonia solani 2.45 2.65 2 R Rhizopus sp. 0.61 0.83 4 R Sclerotium bataticola 1.54 1.35 2 R Trichoderma sp. 1.27 1.75 3 R Z: Out 31 E. alata seed samples. H = High occurrence (> 24 case); M = Moderate occurrence (from 12 to 24 cases); L = Low occurrence (from 6 to 11 cases) and R = Rare occurrence (< 6 cases).  Maintenance of Ephedra alata Seeds Viability via Storage Containers Copyright © 2010 SciRes. AJPS 140 Figure 1. Detection of seedborne fungi associated with E. alata. A) Blotter and B) agar plate techniques used for enumeration of seedborne fungi. C-H) Stereo-microscopy of E. alata seeds acquaint the incidence of seedborne fungi on different seed parts. that agar plate method was more conformable than moist paper (standard blotter) method in yielding more fungal flora (Table 1). The sequential incubation of seed sam- ples yielded twenty fungal species belonging to thirteen genera, which are new to mycoflora of E. alata in Saudi Arabia (Table 1). The genes Aspergillus was the most predominant and represented by 6 species namely A. flavus, A. nidulans, A. niger, A. parasiticus, A. terreus and Aspergillus spp.. Aspergillus was followed by Fusa- rium (F. mon iliforme and F. oxysporum) and Penicillium (Penicillium spp.), respectively. The other fungal genera (Alternaria, Chaetomium, Cladosporium, Drechslera, Epicoccum, Pythium, Rhizoctonia, Rhizopus, Sclerotium, Trichoderma) were rare in their occurrence (Table 1). Up to our knowledge, this is the first investigation for seedborne mycoflora of E. alata especially in Saudi Ara- bia although the contamination of Saudi herbs with toxi- genic mycoflora was reported [8]. Nevertheless, similar mycological studies for many other seeds showed that Aspergillus and Fusarium were the most common genera as seedborne fungi [2; 16]. The component plating of E. alata seeds showed that most prevalent fungi colo- 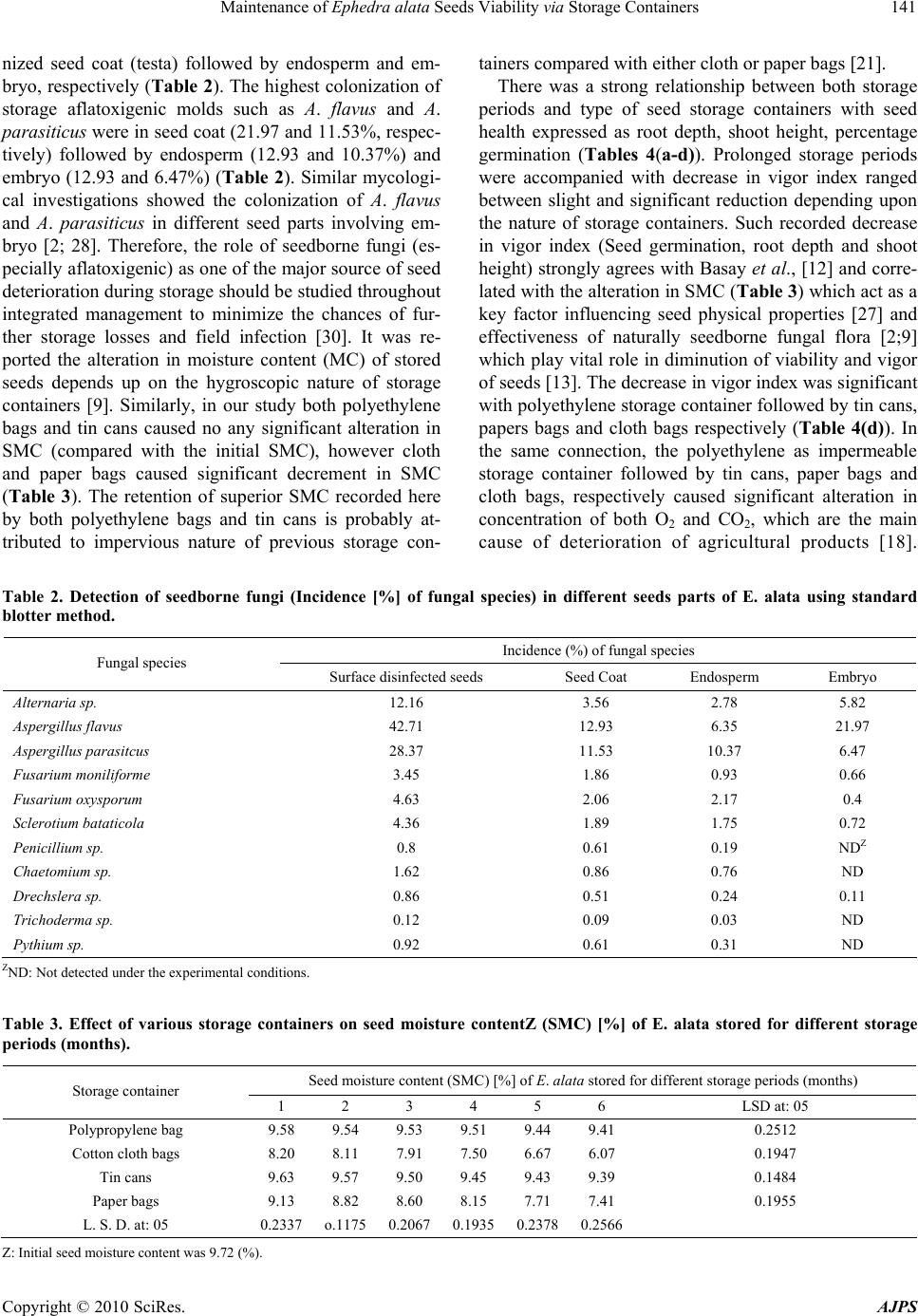 Maintenance of Ephedra alata Seeds Viability via Storage Containers Copyright © 2010 SciRes. AJPS 141 nized seed coat (testa) followed by endosperm and em- bryo, respectively (Table 2). The highest colonization of storage aflatoxigenic molds such as A. flavus and A. parasiticus were in seed coat (21.97 and 11.53%, respec- tively) followed by endosperm (12.93 and 10.37%) and embryo (12.93 and 6.47%) (Table 2). Similar mycologi- cal investigations showed the colonization of A. flavus and A. parasiticus in different seed parts involving em- bryo [2; 28]. Therefore, the role of seedborne fungi (es- pecially aflatoxigenic) as one of the major source of seed deterioration during storage should be studied throughout integrated management to minimize the chances of fur- ther storage losses and field infection [30]. It was re- ported the alteration in moisture content (MC) of stored seeds depends up on the hygroscopic nature of storage containers [9]. Similarly, in our study both polyethylene bags and tin cans caused no any significant alteration in SMC (compared with the initial SMC), however cloth and paper bags caused significant decrement in SMC (Table 3). The retention of superior SMC recorded here by both polyethylene bags and tin cans is probably at- tributed to impervious nature of previous storage con- tainers compared with either cloth or paper bags [21]. There was a strong relationship between both storage periods and type of seed storage containers with seed health expressed as root depth, shoot height, percentage germination (Tables 4(a-d)). Prolonged storage periods were accompanied with decrease in vigor index ranged between slight and significant reduction depending upon the nature of storage containers. Such recorded decrease in vigor index (Seed germination, root depth and shoot height) strongly agrees with Basay et al., [12] and corre- lated with the alteration in SMC (Table 3) which act as a key factor influencing seed physical properties [27] and effectiveness of naturally seedborne fungal flora [2;9] which play vital role in diminution of viability and vigor of seeds [13]. The decrease in vigor index was significant with polyethylene storage container followed by tin cans, papers bags and cloth bags respectively (Table 4(d)). In the same connection, the polyethylene as impermeable storage container followed by tin cans, paper bags and cloth bags, respectively caused significant alteration in concentration of both O2 and CO2, which are the main cause of deterioration of agricultural products [18]. Table 2. Detection of seedborne fungi (Incidence [%] of fungal species) in different seeds parts of E. alata using standard blotter method. Incidence (%) of fungal species Fungal species Surface disinfected seeds Seed Coat Endosperm Embryo Alternaria sp. 12.16 3.56 2.78 5.82 Aspergillus flavus 42.71 12.93 6.35 21.97 Aspergillus parasitcus 28.37 11.53 10.37 6.47 Fusarium moniliforme 3.45 1.86 0.93 0.66 Fusarium oxysporum 4.63 2.06 2.17 0.4 Sclerotium bataticola 4.36 1.89 1.75 0.72 Penicillium sp. 0.8 0.61 0.19 NDZ Chaetomium sp. 1.62 0.86 0.76 ND Drechslera sp. 0.86 0.51 0.24 0.11 Trichoderma sp. 0.12 0.09 0.03 ND Pythium sp. 0.92 0.61 0.31 ND ZND: Not detected under the experimental conditions. Table 3. Effect of various storage containers on seed moisture contentZ (SMC) [%] of E. alata stored for different storage periods (months). Seed moisture content (SMC) [%] of E. alata stored for different storage periods (months) Storage container 1 2 3 4 5 6 LSD at: 05 Polypropylene bag 9.58 9.54 9.53 9.51 9.44 9.41 0.2512 Cotton cloth bags 8.20 8.11 7.91 7.50 6.67 6.07 0.1947 Tin cans 9.63 9.57 9.50 9.45 9.43 9.39 0.1484 Paper bags 9.13 8.82 8.60 8.15 7.71 7.41 0.1955 L. S. D. at: 05 0.2337 o.11750.20670.19350.23780.2566 Z: Initial seed moisture content was 9.72 (%).  Maintenance of Ephedra alata Seeds Viability via Storage Containers Copyright © 2010 SciRes. AJPS 142 Table 4-a. Effect of various storage containers on root depth (cm) of germinating seeds of E. alata stored for different storage periods (months). Root depth (cm) of germinating seeds of E. alata stored for different storage periods (months) Storage container 1 2 3 4 5 6 LSD. at: 05 Polypropylene bag 15.30 14.33 12.77 11.73 9.97 8.27 0.4398 Cotton cloth bags 18.60 18.43 17.97 17.50 15.67 15.27 0.7383 Tin cans 17.53 17.03 16.17 15.90 15.07 13.80 0.8323 Paper bags 18.27 17.60 17.03 16.53 16.00 15.07 0.6134 LSD at: 05 0.4280 0.4892 0.8117 0.4175 0.8044 1.0651 Table 4-b. Effect of various storage containers on shoot height (cm) of germinating seeds of E. alata stored for different stor- age periods (months). Shoot height (cm) of germinating seeds of E. alata stored for different storage periods (months) Storage container 1 2 3 4 5 6 LSD at: 05 Polypropylene bag 9.63 8.97 8.40 7.30 6.37 4.83 0.5204 Cotton cloth bags 10.17 9.97 9.70 9.20 8.33 7.73 0.4836 Tin cans 8.97 8.67 8.07 7.50 6.40 4.90 0.4926 Paper bags 9.67 9.10 8.67 8.13 7.30 6.67 0.4438 L. S. D. at: 05 0.3689 0.4104 0.321 0.4644 0.6221 0.7590 Table 4-c. Effect of various storage containers on percentage germination of E. alata stored for different storage periods (months). Percentage germination of E. ala ta stored for different storage periods (months) Storage container 1 2 3 4 5 6 LSD at: 05 Polypropylene bag 61.30 59.93 57.20 53.70 51.20 43.33 1.9266 Cotton cloth bags 70.17 69.00 65.03 63.00 59.00 55.20 1.2275 Tin cans 65.23 63.20 60.57 56.87 52.27 46.53 1.3697 Paper bags 69.30 65.27 61.70 57.13 53.73 51.07 1.6294 LSD at: 05 0.5040 0.9993 1.4174 2.1543 1.8938 2.2118 Table 4-d. Effect of various storage containers on seed vigor indexZ of E. alata stored for different storage periods (months). Vigor index of E. alata stored for different storage periods (months) Storage container 1 2 3 4 5 6 LSD at: 05 Polypropylene bag 1528.41 1396.45 1210.73 1022.09 836.27 567.67 60.3480 Cotton cloth bags 2018.46 1959.60 1799.26 1682.10 1416.00 1269.60 83.1640 Tin cans 1728.68 1624.24 1467.73 1330.68 1121.99 870.17 73.3190 Paper bags 1935.78 1742.62 1585.69 1409.29 1251.99 1109.85 49.980 L. S. D. at: 05 32.05 60.769 65.249 72.736 92.857 91.266 Z: Vigor index = Seed germination X [mean Root depth (cm) + mean Shoot length (cm)]. The effect of seed storage containers on aflatoxins pro- duction was investigated for seeds of many crops how- ever, this approach does not provide a comprehensive view of the impact of range seeds. In our results, afla- toxins production were found to be inferior with em- ployment of cloth bags followed by paper bags, tin cans and polyethylene bags respectively (Tables 5(a,d)). Such inhibition agrees with the findings of Paramawati et al.,  Maintenance of Ephedra alata Seeds Viability via Storage Containers Copyright © 2010 SciRes. AJPS 143 Table 5-a. Effect of different storage containers and storage periods (month) on the natural contamination of E. alata seeds with aflatoxin B1 (µg/Kg seed). Natural contamination with aflatoxin B1 (µg/Kg seed) of E. alata seeds stored for different storage periods (months) Storage container 1 2 3 4 5 6 LSD at: 05 Polypropylene bag 42.07 38.70 34.20 29.77 24.23 19.27 2.4825 Cotton cloth bags 14.53 12.00 6.20 2.70 0.00 0.00 5.8834 Tin cans 36.00 27.20 23.13 20.93 17.30 11.20 3.0896 Paper bags 27.70 24.93 22.93 14.50 9.17 0.00 3.2088 L. S. D. at: 05 4.3322 2.6583 5.9746 5.7786 2.1357 2.1357 Table 5-b. Effect of different storage containers and storage periods (month) on the natural contamination of E. alata seeds with aflatoxin B2 (µg/Kg seed). Natural contamination with aflatoxin B2 (µg/Kg seed) of E. alata seeds stored for different storage periods (months) Storage container 1 2 3 4 5 6 LSD at: 05 Polypropylene bag 92.17 85.30 77.80 73.27 69.03 63.37 4.1764 Cotton cloth bags 45.07 34.93 28.63 18.50 11.17 9.50 3.5914 Tin cans 71.50 65.10 58.30 51.30 38.10 27.50 6.7281 Paper bags 77.17 66.27 55.67 46.87 37.43 24.27 4.9676 L. S. D. at: 05 6.8605 4.6379 3.6884 6.4785 5.8013 3.2707 Table 5-c. Effect of different storage containers and storage periods (month) on the natural contamination of E. alata seeds with aflatoxin G1 (µg/Kg seed). Natural contamination with aflatoxin G1 (µg/Kg seed) of E. alata seeds stored for different storage periods (months) Storage container 1 2 3 4 5 6 LSD at: 05 Polypropylene bag 35.87 30.03 26.77 21.50 18.40 11.23 2.5326 Cotton cloth bags ND ND ND ND ND ND 0.00 Tin cans 23.10 18.10 12.10 10.70 ND ND 4.3153 Paper bags 26.07 19.57 10.97 9.80 5.73 ND 2.6836 L. S. D. at: 05 5.0457 1.5258 1.3135 1.1019 4.6876 1.2105 ND: Not detected under the experimental conditions. Table 5-d. Effect of different storage containers and storage periods (month) on the natural contamination of E. alata seeds with total aflatoxinsZ (µg/Kg seed). Natural contamination with total aflatoxinsZ (µg/Kg seed) of E. alata seeds stored for different storage periods (months) Storage container 1 2 3 4 5 6 LSD at: 05 Polypropylene bag 170.11 154.03 138.77 124.53 111.67 93.87 7.8536 Cotton cloth bags 59.60 46.93 34.83 21.20 11.17 9.50 7.0388 Tin cans 139.23 113.03 89.77 77.60 60.47 35.47 10.7660 Paper bags 105.17 79.00 62.40 47.80 29.93 11.13 5.5776 L. S. D. at: 05 12.8030 7.2977 9.0200 8.8850 6.5909 3.6033 Z: Total aflatoxins (Sum. of B1 + B2 + G1). [25]. It can explain in terms of the decrement alteration in SMC inferior requisite level for growth and aflatoxins production by seedborne fungi [2;9]. Data in our investi- gation (Tables 6 (a-d)) shows that prolongation of stor- age periods was accompanied with gradual deterioration in the biochemical aspects of seed such as lipids, ash, total nitrogen, and fiber contents. The employment of cloth bags followed by paper bags, tin cans and polyethylene bags respectively diminished such as sharpness in the deterioration of seed biochemical aspects (Tables 6 (a-d)).  Maintenance of Ephedra alata Seeds Viability via Storage Containers Copyright © 2010 SciRes. AJPS 144 Table 6(a). Effect of different storage containers and storage periods (month) on nitrogen content (mg/g dry weight) of E. alata seeds. Nitrogen content (mg/g dry weight) of E. alata seeds stored for different storage periods (months) Storage container 1 2 3 4 5 6 LSD at: 05 Polypropylene bag 32.48 30.06 28.6 28.37 27.48 26.50 0.1773 Cotton cloth bags 48.65 45.54 43.97 43.67 42.08 40.83 0.1002 Tin cans 39.65 35.86 32.88 31.31 30.76 31.073 0.1273 Paper bags 44.21 42.55 41.30 39.89 38.62 36.68 0.1119 L. S. D. at: 05 0.1609 0.0981 0.1092 0.1084 0.0853 0.2270 Table 6(b). Effect of different storage containers and storage periods (month) on fiber content (% of dry weight) of E. alata seeds. Fiber content (% of dry weight) of E. alata seeds stored for different storage periods (months) Storage container 1 2 3 4 5 6 LSD at: 05 Polypropylene bag 5.03 4.49 4.25 5.02 3.84 3.48 1.1738 Cotton cloth bags 5.36 5.09 4.96 4.84 4.76 4.61 0.8601 Tin cans 5.20 4.39 4.07 3.77 3.38 3.13 0.1723 Paper bags 5.27 4.93 4.66 4.37 4.94 4.12 0.3679 LSD at: 05 0.2849 0.2246 0.2517 1.5158 1.1231 0.2852 Table 6(c). Effect of different storage containers and storage periods (month) on lipids content (% of dry weight) of E. alata seeds. Lipids content (% of dry weight) of E. alata seeds stored for different storage periods (months) Storage container 1 2 3 4 5 6 LSD at: 05 Polypropylene bag 6.03 5.12 4.44 3.51 3.02 2.40 0.4421 Cotton cloth bags 7.63 7.21 7.03 6.86 4.29 6.50 0.4052 Tin cans 6.35 5.97 5.41 4.97 4.28 3.81 0.5560 Paper bags 7.20 6.83 6.40 6.06 5.83 5.50 0.3554 LSD at: 05 0.5457 0.4259 0.4288 0.3882 0.3366 0.6253 Table 6(d). Effect of different storage containers and storage periods (month) on ash content (% of dry weight) of E. alata seeds. Ash content (% of dry weight) of E. alata seeds stored for different storage periods (months) Storage container 1 2 3 4 5 6 LSD at: 05 Polypropylene bag 4.69 4.35 4.11 3.92 3.59 3.45 1.6900 Cotton cloth bags 6.52 6.40 6.25 6.20 6.09 5.89 3.5538 Tin cans 5.70 4.94 4.23 3.87 3.71 3.56 1.9654 Paper bags 6.22 5.921 5.67 5.40 5.18 4.91 0.7454 LSD at: 05 4.1092 3.1294 1.3800 1.5175 0.6931 1.4413 Such results were in agreed with our data recorded pre- viously concerning soybean seeds [17]. In this regard, the alteration in SMC due to the employment of different storage containers (Tabl e 3) was the main cause of seed- borne fungal activities [2; 9] including production of hydrolytic enzymes such as proteinase [26], lipase [23] and lignocellulolytic enzymes [19] which were responsi- ble for the biotic degradation of seed contents of protein, lipids and fiber [22]. Our results indicate that these bio- chemical events were correlated with maintenance of  Maintenance of Ephedra alata Seeds Viability via Storage Containers Copyright © 2010 SciRes. AJPS 145 high germination rate during storage. The success of storage container to preserve seed viability recorded in this investigation is still limited and more studies through integrated seed management program to maintain healthy range plants in the grassland is needed hence production vital seeds are necessary for desert ecological mainte- nance. These will be considered in the forthcoming in- vestigation. 4. Acknowledgment We acknowledge Dr. Abeer Hashem, Bot. & Microbiol. Department, Faculty of Science, King Saud University, Riyadh, Saudi Arabia for her excellent assistance in fun- gal identification that contributed in this success of this survey. REFERENCES [1] E. F. Abd_Allah, “Effect of a Bacillus subtilis Isolate on Southern Blight (Sclerotium rolfsii) and Lipid Composi- tion of Peanut Seeds,” Phytoparasitica, Vol. 33, No. 5, 2005, pp. 460-466. [2] E. F. Abd_Allah and Abeer Hashem, “Seed Mycoflora of Lens esculenta and Their Biocontrol by Chitosan,” Phy- toparasitica, Vol. 34, No. 2, 2006, pp. 213-218. [3] L. Abdallah, and M. Chaieb, “Water Status and Growing Phonology of a Saharan Shrub in North Africa,” African Journal of Ecology, Vol. 1, No. 45, 2007, pp. 80-85. [4] A. M. Abdelmonem, “Status of Seed Pathology and Seed Health Testing in Egypt,” Seed Science & Technology, Vol. 28, 2000, pp. 533-547. [5] E. A. Abourashed, T. El-Alfy, I. A. Abir-Khan, and L. Walker, “Ephedra in Perspective—A Current Review,” Phytother. Res. 17, 2003, 703-712. [6] M.R. Al-Masri, “An in vitro evaluation of some drought-tolerant native range plants in terms of ruminal microbial nitrogen, microbial biomass and their fermenta- tion characteristics utilizing a gas-production technique,” Tropical Grasslands 4, 2007, 292-300. [7] Wafaa A. Al-Taisan, A.A. Al-Qarawi and Moodi S. Al- subiee, “Effect of water stress by polyethylene glycol 8000 and sodium chloride on germination of Ephedra alata Decne seeds,” Saudi journal of Biological Science 17, 2010, 253-257. [8] Suaad S. Alwakeel, “The effect of mycotoxins found in some herbal plants on biochemical parameters in blood of female albino mice.” Pakistan Journal of Biological Sci- ences 12 (8), 2009, 637-642. [9] J.E. Amadi, and M.O. Adebola, “Effect of moisture con- tent and storage conditions on the storability of garri,” Af- rican Journal of Biotechnology 7(24), 2008, 4591-4594. [10] Association of Official Analytical Chemists, “Official Methods of Analysis” (11th ed.), Section 26.019 (a), 429. AOAC, Washington, D.C, 1970. [11] Association of Official Analytical Chemists “Official Methods of analysis” (15th ed.). Washington , D.C, 1995. [12] S. Basay, S.G. Okcu, and I. Demir, “Changes in germina- tion percentages, protein and lipid contents of primed pepper seeds during storage,” Acta Agriculturae Scandi- navica 56, 2006, 138-142. [13] M. Begum, and S. Lokesh, “Syngergistic effect of fungi- cides on the incidence of seed mycoflora of okra,” Inter- national Journal of Botany 4(2), 2008, 24-32. [14] W.W. Daniel, “Biostatistics: A foundation for Analysis in the Health Science” 4th ed., John Wiley and Sons, New York, NY. pp. 292-293, 1987. [15] K.H. Domsch, W. Gams, and T.H. Anderson, “Compen- dium of Soil Fungi”. Academic Press, London, 860 pp 1993. [16] S. Figueroa, S. Centeno, M.A. Calvo, A. Rengel, and C. Adelantado, “Mycobiota and concentration of ochratoxin A in concentrated poultry feed from Venzuela,” Pakistan Journal of Biological Sciences 12(7), 2009, 589-594. [17] M.I. Ghonim, A.E.A. Ismail, and E.F. Abd_Allah, “Bio- logical control of Aspergillus flavus in soybean seeds by atoxigenic A. flavus strain,” Zazazig J. Agric Res., Vol. 26, No. 1, 1999, 27-35. [18] P. Giorni, P. Battilani, A. Pietri, and N. Magan, “Effect of aw and CO2 level on Aspergillus flavus growth and afla- toxin production in high moisture maize post-harvest,” International journal of Food Microbiology, Vol. 122, 2008, pp. 109-113. [19] A. Gupte, S. Gupte, and H. Patel, “Ligninolytic enzyme production under solid state fermentation by white rot fungi,” Journal of Scientific & Industrial Research 66, 2007, 611-614. [20] International Seed Testing Association [ISTA] “Interna- tional rules for seed testing rules,” Seed Sci. & Technol. 24, 1996, 1-335. [21] S.D. Malimath, and M.N. Merwade, “Effect of storage containers on seed storability of garden pea (Pisum sati- vum L.),” Karntaka J. Agri. Sci. 20(2), 2007, 384-385. [22] J.E. Mellon, P.J. Cotty, and M.K. Down, “Aspergillus flavus hydrolysis: their roles in pathogenesis and substrate utilization,” Appl. Microbiol. Biotechnol. 2007, 1201- 1208. [23] V. Mohanasrinivasan, P. Dhrisya, K.P. Dipinsha, C.M. Unnithan, K.M. Viswanath, and C.S. Devi, “Comparative study of the lipase yield by solid state and submerged fermentations using fungal species from biopharmaceuti- cal oil waste,” African Journal of Biotechnology 8(1), 2009, 73-76. [24] J. Nabeny, and B.F. Nesbitt, “A spectophotometric method for determining the aflatoxins,” Analyst 90, 1965, 155-160. [25] R. Paramawati, P. Widodo, U. Budiharti, and dan Han- daka, “The role of postharvest machineries and packaging in minimizing aflatoxin contamination in peanut,” Indo- nesian journal of Agricultural Science 7(1), 2006, 15-19. [26] A. Pekkarinen, L. Mannonen, B.L. Jones, and M.L. Niku-Paavola, “Production of proteases by Fusarium  Maintenance of Ephedra alata Seeds Viability via Storage Containers Copyright © 2010 SciRes. AJPS 146 species grown on barley grains and in media containing cereal proteins,” Journal of Cereal Science 31, 2000, 253-261. [27] Z. Prochăzkovă, and L. Bezděčkova, “Effects of moisture content, storage temperature and type of storage bag on the germination and viability of stored European beech (Fagus sylvatica L.) seeds,” Journal of Forest Science 54(7), 2008, 287-293. [28] S. Rasheed, S. Dawar, and A. Ghaffar, “Location of fungi in groundnut seed,” Pak J. Bot. 36(3), 2004, 663-668. [29] S. S. Tag El-Din, A.M. Assaeed, and A.A. Al-Sheick, “Distribution of range plant communities as influenced by edaphic factors in Raudhat Khuraim,” Egypt. J. Appl. Sci. 9(10), 1994, 69-82. [30] M. Wagner, and N. Mitschunas, “Fungal effects on seed bank persistence and potential applications in weed bio- control: a review,” Basic and Applied Ecology 9, 2008, 191-203. |

