 Journal of Modern Physics, 2013, 4, 22-27 http://dx.doi.org/10.4236/jmp.2013.47A2004 Published Online July 2013 (http://www.scirp.org/journal/jmp) Local Stability of Curzon-Ahlborn Cycle with Non-Linear Heat Transfer for Maximum Power Output Regime Delfino Ladino-Luna, Pedro Portillo-Díaz, Ricardo T. Páez-Hernández Universidad Autónoma Metropolitana-Atzcapotzalco, Física de Procesos Irreversibles, Cd. México, México Email: dll@correo.azc.uam.mx, phrt@correo.azc.uam.mx Received May 7, 2013; revised June 9, 2013; accepted July 4, 2013 Copyright © 2013 Delfino Ladino-Luna et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The study of local stability of thermal engines modeled as an endoreversible Curzon and Ahlborn cycle is shown. It is assumed a non-linear heat transf er for heat flux es in the system (eng ine + env iron ments). A semisum of two expression s of the efficiency found in the literature of finite time thermo dynamics for the maximum power output regime is consid- ered in order to make the analysis. Expression of variables for local stability and power output is found even graphic results for important parameters in the analysis of stability, and a phase plane portrait is shown. Keywords: Local Stability; Thermal Engines; Non-Linear Heat Transfer 1. Introduction As it is known the limits in the performance of thermal engines in th e Clas sica l Equ ilibrium Thermodynamics con- text correspond to reversible processes [1-4]. This situa- tion represents a very hard obstacle in the analysis of thermal engines and leads to non adequate values for va- riables of processes whose values far from to the experi- mental values were reported in the literature. These limits have been partially overcome by helping of the named Finite Time Thermodynamics [5-7]. In order to analyze the performance of thermal engines, many papers in this context have considered that the heat flux between the system and its environs is made by Newton heat transfer law [5-14], for the named Curzon and Ahlborn engine [7]. Nevertheless a more real model has to consider all possi- bilities of heat transfer. Thus, some authors have used particularly the Dulong and Petit heat transfer law be- cause it allows a better model than the Newton heat trans- fer model is [15-18]. In [15-17] numerical results appear near to the experimental values reported in the literature for power plants working at maximum power output, and in [17,18] for nuclear plants working at maximum ecolo- gical function [14]. It is important to point out that all of the above-cited papers have been focused on the thermodynamics prop- erties of the system, through an objective function to an a- lyze the performance of thermal engines, and only the steady state has been analyzed. Nevertheless, other authors have analyzed the intrinsic properties of the systems as the response to a perturba- tion on the steady state of important quantities for the per- formance of thermal engines [19,20]. More recently the local stability of thermal engines has been made consi- dering Newton heat transfer [21-24] and Stefan-Boltz- mann heat transfer [25], besides it has been made con- sidering a working substance different to ideal gas [26]. In the present paper, we consider a heat transfer like the Dulong-Petit heat transfer law in order to make the analysis of a thermal engine for local stability. The im- portant quantities in the performance of the thermal en- gine are found by the heat transfer cited, as the expres- sion of power output and the dynamic equations for an endoreversible Curzon and Ahlborn engine. We assume for simplicity an expression of efficiency as a semi-sum of two expressions found by two different authors [16- 18], which contains the same necessary parameters of the present work, including a comparison by plotting of them. To make this paper self-contained, a review of some well- known results on the Carnot, and Curzon and Ahlborn engines concerning to steady state variables is also inclu- ded. 2. Properties of the Steady States 2.1. Steady States Variables Let us consider a system which consists of two reservoirs at temperatures (hot reservoir) and (cold reser- 1 T2 T C opyright © 2013 SciRes. JMP  D. LADINO-LUNA ET AL. 23 voir), which are related as 12 ; and the thermal en- gine working at temperatures x and y, which are related as TT y xyT . There is a resistance for the heat flow between the thermal engine and its reservoirs with a heat conduc- tance denoted by α, as is shown in Figure 1. In case of Carnot engine, 1 and 2 T , and in case of Cur- zon and Ahlborn engine the temperatures are related by 12 . The engine produces the work W. The heat 1 flows from the hot reservoir to the engine and the heat 2 flows from the engine to the cold reservoir, assuming a constant thermal conductance by the given parameter Tx Q y Q T in both fluxes. According to the first and second laws of thermody- namics, for Carnot engine the heats exchanged in the sys- tem and are given by 1 Q2 Q 1 x QW y (1a) and 2. y QW y (1b) For the endorev ersible Curzon and Ahlborn eng ine we can consider an engine working in steady state, so the temperatures are now and with 12 Tx [20]. Here, and thereafter, the variables with over-bars represent steady state quantities. The endoreversible hy- pothesis is now, yT 12 12 J TT (2) which asserts that an engine working between two reser- voirs at temperatures and behaves as a Carnot Figure 1. Thermal engine working between both x and y temperatures. The reservoirs are at temperatures and . The fluxes to the engine and from the engine are respec- tively T1 2 T 1 and 2. engine, despite the fact that it works in finite-time cycles [10]. and 21 are the steady state heat flows from the to the engine and from the engine to , respec- tively, and because the Curzon and Ahlborn engine is usually supposed working in steady state, the fluxes be- fore mentioned are 11 Tx (3a) and 22 .yT (3b) This means that 1 x P (4a) y and 2 y P (4b) y P is the power output in steady state. The efficiency in steady state for this internally reversible Curzon and Ahlborn engine is written as (5) 1 and by using (4b) we can write 2 1 1JWP J 1 P or , (6) and it follows that 121 1 21 TTT x (7a) and 121 11 21 TTT y . (7b) 2.2. Effect of a Non-Linear Heat Transfer Law Consider now a heat transfer law as, 0 d, d k QTT t (8) which contains as a particular case the Dulong and Petit heat transfer law, where ddQt is the rate of heat trans- fer, is the thermal conductance, 0 is the tempera- ture of environs, T is the temperature of th e body and k is a parameter as , which in case of Dulong and Petit heat transfer is as T 1k 1.1 1.6k [27,28]. Heat fluxes 12 ,J can be written now as 11 k Tx and 22 ,1 k JyTk , (9) and (6) with (9) per mits Copyright © 2013 SciRes. JMP  D. LADINO-LUNA ET AL. 24 1 2 , k k Tx xy yT (10) and then, 1 1, 11 k k 1 1 xT (11) and, 1 1, 11 k k 1 1 1yT (12) where has been defini t e 21 TT . On other hand, the Curzon and Ahlborn engine gives more realistic values of efficiency with this heat transfer than it gives with the Newton heat transfer, in case of maximum power output regime [15]. Results in [14,15] are compared with different reported values of power plants. More recently, at maximum power output regime and at maximum ecological function regime, assuming 54k, analytical approximated expressions for the ef- ficiency were found as [16,18], 2981 10 1 1 OPDP (13a) and 2646 1 1. 1 649 36 OEDP (13b) Besides, by a variational approach, the efficiencies in the maximum power output regime and in the ecological function regi me were obtained res pect i vel y [1 7] as, 1 88 MP 12 160, (14a) and 1 1 88 ME 4 041. (14b) Analyzing the results of several studies in the literature of finite time thermodynamics, it is found that the effi- ciency of Curzon and Ahlborn cycle CAN , named Cur- zon-Ahlborn-Novikov efficiency, is adequate for con- ventional plants, and the named ecological efficiency is adequate for modern plants (including nuclear plants). So, we consider hereafter a thermal engine in the maxim um power output r egime. Comparing the efficiencies in (13a) and (14a) the fol- lowing property is found, , OBS MP OPDP (15) and the semisum 12 SOPDPMP is closer than the efficiencies in (15) to experimental efficiencies. Table 1 shows a comparison between efficiencies in (15), for the case of some conventional power plants working in a maximum power output regime, reported in [16,17]. Even more, the difference between the eficien- cies (13a) and (14a) is shown in Figure 2. As can be seen, most of the numerical values of half the sum of the above cited efficiencies are an approximated constant ratio of Curzon and Ahlborn efficiency as, 1 20.88, OPDP MPCAN (16) where the Curzon-Ahlborn-Novikov efficiency is 1 . Besides, if the Carnot efficiency CCAN is considered, a numerical factor of the semisum appears also as an approximated constant ratio of this efficiency. It can be verified that the semisum 12 PDPMP SO is related with the Carnot efficiency as, 1 20.55. OPDP MPC (17) So, in order to analyze the local stability for a Curzon and Ahlborn engine it can be assumed the previous value for the efficiency, when the Dulong and Petit heat trans- fer law is considered. Figure 3 shows the difference of efficiencies in (17) as function of the parameter , where it can be appreciated that the difference goes to zero, when 111 b aba 2 T1 T . Using the linear approximation , in Table 1. Efficiency at maximum power output regime. OPDP OBS Power plant P Steam power plant, West Thurrock, U K 298838 0.37625 0.335770.360 Geothermal steam plant, Lardarello, Italy 353523 0.16198 0.145300.160 Steam power plant, USA298923 0.40380 0.360060.400 Combined cycle plant (steam-mercury), USA 298783 0.35620 0.318040.340 Central steam power (UK 1936-1940) 298698 0.32089 0.286780.280 Figure 2. Graphic comparison of the efficiencies . ,, OPDPMP CAN Copyright © 2013 SciRes. JMP  D. LADINO-LUNA ET AL. 25 Figure 3. Behavior of the difference between the efficiencies P and OPDP as function of . case of 1a, and with (17) can be obtained the ap- proximate expression for the variables in steady state as, 11 2 12 931 , 29 11 TT T TT x (18a) and 12 0.775 ;yTT 1 T2 0.225 (18b) and solving for and T we obtain, 122 , 31 9 y yx T (19a) and 2910 4. 18 31 y Ty y (19b) Thus, the power output can be written as, 22 1153.4530.69PJyx x. 931 xy xy (20) 2.3. The Local Stability of the Curzon and Ahlborn Engine In order to make the analysis of local stability for an en- doreversible Curzon and Ahlborn engine, we follow the procedure developed in [21], obtaining a system of cou- pled differential equations to model the rate of change of intermediate temperature. Let us assume the temperatures x and y as the corresponding to macroscopic objects with the heat capacity C, and two differential equations for x and y as in [21], 11 Tx J d1 d x tC (21a) and 22 ,Jy T 1 d1 d y tC (21b) which are cancelled when x, y, and 2 take their steady state values. Because the assumption of endor- eversibility, the heat flux from x to the working fluid is 1 and the heat flux from the thermal engine to y is 2 , so these fluxes can be written as 1, x P y (22a) and 2 y P. (22b) y It is reasonable to assume that the power output from the Curzon and Ahlborn engine is related to temperatures x and y as the power output at steady state P depends on and in the maximum power output regime, then we have, 22 153.4530.6 9. 931 xy Pyxx xy (23) Substituting (22) and (23) into (21) we obtain the cou- pled differential equations for temperatures x and y of a Curzon and Ahlborn engine performing in the maximum power output regime, 2 11 6201801232 3069 d, d2 319 Ty Txxyy x tC yx (24a) and 22 32 22 36083069 620180d. d2 319 xyxyxTy Txy tC xyx (24b) To analyze the system’s stability near to steady state we define two adequate functions. The differential equa- tions in the maximum power output regime (21) are de- fined as the functions ,xy , and xy , so (24) can be written as the coupled equations from which the ana- lysis of stability can be made, 2 11 6201801232 3069 ,2319 Ty Txxyy fxyCyx (25a) and 22 32 22 36083069 620180 ,. 2319 yxyxyxTyT x gxy Cxyx (25b) 3. Linearization and Stability Analysis In order to establish the consequences of a non-linear heat transfer in a thermal engine working in the maxi- mum power output regime; we need to find the relaxation times for the corresponding eigenvectors in the stability analysis [21-25]. Mor eover, if both eig envalues are nega- tive real numbers, perturbations decay exponentially. In Copyright © 2013 SciRes. JMP 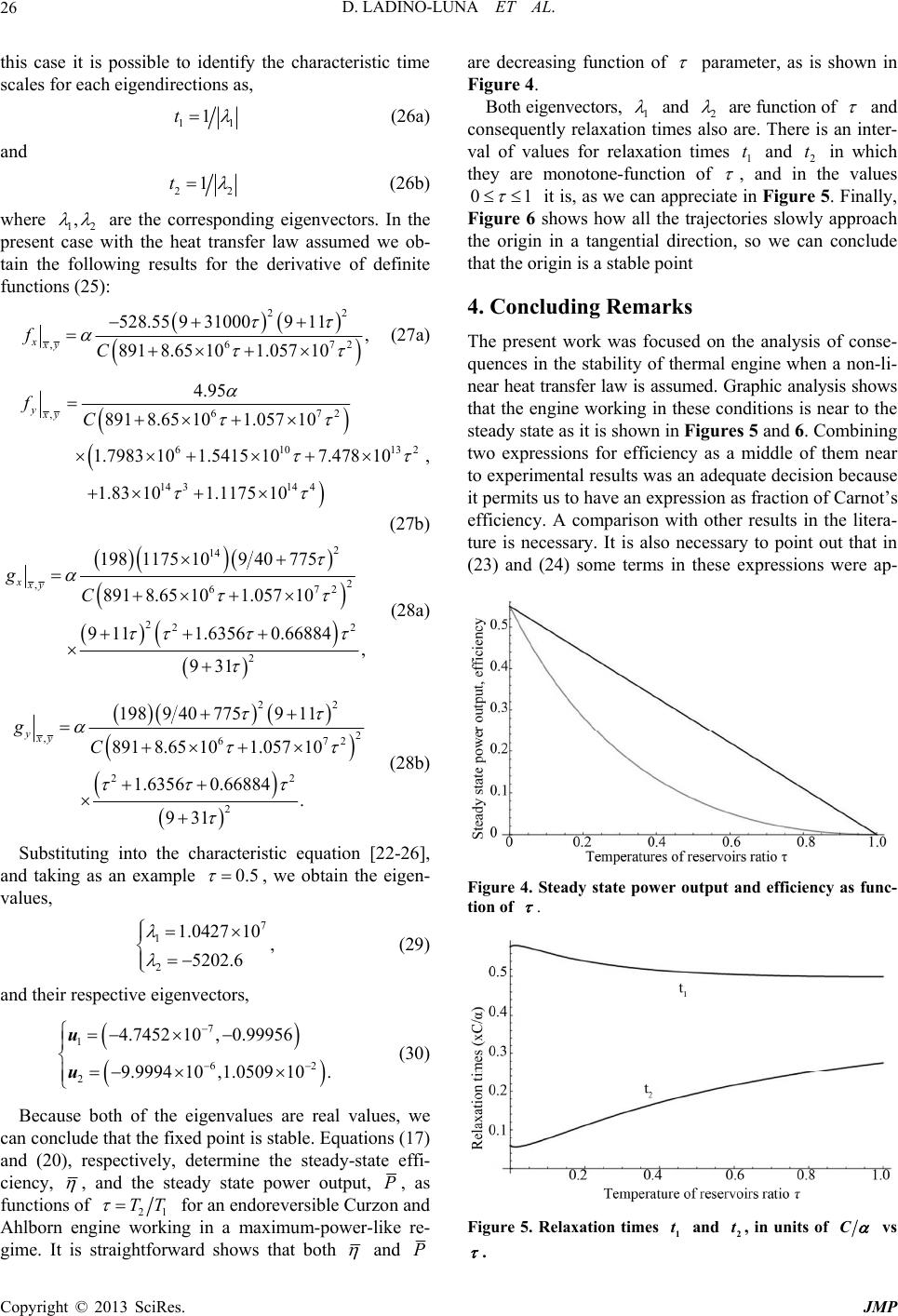 D. LADINO-LUNA ET AL. 26 this case it is possible to identify the characteristic time scales for each eigendirections as, 11 1t (26a) and 22 1t , (26b) where 12 are the corresponding eigenvectors. In the present case with the heat transfer law assumed we ob- tain the following results for the derivative of definite functions (25): 22 672 9 11, 57 10 , 528.55 931000 8918.65 101.0 xxy fC (27a) 67 , 61 14 314 4 4.95 8918.65 101.05710 1.7983 101.5415 107 1.83 101.1175 10 yxy fC 2 0132 .478 10 , (27b) 2 2 672 22 775 10 0.66884 , 14 , 2 2 1981175109 40 8918.65 101.057 9 111.6356 931 xxy g C (28a) 22 2 672 22 9 11 .057 10 . 0.5 , 2 198940775 8918.65 101 1.6356 0.66884 931 yxy g C (28b) Substituting into the characteristic equation [22-26], and taking as an example , we obtain the eigen- values, 7 1.042710 , 5202.6 62 0.99956 .0509 10. 1 2 (29) and their respective eigenvectors, 7 1 2 4.7452 10, 9.9994 10,1 u u (30) Because both of the eigenvalues are real values, we can conclude that the fixed po int is stable. Equation s (17) and (20), respectively, determine the steady-state effi- ciency, , and the steady state power output, P, as functions of 21 TT for an endore versible Curzon and Ahlborn engine working in a maximum-power-like re- gime. It is straightforward shows that both and are decreasing function of parameter, as is shown in Figure 4. P Both eigenvectors, 1 and 2 are function of and consequently relaxation times also are. There is an inter- val of values for relaxation times 1 and 2 t in which they are monotone-function of t , and in the values 01 it is, as we can appreciate in Figure 5. Finally, Figure 6 shows how all the trajectories slowly approach the origin in a tangential direction, so we can conclude that the origin is a stable point 4. Concluding Remarks The present work was focused on the analysis of conse- quences in the stability of thermal engine when a non-li- near heat transfer law is assumed. Graphic analysis shows that the engine working in these conditions is near to th e steady state as it is shown in Figures 5 and 6. Combining two expressions for efficiency as a middle of them near to experimental results was an adequate decision because it permits us to have an expression as fraction of Carnot’s efficiency. A comparison with other results in the litera- ture is necessary. It is also necessary to point out that in (23) and (24) some terms in these expressions were ap- Figure 4. Steady state power output and efficiency as func- tion of . Figure 5. Relaxation times and , in units of t1t2C vs . Copyright © 2013 SciRes. JMP  D. LADINO-LUNA ET AL. Copyright © 2013 SciRes. JMP 27 [10] M. Rubin, Physical Review A, Vol. 19, 1979, pp. 1277- 1289. doi:10.1103/PhysRevA.19.1277 [11] P. Salamon, A. Nitzan, B. Andresen and R. S. Berry, Phy- sical Review A, Vol. 21, 1980, pp. 2115-2129. doi:10.1103/PhysRevA.21.2115 [12] J. L. Torres, Revista Mexicana de Física, Vol. 34, 1988, pp. 18-24. [13] F. Angulo-Brown, Revista Mexicana de Física, Vol. 37, 1991, pp. 87-96. [14] F. Angulo-Brown, Journal of Applied Physics, Vol. 69, 1991, pp. 7465-7469. doi:10.1063/1.347562 [15] F. Angulo-Brown and R. T. Páez-Hernández, Journal of Applied Physics, Vol. 74, 1993, pp. 2216-2219. doi:10.1063/1.354728 [16] D. Ladino-Luna, Revista Mexicana de Física, Vol. 48, 2003, pp. 87-91. Figure 6. Qualitative phase portrait of xt and yt for a Curzon-Ahlborn cycle using the efficiency in (17). [17] L. A. Arias-Hernández, G. Ares and F. Angulo-Brown, Open Systems & Information Dynamics, Vol. 10, 2003, pp. 351-375. doi:10.1023/B:OPSY.0000009556.27759.11 proximated without losing the objective of numerical cal- culation. [18] D. Ladino-Luna, Journal of the Energy Institute, Vol. 81, 2008, pp. 114-117. doi:10.1179/174602208X301961 [19] A. Lifschitz and E. Hameiri, Physical Fluids A, Vol. 3, 1991, pp. 2644-2651. doi:10.1063/1.858153 5. Acknowledgements [20] G. Gallavotti, “New Methods in Noequilibrium Gases and Fluids,” arXiv:chao-dyn/9610018v1 [accesed 30.10.1996]. Authors thank the partial support of CONACYT (México) by SNI program. [21] M. Santillán, G. Maya and F. Angulo-Brown, Journal of Physics D, Vol. 34, 2001, pp. 2068-2072. doi:10.1088/0022-3727/34/13/318 REFERENCES [22] R. T. Páez-Hernández, F. Angulo-Brown and M. Santil- lán, The Journal of Non-Equilibrium Thermodynamics, Vol. 31, 2006, pp. 173-188. [1] S. Carnot, “Réflexions sur la Puissance Motrice du feu et sur les Machines Propres à Développer Cette Puissanc,” Spanish Translation, IPN Press, México, 1998. [23] Y. Huang and D. Sun, The Journal of Non-Equilibrium Thermodynamics, Vol. 33, 2008, pp. 61-74. [2] R. Clausius, “Théorie Mécanique de la Chaleur, 1ére Par- tie,” Lacroix, 1868. [24] Y. Huang, D. Sun and Y. Kang, Journal of Applied Phys- ics, Vol. 102, 2007, pp. 349051-349056. doi:10.1063/1.2767622 [3] E. Fermi, “Thermodynamics,” Columbia University Press, New York, 1936. [4] R. C. Tolman and P. C. Fine, Reviews of Modern Physics, Vol. 20, 1948, pp. 51-77. doi:10.1103/RevModPhys.20.51 [25] L. Guzmán-Vargas, I. Reyes-Ramírez and N. Sánchez, Journal of Physics D: Applied Physics, Vol. 38, 2005, pp. 1282-1295. doi:10.1088/0022-3727/38/8/028 [5] I. I. Novikov, Journal of Nuclear Energy II, Vol. 7, 1958, pp. 125-128. [26] R. T. Páez-Hernández, D. Ladino-Luna and P. Portillo- Díaz, Physica A, Vol. 390, 2011, pp. 3275-3282. doi:10.1016/j.physa.2011.05.019 [6] P. Chambadal, “Les Centrales Nucleares,” Armand Colin, 1957. [27] L. W. Taylor, “Manual of Adva nced Undergraduate Expe- riments in Physics,” Addison-Wesley, 1959, p. 115. [7] F. L. Curzon and B. Ahlborn, American Journal of Phys- ics, Vol. 43, 1975, pp. 22-24. doi:10.1119/1.10023 [28] M. Nelson and P. Parker, “Advanced Level Physics,” 4th Edition, Heinemann, New Orleans, 1977, p. 194. [8] D. Gutkowics-Krusin, I. Procaccia and J. Ross, Journal of Chemical Physics, Vol. 69, 1978, pp. 3898-3906. doi:10.1063/1.437127 [9] M. Rubin, Physical Review A, Vol. 19, 1979, pp. 1272- 1276. doi:10.1103/PhysRevA.19.1272
|