Paper Menu >>
Journal Menu >>
 American Journal of Plant Sciences, 2010, 1, 87-94 doi:10.4236/ajps.2010.12011 Published Online December 2010 (http://www.SciRP.org/journal/ajps) Copyright © 2010 SciRes. AJPS 87 Databasing Molecular Identities of Sugarcane (Saccharum spp.) Clones Constructed with Microsatellite (SSR) DNA Markers Yong-Bao Pan Sugarcane Research Laboratory, Houma, USA. Email: yongbao.pan@ars.usda.gov Received October 1st, 2010; revised October 31st, 2010; accepted November 13th, 2010. ABSTRACT This paper reports the development of the first SSR marker-based sugarcane (Saccharum spp.) molecular identity da- tabase in the world. Since 2005, 1,025 sugarcane clones were genotyped, including 811 Louisiana, 45 Florida, 39 Texas, 130 foreign, and eight consultant/seed company clones. Genotyping was done on a fluorescence-capillary elec- trophoresis detection platform involving 21 highly polymorphic SSR markers that cou ld potentially amplify 144 distinc- tive DNA fragments. Genotyping data were processed with the GeneMapper™ software to reveal electrophoregrams that were manually checked against the 144 fragments. The presence (A) or absence (C) of these 144 fragment s in any sugarcane clone was recorded in an affixed sequence order as a DNAMAN® file to represent its molecula r identity be- ing achieved into a local molecular identity database. The molecular identity database has been updated annually by continued genotyping of newly assigned sugarcane clones. The database provides molecular descriptions for new cul- tivar registration articles, enables sugarcane breeders to identify mis-labeled sugarcane clones in crossing programs and determine the patern ity of cross progeny, and ensures the desired cultivars are grown in farmers’ fields. Keywords: Sugarcane (Saccharum spp.), Breeding, SSR Marker, Molecular Identity Database 1. Introduction Sugarcane (Saccharum spp.) is a complex aneu-poly- ploidy plant (2n = 8x or 10x = 100-130) that propagates asexually through planting of vegetative cuttings (setts) of mature stalks [1,2]. A sugarcane breeding cycle in Louisiana takes 12 years. This cycle begins with cross hybri dization and continues with field evaluation and selection, advancement, and multi-year, multi-location testing, and ending with the release of a new cultivar [3]. During this cycle, exchange and shipment of elite clones and breeding lines in the form of stalk cuttings (setts) across different test locations occur regularly for the purposes of verifying parental source or desired use of a clone in an experiment. Traditional tools for sugarcane breeders to identify different varieties rely on anatomical and morphological characters [1,4]. In Louisiana, the morphological descriptors, stalk wax, leaf sheath wax, leaf sheath margin, leaf sheath hair (pubescence), dewlap appearance, stalk color, auricle size and color, and other distinguishing characteristics, are used by Louisiana sug- arcane breeders [5]. Others may use sugarcane descrip- tors available under the USDA-ARS GRIN system (http://www.ars-grin.gov/npgs/descriptors/sugarcane). Al- though these morphological descriptors may serve breeders who are directly involved in the evaluation and selection of those clones, breeders from other locations or researchers in other disciplines may not be familiar with these morphological traits, especially traits for which differential expression is already known to be strongly influenced by the environment. Therefore, it is not uncommon that mislabeling or misidentification of sugarcane clones occurs from time to time, whether on crossing carts or in the field plots (Jim Miller, personal communication, 2003). It might be worth noting that cumulative probability of this error may be high for pa- rental clones that are propagated many times over the years (Phil Jackson, personal communication, 2010). Because of this, sugarcane pedigree information some- times may not be so reliable (Karl J. Nuss, personal communication, 2003). Thus, to ensure correct variety identity and its genetic pedigree, a procedure for accurate identification using molecular data is urgently needed [6-8].  Databasing Molecular Identities of Sugarcane (Saccharum spp.) Clones Constructed with Microsatellite (SSR) DNA Markers Copyright © 2010 SciRes. AJPS 88 Microsatellite or simple sequence repeats (SSRs) DNA markers are short DNA fragments that contain various numbers of tandem repeat units of di-, tri-, tetra- or composite-nucleotide motifs [9,10]. SSR markers are useful for genotyping sugarcane because they are abun- dant, co-dominantly inherited, and highly reproducible [11,12]. Since the beginning of the century, a high- throughput molecular genotyping technology has been developed for sugarcane (6, 8). By using a fluores- cence/capillary electrophoresis (CE)-based genotyping system, a total of 144 distinctive SSR DNA fragments were consistently amplified among the U.S. sugarcane germplasm from primer pairs of 21 polymorphic SSR DNA markers [13]. The 144 DNA fragments were ar- ranged in a linear order in an Excel spreadsheet, which was used to score the presence (denoted by A) or absence (C) of each fingerprint from a sugarcane clone. The unique sequence of As or Cs was then converted to a DNAMAN® (Lynnon Biosoft, Vaudreuil, Canada) file to represent the molecular identity of that clone. This paper describes the development of the first sug- arcane molecular identity database that has been used by the sugarcane breeders as a molecular breeding tool. Unlike the anatomical and morphological traits that are influenced by environment, SSR DNA marker-based molecular identities represent stable genetic fingerprints that are not affected by geographical region or seasonal changes. With the advent of this molecular breeding tool [6], U.S. sugarcane breeders have been able to provide a molecular descriptor for new variety releases, identify any sugarcane clone that has been mislabeled [7-8], iden- tify S. spontaneum cytoplasm-derived hybrids for trait introgression without violating the noxious weed regula- tions, and determine paternity of clones derived from polycrosses [14]. 2. Materials and Methods 2.1. SSR Markers and Genotyping Sample Collection Primer pairs of 21 highly polymorphic SSR markers de- veloped by the International Consortium of Sugarcane Biotechnologists [11] based on the genomic DNA se- quence of sugarcane cultivars Q124 and R570 were used. The nucleotide sequences and annealing temperatures of these primer pairs are listed in Table 1. The 5' ends of Table 1. Sugarcane SSR markers, anne aling temperatures, and primer seque nc e s. SSR Name* Anneal (℃) Forward Primer (5' to 3') Reverse Primer (5' to 3') SMC119CG 58 TTC ATC TCT AGC CTA CCC CAA AGC AGC CAT TTA CCC AGG A SMC1604SA 58 AGG GAA AAG GTA GCC TTG G TTC CAA CAG ACT TGG GTG G SMC18SA 62 ATT CGG CTC GAC CTC GGG AT AGT CGA AAG GTA GCG TGG TGT TAC SMC24DUQ 64 CGC AAC GAC ATA TAC ACT TCG G CGA CAT CAC GGA GCA ATC AGT SMC278CS 64 TTC TAG TGC CAA TCC ATC TCA GA CAT GCC AAC TTC CAA ACA GAC T SMC31CUQ 62 CAT GCC AAC TTC CAA TAC AGA CT AGT GCC AAT CCA TCT CAG AGA SMC334BS 60 CAA TTC TGA CCG TGC AAA GAT CGA TGA GCT TGA TTG CGA ATG SMC336BS 62 ATT CTA GTG CCA ATC CAT CTC A CAT GCC AAC TTC CAA ACA GAC SMC36BUQ 64 GGG TTT CAT CTC TAG CCT ACC TCA GTA GCA GAG TCA GAC GCT T SMC486CG 58 GAA ATT GCC TCC CAG GAT TA CCA ACT TGA GAA TTG AGA TTC G SMC569CS 62 GCG ATG GTT CCT ATG CAA CTT TTC GTG GCT GAG ATT CAC ACT A SMC7CUQ 60 GCC AAA GCA AGG GTC ACT AGA AGC TCT ATC AGT TGA AAC CGA SMC597CS 64 GCA CAC CAC TCG AAT AAC GGA T AGT ATA TCG TCC CTG GCA TTC A SMC703BS 62 GCC TTT CTC CAA ACC AAT TAG T GTT GTT TAT GGA ATG GTG AGG A SMC851MS 58 ACT AAA ATG GCA AGG GTG GT CGT GAG CCC ACA TAT CAT GC mSSCIR66 48 AGG TGA TTT AGC AGC ATA CAC AAA TAA ACC CAA TGA mSSCIR3 60 ATA GCT CCC ACA CCA AAT GC GGA CTA CTC CAC AAT GAT GC SMC1751CL 60 GCC ATG CCC ATG CTA AAG AT ACG TTG GTC CCG GAA CCG SMC22DUQ 62 CCA TTC GAC GAA AGC GTC CT CAA GCG TTG TGC TGC CGA GT mSSCIR43 52 ATT CAA CGA TTT TCA CGA G AAC CTA GCA ATT TAC AAG AG mSSCIR74 54 GCG CAA GCC ACA CTG AGA ACG CAA CGC AAA ACA ACG  Databasing Molecular Identities of Sugarcane (Saccharum spp.) Clones Constructed with Microsatellite (SSR) DNA Markers Copyright © 2010 SciRes. AJPS 89 the forward primers were labeled with one of three fluo- rescent phosphoramidite dyes, FAM, VIC, or NED (Ap- plied Biosystems, Foster City, CA). For U.S. cultivars and advanced breeding clones, leaf samples were collected from healthy younger leaves without disease symptoms from sugarcane plants maintained on the crossing carts, breeding nurseries, varietal trials, quarantine facilities, or commercial fields. For foreign sugarcane cultivars, either leaf samples collected from clones grown at USDA-ARS, SRL or genomic DNA samples obtained from foreign sugarcane breeding programs were used. 2.2. Leaf DNA Extraction Leaf DNA was extracted by either using CTAB-beta mercaptoethanol [15] or hot NaOH-Tween 20 buffer [16]. For the CTAB-beta mercaptoethanol buffer procedure, total nucleic acids were extracted from approximately 200 mg fresh leaf tissue by blending in a 2-ml microfuge tube containing 1 ml CTAB extraction buffer [2% CTAB, 1.4 M NaCl, 20 mM EDTA, 100 mM Tris-HCl (pH 8.0), 2 μl beta-mercaptoethanol added prior to extraction] and a 4.5 mm diameter sterile chrome-steel bead by violently shaking the tube using a Mini-Bead-BeaterTM (BioSpec Products, Inc., Bartleville, OK) for 1 min. The leaf ho- mogenate was incubated at 60℃ for 30 min, extracted once with 0.75 ml chloroform/isoamyl alcohol (24/1) by centrifuging at 6,000 x g for 10 min at 4℃and transfer- ring 600 μl aqueous phase to a new microfuge tube that contained 500 μl of cold isopropyl alcohol. The mixture was incubated at –20℃for at least 1 hr before centrifug- ing for 15 min at 12,000 x g. The resulting pellet was washed with 500 μl of 70% ethanol plus 10 mM sodium acetate and centrifuged for 10 min at 12,000 x g to col- lect the nucleic acid pellet. Excess wash solution was evaporated in a DNA 120 SpeedVac System (Savant Instruments, Inc., Holbrook, NY) and the pellet was re- hydrated in 200 μl sterile water. The DNA concentration was determined using NanoDrop1000 (Thermo Scientific, Wilmington, DE) and adjusted to 10 μg/μl accordingly. For the hot NaOH-Tween 20 buffer procedure, small pieces (about 30 mm2) of leaf tissue were excised from the youngest fully expanded leaves and dislodged into sample wells of a 96-well microplate that was pre-loaded with 50 μl of a freshly prepared denaturing buffer (100 mM NaOH and 2% Tween-20). The plates were sealed with aluminum sealing tape, incubated at 95℃ for 10 min, placed on ice for three min, and spun at 1,480 x g for 1 min. Fifty μl of a neutralization buffer (100 mM Tris-HCl and 2 mM EDTA) were then added to each well. The plates were re-sealed with aluminum sealing tape; the buffers were mixed by vortex, and spun at 1,480 x g for 1 min. The resulting supernatants were transferred to a fresh sterile 96-well microplate. 2.3. Semi-Automatic PCR and CE Fifty-μl aliquots that were either diluted DNA samples from the CTAB procedure or supernatant from the NaOH-Tween 20 procedure were transferred into the wells of 96-well microplates. Plates were sent to the USDA-ARS, Mid-South Area Genomics Laboratory in Stoneville, MS for high throughput PCR and CE-based fragment analyses. A robot machine, the Hamilton’s Mi- crolab Star Liquid Handling Station (Hamilton Company, Reno, NV), was used to consolidate the DNA samples from four 96-well plates into a single 384-well plate and prepare 384-well PCR amplification reaction plates con- taining a 5-μl PCR reaction mixture within each well. The PCR reaction mixture consisted of 0.25 μl of the DNA sample, 0.5 μl of 10X Buffer, 0.3 μl of 25 mM MgCl2, 0.1 μl of 10 mM dNTPs, 0.41 μl each of 3 pm/μl forward and reverse primers, 0.5 μl of 10 mg/ml BSA-V, 0.5 μl of 100 μg/μl PVP-40, 0.025 μl of 5 Units/μl Taq, and 2.0 μl of PCR water. PCR amplification reactions were conducted on a DNA Engine Tetra equipped with four 384-well Alpha blocks with heated lids (Bio-Rad Laboratories, Hercules, CA) under a program of 95℃ for 15 min, 40 cycles of 94℃ for 15 sec, annealing for 15 sec, and 72℃ for 1 min, final extension at 72℃ for 10 min, and holding at 4℃. When PCR amplification was complete, the robot was used again to prepare 384-well CE sample plates by first diluting the amplified SSR DNA fragments and then mixing in each well one μl of the diluted products with nine μl Hi-Dye formamide so- lution premixed with the GeneScan™ Rox™ 500 Size Standard. The CE sample plates were subjected to auto- mated fragment analysis by ABI3730XL following manufacturer’s instruction to produce Genescan files (Applied Biosystems, Inc., Foster City, CA). 2.4. GeneMapper® Analysis, Construction of Molecular Identity (ID), and Clone Identity Check Genescan files were downloaded online from the file download site of the MSA Genomics Laboratory home page (https://msa.ars.usda.gov/computerhelp/upload/) and archived into individual folders named after sugarcane clones before being processed with the GeneMapper™ software (Applied Biosystems, Inc., Foster City, CA). The software calibrated SSR fragments based on the GeneScan™ Rox™ 500 Size Standard and revealed SSR fragments in the Sample Plot Window, which were in- terpreted and scored manually. True SSR fragments that could be scored exhibited measurable fluorescence peaks. When both “plus-adenine” and “Minus-adenine” DNA 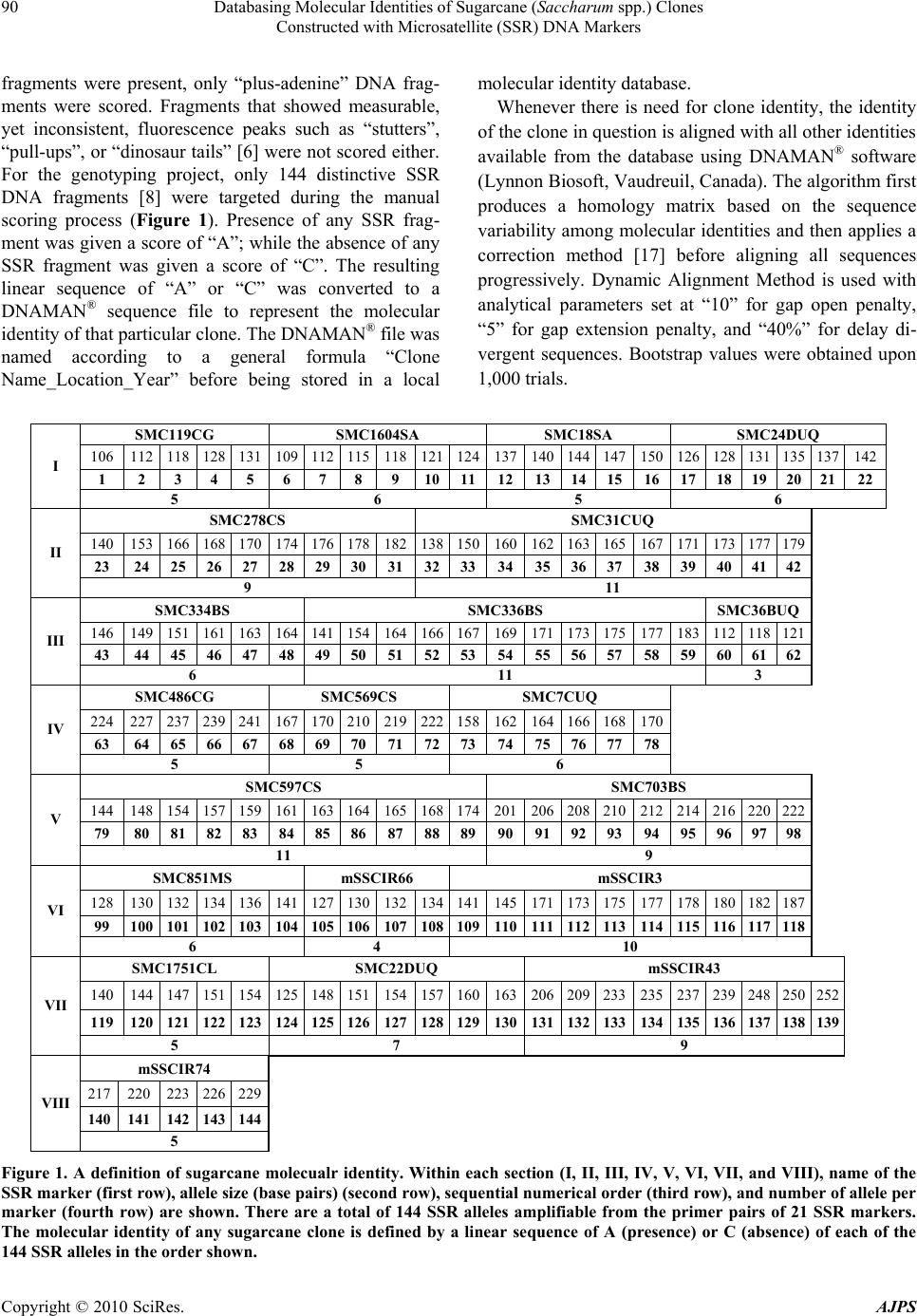 Databasing Molecular Identities of Sugarcane (Saccharum spp.) Clones Constructed with Microsatellite (SSR) DNA Markers Copyright © 2010 SciRes. AJPS 90 fragments were present, only “plus-adenine” DNA frag- ments were scored. Fragments that showed measurable, yet inconsistent, fluorescence peaks such as “stutters”, “pull-ups”, or “dinosaur tails” [6] were not scored either. For the genotyping project, only 144 distinctive SSR DNA fragments [8] were targeted during the manual scoring process (Figure 1). Presence of any SSR frag- ment was given a score of “A”; while the absence of any SSR fragment was given a score of “C”. The resulting linear sequence of “A” or “C” was converted to a DNAMAN® sequence file to represent the molecular identity of that particular clone. The DNAMAN® file was named according to a general formula “Clone Name_Location_Year” before being stored in a local molecular identity database. Whenever there is need for clone identity, the identity of the clone in question is aligned with all other identities available from the database using DNAMAN® software (Lynnon Biosoft, Vaudreuil, Canada). The algorithm first produces a homology matrix based on the sequence variability among molecular identities and then applies a correction method [17] before aligning all sequences progressively. Dynamic Alignment Method is used with analytical parameters set at “10” for gap open penalty, “5” for gap extension penalty, and “40%” for delay di- vergent sequences. Bootstrap values were obtained upon 1,000 trials. SMC119CG SMC1604SA SMC18SA SMC24DUQ 106 112 118 128 131 109 112 115118121 124137140 144 147150126 128 131 135 137142 1 2 3 4 5 6 7 89 1011121314151617 18 19 20 2122 I 5 6 5 6 SMC278CS SMC31CUQ 140 153 166 168 170 174 176 178182138 150160162 163 165167171 173 177 179 23 24 25 26 27 28 29 30313233343536373839 40 41 42 II 9 11 SMC334BS SMC336BS SMC36BUQ 146 149 151 161 163 164 141 154164166 167169171 173 175177183 112 118 121 43 44 45 46 47 48 49 50515253545556575859 60 61 62 III 6 11 3 SMC486CG SMC569CS SMC7CUQ 224 227 237 239 241 167 170 210219222 158162164 166 168170 63 64 65 66 67 68 69 707172737475767778 IV 5 5 6 SMC597CS SMC703BS 144 148 154 157 159 161 163 164165168 174201206 208 210212214 216 220 222 79 80 81 82 83 84 85 86878889909192939495 96 97 98 V 11 9 SMC851MS mSSCIR66 mSSCIR3 128 130 132 134 136 141 127 130132134 141145171 173 175177178 180 182 187 99 100 101 102103 104 105 106107108109110111112113114115 116 117 118 VI 6 4 10 SMC1751CL SMC22DUQ mSSCIR43 140 144 147 151 154 125 148 151154157 160163206 209 233235237 239 248 250 252 119 120 121 122123 124 125 126127128129130131132133134135 136 137 138 139 VII 5 7 9 mSSCIR74 217 220 223 226229 140 141 142 143 144 VIII 5 Figure 1. A definition of sugarcane molecualr identity. Within each section (I, II, III, IV, V, VI, VII, and VIII), name of the SSR marker (first row), allele size (base pairs) (second row), sequential numerical order (third row), and number of allele per marker (fourth row) are shown. There are a total of 144 SSR alleles amplifiable from the primer pairs of 21 SSR markers. The molecular identity of any sugarcane clone is defined by a linear sequence of A (presence) or C (absence) of each of the 144 SSR alleles in the order shown.  Databasing Molecular Identities of Sugarcane (Saccharum spp.) Clones Constructed with Microsatellite (SSR) DNA Markers Copyright © 2010 SciRes. AJPS 91 3. Results 3.1. Number of Clones Genotyped From 2005 to 2008, a total of 1,004 samples were geno- typed targeting the 144 specific DNA fragments that were potentially amplifiable from the primer pairs of 21 SSR markers. These included 237 samples in 2005, 238 in 2006, 339 in 2007, and 190 in 2008. Most of the genotyping (803 samples or 78.3%) was conducted on cultivars and newly assigned breeding lines from the Louisiana breeding programs. In addition, 45 (4.4%) Florida, 39 (3.8%) Texas, 130 (12.7%) foreign, and eight (0.8%) cultivar samples from consultants and seedcane companies were also genotyped (Table 2). Genotyping continues annually for the Louisiana sugarcane breeding program and on request for Florida, Texas, or foreign sugarcane breeding programs. Depending upon the needs for rigor identification, multiple samples are collected from the same clone grown at up to four different loca- tions, in the same or different years. 3.2. A SSR Molecular Identity Database A local SSR identity database was constructed in 2005 by creating a folder named as “Genotyping Database” inside the “C:/My documents/Breeding” folder located in the hard drive disk of a desktop PC operated by the Mi- crosoft Windows program. The “C:/My documents/ Breeding/Genotyping Database” folder is expandable by creating additional sub-folders that are named after cal- endar year, for example, sub-folders <2005>, <2006>, <2007>, etc. (Figure 2). Within each sub-folder are mo- lecular identity files or DNAMAN® files of sugarcane clones that are genotyped in that year. For example, the molecular identity of HoCP 00-950 [18], “CACA- CAACCCCCCAAAAAACCACCAACCC CCCCCAC- ACCCCCACACACCCCACACCCCCCAA ACAAAC- CCCACCAACCCCCACAACCACCACAAA ACCCA- CAACCACCCCCCCACCCACAAAACAAAA ACCA- CACCAAAAAAAA”, can be found in subfolders <2005> as “HoCP00-950_H_05” or “HoCP00-950_ Q_05”, <2006> as “HoCP00-950_H_06” or “HoCP00- 950_Q_06”, and <2008> as “HoCP00-950_CP_08”, where H = Houma, Q = quarantine, CP = Canal Point. 4. Discussion Conventional sugarcane breeding takes 12 years from initial cross hybridization to a new cultivar release [3]. This paper reports the development of the first SSR marker-based molecular identity database in sugarcane that can serve as an additional tool to ensure that breed- ers have the correct clones involved in their crosses as well as varietal trials. Unlike the anatomical and mor- phological traits, SSR DNA marker-based molecular identities represent stable genetic fingerprints that are not affected by location or seasonal changes [7-8]. Since its initial establishment in 2005, the database has been Table 2. Number of sugarcane clones genotyped with SSR markers (2005-2008). Sample Collection Site 2005 2006 2007 2008TOTAL USDA at Houma, FL 57 41 126 131355 USDA at Canal Point, FL 78 97 91 49 315 LSU 52 46 28 7 133 Florida 3 34 6 2 45 Texas 32 7 0 0 39 Consultants/Companies 2 0 3 3 8 Foreign 13 20 85 12 130 TOTAL 237 245 339 2041,025 Figure 2. A local genotyping database at C:\My documents\Breeding\Genotyping database, in which there are five folders, namely, 2005, 2006, 2007, 2008, and New Folder. Part of the Folder 2006 is shown listing molecular identity files of a few sugacrane clones that were genotyped in 2006.  Databasing Molecular Identities of Sugarcane (Saccharum spp.) Clones Constructed with Microsatellite (SSR) DNA Markers Copyright © 2010 SciRes. AJPS 92 updated each year with newly acquired molecular identi- ties. It has also been demonstrated that if a Louisiana sugarcane clone was correctly labeled, then the same molecular identity would be produced using the same fluorescence- and CE-based SSR genotyping protocol and the molecular identity would group together with those produced in prior years or different field plots from the same sugarcane clone. One recent example is shown in Figure 3 that deals with identity checks of three sug- arcane clones, namely, ST-283, ST-299, and ST-950. After these clones were genotyped using the standard protocol, the resulting molecular identities were blindly aligned with those of all other Louisiana sugarcane clones constructed in 2005, 2006, and 2007 using the multiple sequence alignment program of DNAMAN® software (Lynnon Biosoft, Vaudreuil, Canada). The re- sults verified that ST-283 was indeed cultivar L 01-283 (Panel A) and ST-299 was cultivar L 01-299 (Panel A). However, ST-950 was not cultivar HoCP 00-950 but clone Ho 01-564 (Panel B). There are three other demonstrated applications of the reported molecular identity database. The primary and most important application of the molecular identity da- tabase is to protect sugarcane breeders’ rights by provid- ing a molecular descriptor in their cultivar registration. These include Louisiana sugarcane cultivar Ho 95-988 [18], HoCP 96-540 [19], Ho 00-950 [20], HoCP 91-552 [21], and Ho 00-961 [22]. In addition, molecular de- scriptors were also included in sugarcane cultivar regis- tration articles from the Florida sugarcane breeding pro- gram, including CPCL 97-2730 [23], CP 00-1101 [24], CP 88-1165 [25], CP 00-1446 [26], and CP 00-2180 [27]. All the molecular descriptors of newly released Louisi- ana sugarcane cultivars are produced from SSR DNA marker-based genotyping that are stored in the local mo- lecular identity database. The second application of the molecular identity data- base is to facilitate the exploration of S. spontaneum cy- toplasm through conventional breeding and more general, to determine whether progeny are from proposed parents for any type of sugarcane cross, in particular, cross in- volving related wild species. Prior to the advent of SSR genotyping technology, there was no report on the use of the cytoplasmic genome of S. spontaneum clones in sug- arcane breeding. Also, no genetic stock with S. sponta- neum cytoplasm had ever been released. This is because S. spontaneum clones are designated as regulated nox- ious weeds with substantial self-pollination and vigorous Figure 3. Molecular identity verification of three sugarcane clones, ST-283, ST-299, and ST-950 conducted in 2007. The molecular identities of ST-283, ST-299, and ST-950 were aligned with those of all Louisiana sugarcane clones that were genotyped in 2005, 2006, and 2007 using the multiple sequence alignment program of DNAMAN® software (Lynnon Biosoft, Vaudreuil, Canada). Results showed that ST-283 was cultivar L 2001-283 (Panel A), ST-299 was cultivar L 2001-299 (Panel A), and ST-950 was clone Ho 01-564 (Panel B). The dynamic alignment method is used with analytical parameters set at “10” for gap open penalty, “5” for gap extension penalty, and “40%” for delay divergent sequences. The numerical values on the branches are bootstrapping (confide nce) values based on 1,000 trials.  Databasing Molecular Identities of Sugarcane (Saccharum spp.) Clones Constructed with Microsatellite (SSR) DNA Markers Copyright © 2010 SciRes. AJPS 93 rhizomes [28]. With the advent of SSR genotyping tech- nology, sugarcane breeders have been able to use DNA marker information to identify true F1 progeny from selfs arising from crosses in which S. spontaneum clones were maternal parents before evaluation in the field en- suring the noxious weed regulations were not violated. A few S. spontaneum cytoplasm-derived clones have been reported, of which US 99-51 [29] and Ho 02-113 (un- published data) produced consistently high yields of total dry mass. A third but potential use of the molecular identity da- tabase is to determine the paternity of sugarcane progeny, particularly those from polycrosses [14]. When only a few tassels are available from desirable parents, sugar- cane breeders must decide whether to make a limited number of bi-parental crosses or intersperse the tassels in a polycross to obtain a greater number of crosses and more seeds. Without the molecular identity information for the parental clones, breeders are not able to defini- tively determine the paternity information for polycross progeny. Using seven highly polymorphic SSR markers that produced parent-specific SSR alleles, Tew and Pan [14] were able to determine the paternity for 79 to 99% of the progeny from a seven-parent polycross, depending upon the maternal parent. The ability to identify paternity of polycross progeny with SSR DNA markers can be used in sugarcane breeding to maximize the number of desirable crosses from a limited source of flowers with minimal loss of pedigree information. 5. Acknowledgements The high-throughput PCR and fragment analysis were conducted by Sheron Simpson at the USDA-ARS, Mid- South Area Genomics Laboratory, Stoneville, MS, di- rected by Brian E. Scheffler. Lionel Lomax and Jennifer Shaw provided technical assistance. The study was par- tially supported by the sugarcane grower/miller check-off funds administered by the American Sugar Cane League and the Florida Sugar Cane League. Product names and trademarks are mentioned to report factually on available data; however, the USDA neither guarantees nor warrants the standard of the product, and the use of the name by USDA does not imply the ap- proval of the product to the exclusion of others that may also be suitable. REFERENCES [1] D. J. Heinz, “Sugarcane Improvement through Breeding,” Elsevier, Amsterdam. [2] L. Grivet and P. Arruda, “Sugarcane Genomics: Depict- ing the Complex Genome of an Important Tropical Crop,” Current Opinion in Plant Biology, Vol. 5, No. 2, 2002, pp. 122-127. [3] K. P. Bischoff and K. A Gravois, “The Development of New Sugarcane Varieties at the LSU Ag Center,” Journal of American Society Sugar Cane Technologists, Vol. 24, 2004, pp. 142-164. [4] J. C. Skinner, “Description of Sugarcane Clones. III. Bo- tanical Description,” Proceedings International Society Sugar Cane Technologists, Vol. 14, 1972, pp. 124-127. [5] C. LaBorde, B. Legendre, K. Bischoff, K. Gravois and T. Robert, “Sugarcane Variety Identification Guide 2008,” Louisiana State University AgCenter Publication, Baton Rouge, 2008. [6] Y.-B. Pan, G. M. Cordeiro, E. P. Richard Jr. and R. J. Henry, “Molecular Genotyping of Sugarcane Clones with Microsatellite DNA Markers,” Maydica, Vol. 48, No. 4, 2003, pp. 319-329. [7] Y.-B. Pan, J. D. Miller, R. J. Schnell, E. P. Richard Jr. and Q. Wei, “Application of Microsatellite and RAPD Fingerprints in the Florida Sugarcane Variety Program,” Sugar Cane International, March-April 2003, pp. 19-28. [8] Y.-B. Pan, B. S. Scheffler and E. P. Richard Jr., “High Throughput Genotyping of Commercial Sugarcane Clones with Microsatellite (SSR) DNA Markers,” Sugar Tech, Vol. 9, No. 2, 2007, pp. 176-181. [9] A. Edwards, A. Civitello, H. A. Hammond and C. T. Caskey, “DNA Typing and Genetic Mapping with Trimeric and Tetrameric Tandem Repeats,” American Journal Human Genetics, Vol. 49, No. 4, 1991, pp. 746- 756. [10] M. H. Polymeropoulos, H. Xiao, D. S. Rath and C. R. Merril, “Tetranucleotide Repeat Polymorphism at the Human Tyrosine Hydroxylase Gene,” Nucleic Acids Re- search, Vol. 19, No. 13, 1991, p. 3753. [11] G. M. Cordeiro, G. O. Taylor and R. J. Henry, “Charac- terisation of Microsatellite Markers from Sugarcane (Sac- charum sp.), a Highly Polyploid Species,” Plant Science, Vol. 155, No. 2, 2000, pp. 161-168. [12] G. M. Cordeiro, R. Casu, C. L. McIntyre, J. M. Manners and R. J. Henry, “Microsatellite Markers from Sugarcane (Saccharum sp.) ESTs Transferable to Erianthus and Sorghum,” Plant Science, Vol. 160, No. 6, 2001, pp. 1115-1123. [13] Y.-B. Pan, “Highly Polymorphic Microsatellite DNA Markers for Sugarcane Germplasm Evaluation and Vari- ety Identity Testing,” Sugar Tech, Vol. 8, No. 4, 2006, pp. 246-256. [14] T. L. Tew and Y.-B. Pan, “Microsatellite (Simple Se- quence Repeat) Marker–Based Paternity Analysis of a Seven-Parent Sugarcane Polycross,” Crop Science, Vol. 50, No. 4, 2010, pp. 1401-1408. [15] Y.-B. Pan, D. M. Burner and B. L. Legendre, “An As- sessment of the Phylogenetic Relationship among Sugar- cane and Related Taxa Based on the Nucleotide Sequence of 5s Rrna Intergenic Spacers,” Genetica, Vol. 108, No. 3, 2000, pp. 285-295.  Databasing Molecular Identities of Sugarcane (Saccharum spp.) Clones Constructed with Microsatellite (SSR) DNA Markers Copyright © 2010 SciRes. AJPS 94 [16] Z. Xin, J. P. Velten, M. J. Oliver and J. J. Burke, “High-Throughput DNA Extraction Method Suitable for PCR,” BioTechniques, Vol. 34, No. 4, 2003, pp. 820-826. [17] T. H. Jukes and C. R. Cantor, “Evolution of Protein Molecules,” In: H. N. Munro, Ed., Mammalian Protein Metabolism, Academic Press, New York, 1969, pp. 21- 132. [18] T. L. Tew, D. M. Burner, B. L. Legendre, W. H. White, M. P. Grisham, E. O. Dufrene Jr., D. D. Garrison, J. C. Veremis, Y.-B. Pan and E. P. Richard Jr., “Registration of ‘Ho 95-988’ Sugarcane,” Crop Science, Vol. 45, No. 4, 2005, pp. 1660-1661. [19] T. L. Tew, W. H. White, B. L. Legendre, M. P. Grisham, E. O. Dufrene Jr., D. D. Garrison, J. C. Veremis, Y.-B. Pan, E. P. Richard Jr. and J. D. Miller, “Registration of ‘HoCP 96-540’ Sugarcane,” Crop Science, Vol. 45, No. 2, 2005, pp. 785-786. [20] T. L. Tew, W. H. White, M. P. Grisham, E. O. Dufrene, D. D. Garrison, Y.-B. Pan, E. P. Richard Jr., B. L. Leg- endre, and J. D. Miller, “Registration of ‘HoCP 00-950’ sugarcane,” Journal Plant Registrations, Vol. 3, No. 1, 2009, pp. 42-50. [21] T. L. Tew, E. O. Dufrene, W. H. White, R. M. Cobill, B. L. Legendre, M. P. Grisham, D. D. Garrison, Y.-B. Pan, E. P. Richard Jr., and J. D. Miller, “Registration of ‘HoCP 91-552’ High-Fiber Sugarcane,” Journal Plant Registra- tions, in press. [22] W. H. White, R. M. Cobill, T. L. Tew, D. M. Burner, M. P. Grisham, E. O. Dufrene, Y.-B. Pan, E. P. Richard Jr. and B. L. Legendre, “Registration of ‘Ho 00-961’ Sugar- cane,” Journal Plant Registrations, in press. [23] S. B. Milligan, R. W. Davidson, S. J. Edmé, J. C. Com- stock, C. J. Hu, D. G. Holder, B. Glaz, N. C. Glynn and R. A. Gilbert, “Registration of ‘CPCL 97-2730’ Sugarcane,” Journal Plant Registrations, Vol. 3, No. 2, 2009, pp. 158-164. [24] R. A. Gilbert, J. C. Comstock, B. Glaz, S. J. Edmé, R. W. Davidson, N. C. Glynn, J. D. Miller and P. Y. P. Tai, “Registration of ‘CP 00-1101’ Sugarcane,” Journal Plant Registrations, Vol. 2, No. 2, 2008, pp. 95-101. [25] Juárez, J. L., J. D. Miller, H. Orozco, E. Solares, P. Y. P. Tai, J. C. Comstock, B. Glaz, J. L. Quemé de León, W. Ovalle, S. J. Edmé, N. C. Glynn, and C. W. Deren, “Reg- istration of ‘CP 88-1165’ Sugarcane,” Journal Plant Reg- istrations, Vol. 2, No. 2, 2008, pp. 102-109. [26] J. C. Comstock, B. Glaz, S. J. Edmé, R. W. Davidson, R. A. Gilbert, N. C. Glynn, J. D. Miller, and P. Y. P. Tai, “Registration of ‘CP 00-1446’ Sugarcane,” Journal Plant Registrations, Vol. 3, No. 1, 2009, pp. 28-34. [27] B. Glaz, S. J. Edmé, R. W. Davidson, R. A. Gilbert, J. C. Comstock, N. C. Glynn, J. D. Miller and P. Y.P. Tai, “Registration of ‘CP 00-2180’ Sugarcane,” Journal Plant Registrations, Vol. 3, No. 1, 2009, pp. 35-41. [28] P. Y. P. Tai, J. D. Miller and B. L. Legendre, “Preserva- tion of Saccharum spontaneum Germplasm through Stor- age of True Seeds,” Sugar Cane, Vol. 6, 1994, pp. 3-8. [29] Y.-B. Pan, D. M. Burner, Q. Wei, G. M. Cordeiro, B. L. Legendre and R. J. Henry, “New Saccharum Hybrids in S. spontaneum Cytoplasm Developed through a Combina- tion of Conventional and Molecular Breeding Ap- proaches,” Plant Genetic Resources, Vol. 2, No. 2, 2004, pp. 131-139. |

