 Open Journal of Metal, 2013, 3, 68-76 http://dx.doi.org/10.4236/ojmetal.2013.32A1009 Published Online July 2013 (http://www.scirp.org/journal/ojmetal) Assessment of Heavy Metals Immobilization in Artificially Contaminated Soils Using Some Local Amendments Noha H. Abdel-Kader, Reda R. Shahin*, Hasan A. Khater Soils Department, Faculty of Agriculture, Cairo University, Giza, Egypt Email: *dredashahin@gmail.com Received May 17, 2013; revised June 23, 2013; accepted July 2, 2013 Copyright © 2013 Noha H. Abdel-Kader et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Three alluvial soil samples with different textures were artificially polluted with chloride solutions of Cd, Pb, Co and chromate solution for Cr. The aqua-regia extracted concentration ranges in the artificially polluted soils were 1134 - 1489 mg·kg−1 for Pb, 854 - 938 mg·kg−1 for Cr, 166 - 346 mg·kg−1 for Co and 44 - 54 mg·kg−1 for Cd. The aqua-regia extracted metals were the highest in the spiked clay soil due to its high adsorption capacity. Rock phosphate (PR), lime- stone (LS) and Portland-cement (Cem) were mixed with the spiked soils at 1% and 2% rates (w/w) and incubated at 30 C for 2, 7, 14, 30, 60, 150 and 360 days. The extracted DTPA metals significantly decreased with different magnitudes with increasing the incubation period accompanied by increases in both pH and EC. The data showed that cement (Cem) treatment dropped the DTPA-Pb from @ 1000 to @ 400 mg·kg−1 in all the studied soils (60% decrease) in the first 2 months while it gradually decreased from 400 to 200 mg·kg−1 (20% decrease) in the next 10 months. Limestone (LS) and rock phosphate (PR) materials were relatively less effective in lowering DTPA-Pb after 12 months of incubation. The data showed also that cement (Cem) treatment was the most effective one in lowering DTPA-Cd by @ 60% as compared to the un-amended soils after 12 months of soil incubation. Extractable DTPA-Co and Cr showed consistent decreases with time down to nearly 50% of un-amended soils due to the effect of the added amendments after 12 months of incubation with superior reductions for the cement treatment in all the investigated soils. The statistical analysis confirmed that in all the studied metals and treatment, cement treatment (Cem) was significantly the most ef- fective in lowering the DTPA extracted metals as indicated from LSD test. It was found that up to 73% and 57% of the applied Pb and Cd, respectively, were fixed by only 1% cement. However, the present study showed that from the prac- tical and economic points of view, that 1% Cement was the best treatment to immobilize Pb and Cd from all the artifi- cially polluted soils. Keywords: Heavy Metals; Immobilization Efficiency; Rock Phosphate; Portland Cement; Lime-Stone 1. Introduction The contamination of soils with heavy metals is now worldwide concerned due to its hazard to ecosystem in- cluding soil, water, plant, animal and human life. The common international technologies of the remediation of the heavy metals contaminated sites are physical, chemi- cal and biological techniques. The immobilization tech- nique is commonly recognized for the in-situ remediation of heavy metals contaminated soils (Unite State Envi- ronmental Protection Agency (EPA), [1]. Immobiliza- tion is the reduction of the solubility of heavy metals through chemical reactions (ion-exchange, adsorption, precipitation and complexation processes) making them less harmful or less mobile (Hashimoto et al. [2] and Wang et al. [3]). The mostly applied amendments in- clude clay material, cement, zeolites, phosphates, and organic composts GWRTAC [4] and Finžgar et al. [5]. Zhang and Pu [6] used limestone (AL), rock phosphate (RP), palygorskite (PG), and calcium magnesium phos- phate (CMP) to stabilize heavy metals in two urban soils (calcareous soil and acidic soil) polluted with cadmium, copper, zinc and lead for 12 months. The results showed that application of those materials reduced exchangeable forms in the order of Pb > Cd > Cu > Zn. Phosphate and and palygorskite treatments were more efficient than limestone and rock phosphate in stabilizing heavy metals. Padmavathiamma and Li [7] found that lime application to H.M. polluted soil lowered plant available Pb and Mn, while rock phosphate decreased plant available Pb and increased plant Mn. Houben et al. [8] found that the ad- *Corresponding author. C opyright © 2013 SciRes. OJMetal  N. H. ABDEL-KADER ET AL. 69 dition of CaCO3 to heavy metals contaminated soils sig- nificantly reduced both the leaching and the availability of Cd, Zn and Pb metals. Chen et al. [9] applied rock phosphate with a rate of 2500 mg P2O5 kg−1 soil and found that it could successfully reduce the bioavailability and increase the geochemical stability of Pb, Zn and Cd in soil. In addition, Chen et al. [10] showed that rock phosphate of the smallest grain size (<35 microns) was superior to all of other grain sizes more than 35 microns for reducing uptake in plant (Brassica oleracea L.) shoots for Cd (19.6% - 50.0%), Pb (21.9% - 51.4%) and Zn (22.4% - 34.6%), respectively, as compared with the soil without application of rock phosphate. Cao et al. [11] stated that rock phosphate amendment significantly re- duced Pb water solubility, phyto-availability, and bio- accessibility by 72% - 100%, 15% - 86%, and 28% - 92%, respectively due to the formation of insoluble Pb- phosphate minerals and reduced water soluble Cu and Zn by 31% - 80% and 40% - 69%, respectively. Al-Oud and Hilal [12] found that the admixing 0.5% of cement could reduce the 0.5 N HNO3 extracted Pb by values up to 65% in the polluted sandy soils in Saudi Arabia. Alpaslan and Yukselen [13] conducted several leaching experiments for mixtures of different additives (lime, activated carbon, clay, zeolite, sand and cement) with artificially Pb con- taminated (spiked) soil samples. They stated that lime and cement were significantly effective in Pb immobili- zation with 88% efficiency at 1:21 lime:soil ratio and 99% efficiency at 1:15 cement:soil ratio, respectively. The objective of the present work is to evaluate the ef- ficiency of different local amendments to stabilize or immobilize heavy metals in different soil types artifi- cially spiked with Pb, Cd, Cr and Co heavy metals. 2. Materials and Methods Three soil samples with different textures were taken from the most common polluted spots at Bahr el Bakar and Helwan. The collected samples were subjected to the physical and chemical analyses according to Sparks et al. [14] and their characteristics are presented in Table 1. Solutions of different concentrations of cadmium (Cd), lead (Pb), and cobalt (Co) were prepared using metal chlorides and Cr as chromate, then sprayed onto the soil samples with continuous mixing to homogenize the dis- tribution of the applied heavy metals. The spiked soils were allowed to be air-dried after each portion of sprayed metals solution. The total amounts of the metals were applied to exceed their maximum concentrations in soils as reported by EPA [15]. After spiking, the soil was su- persaturated with deionized water and then mixed peri- odically for two weeks. The wetting and air dry cycle procedure was repeated five times to allow sufficient mixing of the applied metals and soil to imitate field conditions (Shanbleh and Kharabsheh, [16]; Lin et al., [17]). The aqua regia extraction was conducted and based on the procedure recommended by Cottenie et al. [18] which standardized by the International Organization for Standardisation (ISO 11466, [19]). In this procedure the soil sample intake was of 1 g, which were placed in 100 ml Pyrex digestion tubes, then 3 ml distilled water was added to obtain slurry. Thereafter, 7.5 ml of 37% HCl and 2.5 ml of 70% HNO3 (3:1) mixture were added to the tube which was covered and left overnight. Then, the suspension was digested at 130˚C for 2 h, in a reflux condenser. The obtained suspension was then filtered through an ashless Whatman 41 filter, diluted to 25 ml with 0.5 mol·L−1 HNO3, and stored in polyethylene bot- tles at 4˚C for analyses. The available metal contents of soils were extracted in DTPA (diethylene-triamine-penta-acetic acid) according to Lindsay and Norwell [20] as modified by ISO 14870 [21]. The DTPA-TEA solution was prepared by mixing of 0.005 mol·L−1 DTPA, 0.01 mol·L−1 CaCl2, 0.1 mol·L−1 tri-ethanolamine (TEA) with pH adjusted to 7.3 with 1 mol·L −1 HCl solution. An amount of 5 g of soil sample (<2 mm) was weighted into a 125 mL Erlenmeyer flask, then 20 ml of DTPA-TEA extracting solution was added and shaken for 2 h at room temperature using a platform shaker. The soil suspension was centrifuged at 2000 rpm for 15 min. and the clear supernatant was diluted to 50 mL with re-distilled water. Both aqua-regia and DTPA- extractable contents were determined by a Perkin-Elmer model 1100B flame atomic absorption spectrometer (AAS). Analysis of variance and LSD were used to compare treatment means. All the statistical analyses were carried out using Costat software [22]. The chemical and physical properties of the artificially contaminated soils are presented in Table 2. Three local amendments were chosen, i.e. Phosphate rock (PR), Limestone (LS), and Portland cement (Cem). Portions of 100 g from the artificially contaminated soils were mixed with 1 g and 2 g of each amendment. A con- trol treatment (C0) for each soil with no amendment ad- dition was also prepared. Each treatment was carried out in triplicates. Replications of the homogeneous soil mix- tures were watered to saturation level and placed in sealed small polyethylene bags in order to maintain the moisture level. The sealed soil mixture bags were incu- bated for 2, 7, 14, 30, 60, 150 and 360 days at 31˚C ± 2˚C. The soil bags were thoroughly mixed during the in- cubation process. At each period, a replicate from each treatment was removed from the incubator and the DTPA-TEA extractable fractions of the studied metals in soils were determined after incubation. The difference in initial concentration and final concentration, knowing dry mass of soil, allowed for the calculation of the exact amount of heavy metals adsorbed per gram of soil. A tandard solution was run with each batch to justify the s Copyright © 2013 SciRes. OJMetal 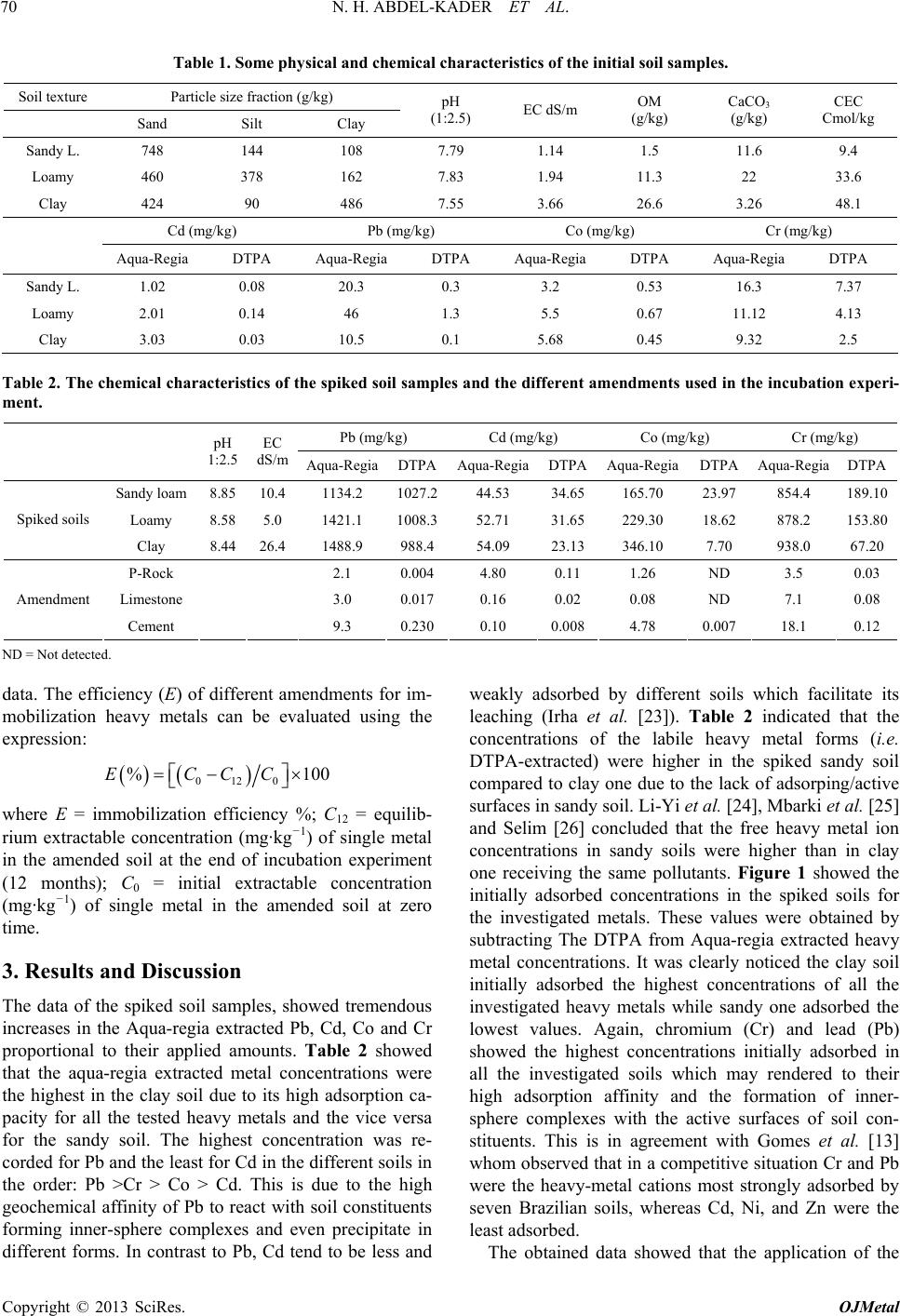 N. H. ABDEL-KADER ET AL. Copyright © 2013 SciRes. OJMetal 70 Table 1. Some physical and chemical characteristics of the initial soil samples. Soil texture Particle size fraction (g/kg) Sand Silt Clay pH (1:2.5) EC dS/m OM (g/kg) CaCO3 (g/kg) CEC Cmol/kg Sandy L. 748 144 108 7.79 1.14 1.5 11.6 9.4 Loamy 460 378 162 7.83 1.94 11.3 22 33.6 Clay 424 90 486 7.55 3.66 26.6 3.26 48.1 Cd (mg/kg) Pb (mg/kg) Co (mg/kg) Cr (mg/kg) Aqua-Regia DTPA Aqua-Regia DTPA Aqua-Regia DTPA Aqua-Regia DTPA Sandy L. 1.02 0.08 20.3 0.3 3.2 0.53 16.3 7.37 Loamy 2.01 0.14 46 1.3 5.5 0.67 11.12 4.13 Clay 3.03 0.03 10.5 0.1 5.68 0.45 9.32 2.5 Table 2. The chemical characteristics of the spiked soil samples and the different amendments used in the incubation experi- ment. Pb (mg/kg) Cd (mg/kg) Co (mg/kg) Cr (mg/kg) pH 1:2.5 EC dS/m Aqua-Regia DTPAAqua-Regia DTPAAqua-RegiaDTPA Aqua-RegiaDTPA Sandy loam 8.8510.4 1134.2 1027.244.53 34.65165.70 23.97 854.4 189.10 Loamy 8.585.0 1421.1 1008.352.71 31.65229.30 18.62 878.2 153.80 Spiked soils Clay 8.4426.4 1488.9 988.454.09 23.13346.10 7.70 938.0 67.20 P-Rock 2.1 0.0044.80 0.11 1.26 ND 3.5 0.03 Limestone 3.0 0.0170.16 0.02 0.08 ND 7.1 0.08 Amendment Cement 9.3 0.2300.10 0.0084.78 0.007 18.1 0.12 ND = Not detected. data. The efficiency (E) of different amendments for im- mobilization heavy metals can be evaluated using the expression: 0120 % 100ECCC where E = immobilization efficiency %; C12 = equilib- rium extractable concentration (mg·kg−1) of single metal in the amended soil at the end of incubation experiment (12 months); C0 = initial extractable concentration (mg·kg−1) of single metal in the amended soil at zero time. 3. Results and Discussion The data of the spiked soil samples, showed tremendous increases in the Aqua-regia extracted Pb, Cd, Co and Cr proportional to their applied amounts. Table 2 showed that the aqua-regia extracted metal concentrations were the highest in the clay soil due to its high adsorption ca- pacity for all the tested heavy metals and the vice versa for the sandy soil. The highest concentration was re- corded for Pb and the least for Cd in the different soils in the order: Pb >Cr > Co > Cd. This is due to the high geochemical affinity of Pb to react with soil constituents forming inner-sphere complexes and even precipitate in different forms. In contrast to Pb, Cd tend to be less and weakly adsorbed by different soils which facilitate its leaching (Irha et al. [23]). Table 2 indicated that the concentrations of the labile heavy metal forms (i.e. DTPA-extracted) were higher in the spiked sandy soil compared to clay one due to the lack of adsorping/active surfaces in sandy soil. Li-Yi et al. [24], Mbarki et al. [25] and Selim [26] concluded that the free heavy metal ion concentrations in sandy soils were higher than in clay one receiving the same pollutants. Figure 1 showed the initially adsorbed concentrations in the spiked soils for the investigated metals. These values were obtained by subtracting The DTPA from Aqua-regia extracted heavy metal concentrations. It was clearly noticed the clay soil initially adsorbed the highest concentrations of all the investigated heavy metals while sandy one adsorbed the lowest values. Again, chromium (Cr) and lead (Pb) showed the highest concentrations initially adsorbed in all the investigated soils which may rendered to their high adsorption affinity and the formation of inner- sphere complexes with the active surfaces of soil con- stituents. This is in agreement with Gomes et al. [13] whom observed that in a competitive situation Cr and Pb were the heavy-metal cations most strongly adsorbed by seven Brazilian soils, whereas Cd, Ni, and Zn were the least adsorbed. The obtained data showed that the application of the 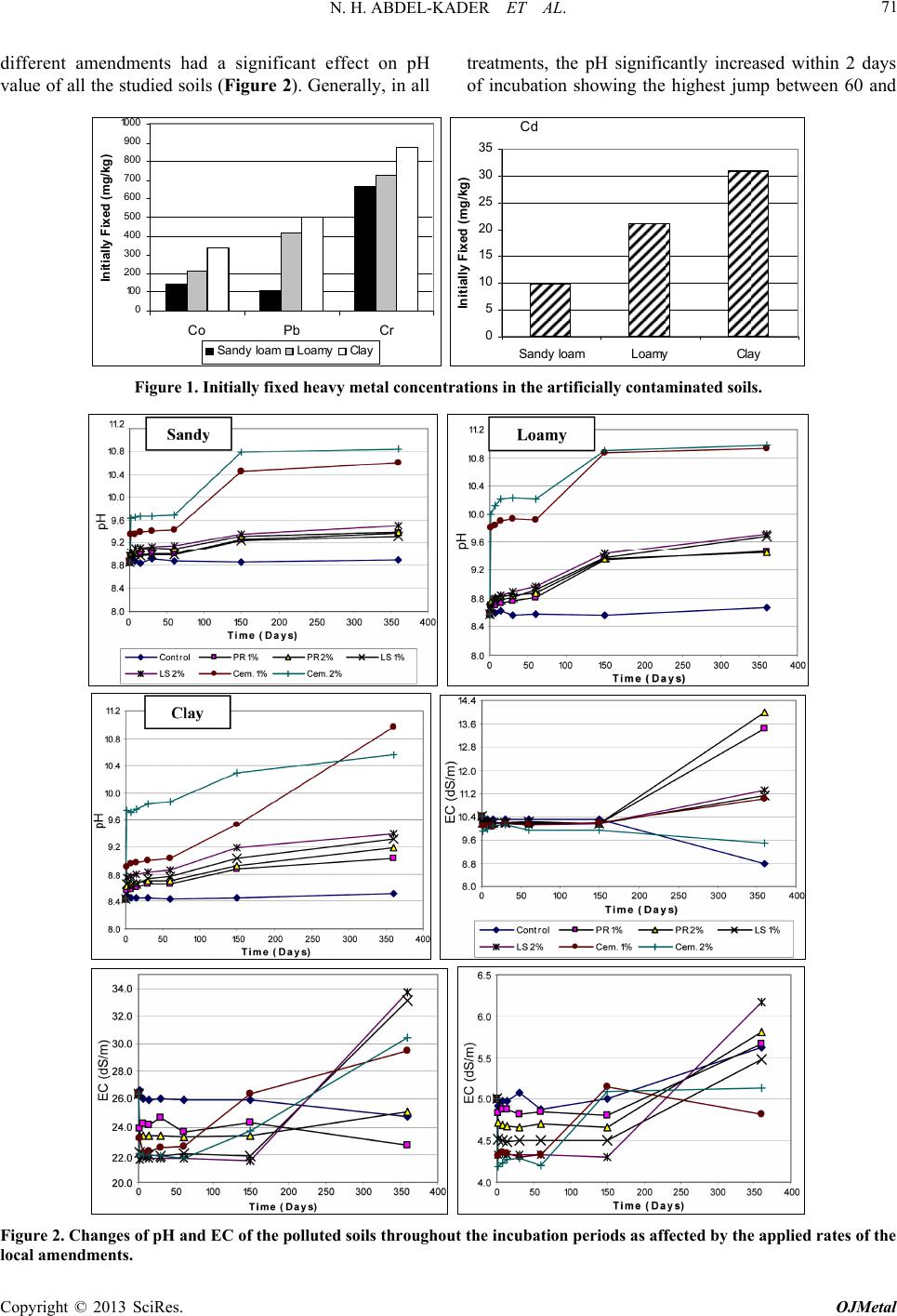 N. H. ABDEL-KADER ET AL. 71 different amendments had a significant effect on pH value of all the studied soils (Figure 2). Generally, in all treatments, the pH significantly increased within 2 days f incubation showing the highest jump between 60 and o 0 10 0 200 300 400 500 600 700 800 900 1000 Co PbC Initially Fixed (mg/kg) Sa ndy loamLoamy Clay Cd 0 5 10 15 20 25 30 35 Sand y loamLoamyClay Initially Fixed (mg/kg) Figure 1. Initially fixed heavy metal concentrations in the artificially contaminated soils. Figure 2. Changes of pH and EC of the polluted soils throughout the incubation periods as affected by the applied rates of the local amendments. Copyright © 2013 SciRes. OJMetal 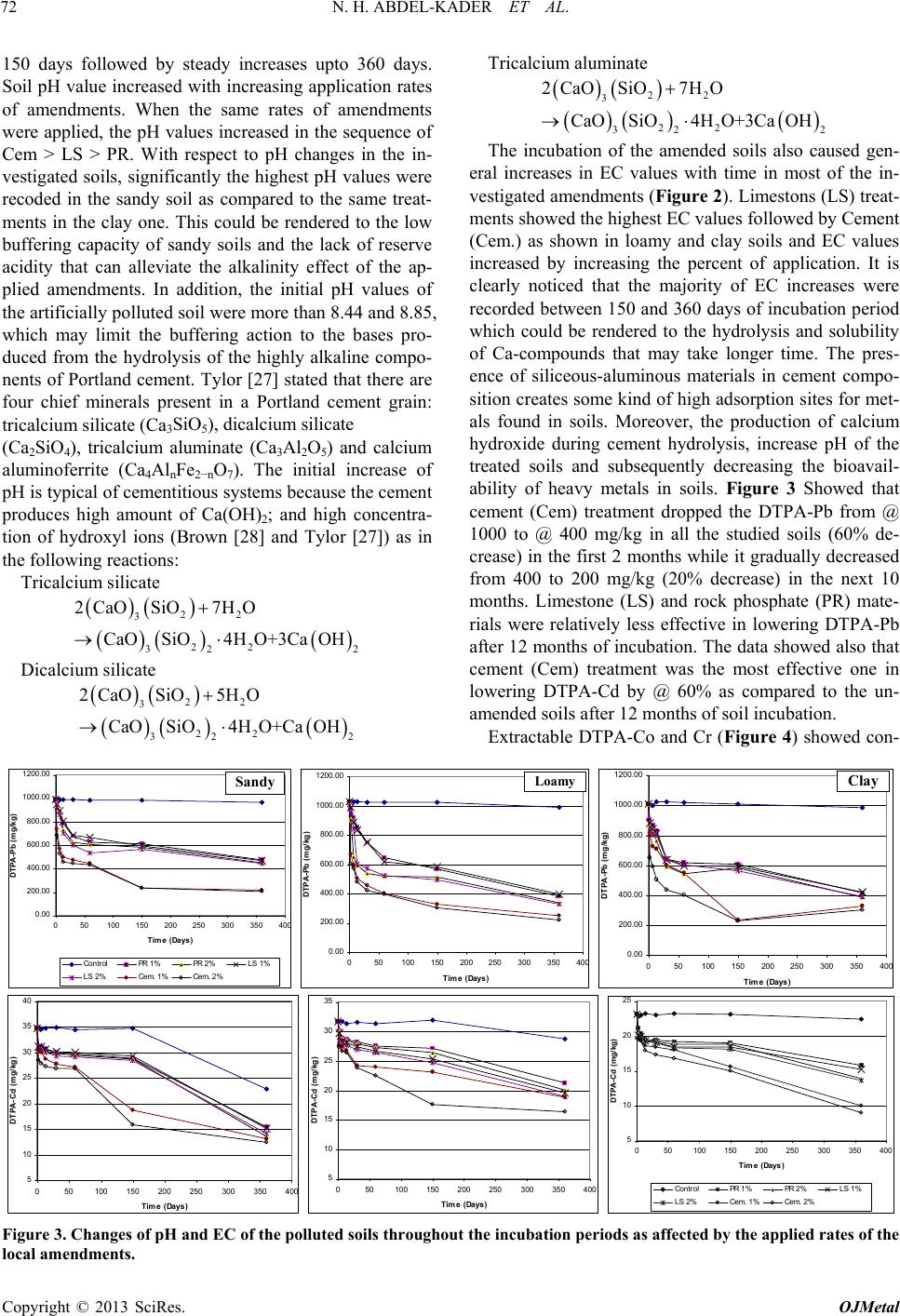 N. H. ABDEL-KADER ET AL. 72 150 days followed by steady increases upto 360 days. Soil pH value increased with increasing application rates of amendments. When the same rates of amendments were applied, the pH values increased in the sequence of Cem > LS > PR. With respect to pH changes in the in- vestigated soils, significantly the highest pH values were recoded in the sandy soil as compared to the same treat- ments in the clay one. This could be rendered to the low buffering capacity of sandy soils and the lack of reserve acidity that can alleviate the alkalinity effect of the ap- plied amendments. In addition, the initial pH values of the artificially polluted soil were more than 8.44 and 8.85, which may limit the buffering action to the bases pro- duced from the hydrolysis of the highly alkaline compo- nents of Portland cement. Tylor [27] stated that there are four chief minerals present in a Portland cement grain: tricalcium silicate (Ca3SiO5), dicalcium silicate (Ca2SiO4), tricalcium aluminate (Ca3Al2O5) and calcium aluminoferrite (Ca4AlnFe2−nO7). The initial increase of pH is typical of cementitious systems because the cement produces high amount of Ca(OH)2; and high concentra- tion of hydroxyl ions (Brown [28] and Tylor [27]) as in the following reactions: Tricalcium silicate 22 3 22 32 2CaOSiO7HO CaOSiO4H O+3CaOH 2 2 Dicalcium silicate 22 3 22 32 2CaO SiO5HO CaOSiO4H O+CaOH Tricalcium aluminate 22 3 22 32 2CaOSiO7HO CaOSiO4H O+3CaOH 2 The incubation of the amended soils also caused gen- eral increases in EC values with time in most of the in- vestigated amendments (Figure 2). Limestons (LS) treat- ments showed the highest EC values followed by Cement (Cem.) as shown in loamy and clay soils and EC values increased by increasing the percent of application. It is clearly noticed that the majority of EC increases were recorded between 150 and 360 days of incubation period which could be rendered to the hydrolysis and solubility of Ca-compounds that may take longer time. The pres- ence of siliceous-aluminous materials in cement compo- sition creates some kind of high adsorption sites for met- als found in soils. Moreover, the production of calcium hydroxide during cement hydrolysis, increase pH of the treated soils and subsequently decreasing the bioavail- ability of heavy metals in soils. Figure 3 Showed that cement (Cem) treatment dropped the DTPA-Pb from @ 1000 to @ 400 mg/kg in all the studied soils (60% de- crease) in the first 2 months while it gradually decreased from 400 to 200 mg/kg (20% decrease) in the next 10 months. Limestone (LS) and rock phosphate (PR) mate- rials were relatively less effective in lowering DTPA-Pb after 12 months of incubation. The data showed also that cement (Cem) treatment was the most effective one in lowering DTPA-Cd by @ 60% as compared to the un- amended soils after 12 months of soil incubation. Extractable DTPA-Co and Cr (Figure 4) showed con- 0.00 200.00 400.00 600.00 800.00 1000.00 1200.00 050100 150 200250 300 350 40 Time (Days) DTPA-Pb (mg/kg) Control PR 1%PR 2%LS 1% LS 2%Cem. 1%Cem. 2% Sandy 0.00 200.00 400.00 600.00 800.00 1000.00 1200.00 050100 150 200 250 300 350 40 Time (Days) DTPA-Pb (mg/kg) Loamy 0.00 200.00 400.00 600.00 800.00 1000.00 1200.00 050100 150200250 30035040 Time (Da ys) DTPA-Pb (mg/ kg) Clay 5 10 15 20 25 30 35 40 050100 150 200 250300 350 40 Time (Days) DTPA-Cd (mg/kg) 5 10 15 20 25 30 35 050100 150 200 250300 350 400 Time (Days) DTPA -Cd (mg/kg) 5 10 15 20 25 050100 150 200 250300 350 400 Time (Days) DTPA -Cd (mg/kg) Control PR 1%PR 2%LS 1% LS 2%Cem. 1%Cem. 2% Figure 3. Changes of pH and EC of the polluted soils throughout the incubation periods as affected by the applied rates of the local amendments. Copyright © 2013 SciRes. OJMetal 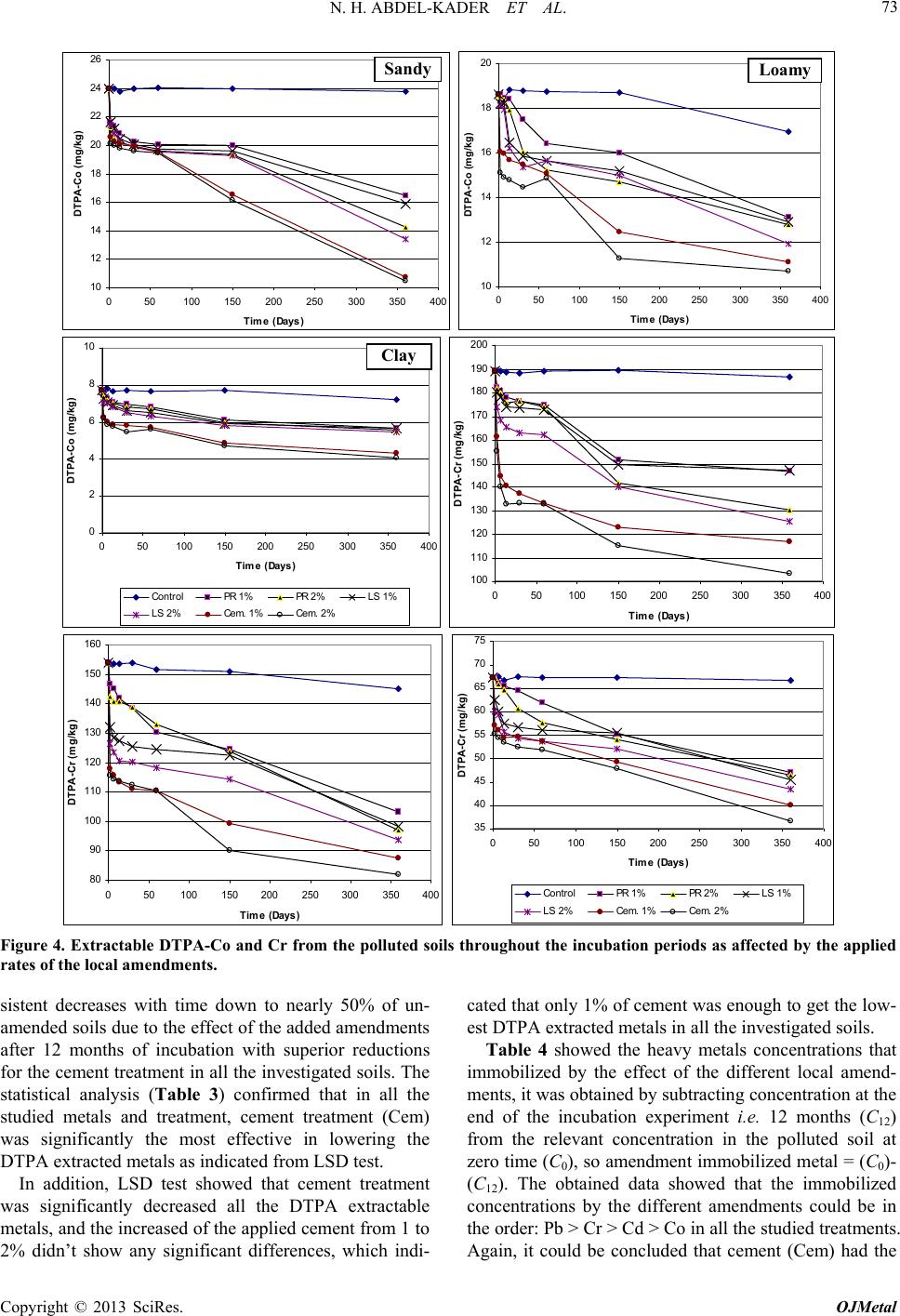 N. H. ABDEL-KADER ET AL. 73 10 12 14 16 18 20 22 24 26 050100 150 200 250300350 400 Time (Days) DTPA-C o (m g /kg ) Sandy 10 12 14 16 18 20 050100150200 250 300 350400 Time (Days) DTPA-Co (mg/kg) Loamy 0 2 4 6 8 10 050100 150200 250 300 350 400 Time (Days) DTPA-Co (mg/kg) Control PR 1%PR 2%LS 1% LS 2%Cem. 1%Cem. 2% Clay 100 110 120 130 140 150 160 170 180 190 200 050100150 200250300350 400 Time (Days) DTPA-Cr (mg/kg) 80 90 100 110 120 130 140 150 160 050100150200 250 300350400 Time (Days) DTPA-Cr (mg/kg) 35 40 45 50 55 60 65 70 75 050100 150 200 250300 350 400 Time (D ays) DTPA-Cr (mg/kg ) Control PR 1%PR 2%LS 1% LS 2%Cem. 1%Cem. 2% Figure 4. Extractable DTPA-Co and Cr from the polluted soils throughout the incubation periods as affected by the applied rates of the local amendments. sistent decreases with time down to nearly 50% of un- amended soils due to the effect of the added amendments after 12 months of incubation with superior reductions for the cement treatment in all the investigated soils. The statistical analysis (Table 3) confirmed that in all the studied metals and treatment, cement treatment (Cem) was significantly the most effective in lowering the DTPA extracted metals as indicated from LSD test. In addition, LSD test showed that cement treatment was significantly decreased all the DTPA extractable metals, and the increased of the applied cement from 1 to 2% didn’t show any significant differences, which indi- cated that only 1% of cement was enough to get the low- est DTPA extracted metals in all the investigated soils. Table 4 showed the heavy metals concentrations that immobilized by the effect of the different local amend- ments, it was obtained by subtracting concentration at the end of the incubation experiment i.e. 12 months (C12) from the relevant concentration in the polluted soil at zero time (C0), so amendment immobilized metal = (C0)- (C12). The obtained data showed that the immobilized concentrations by the different amendments could be in the order: Pb > Cr > Cd > Co in all the studied treatments. gain, it could be concluded that cement (Cem) had the A Copyright © 2013 SciRes. OJMetal 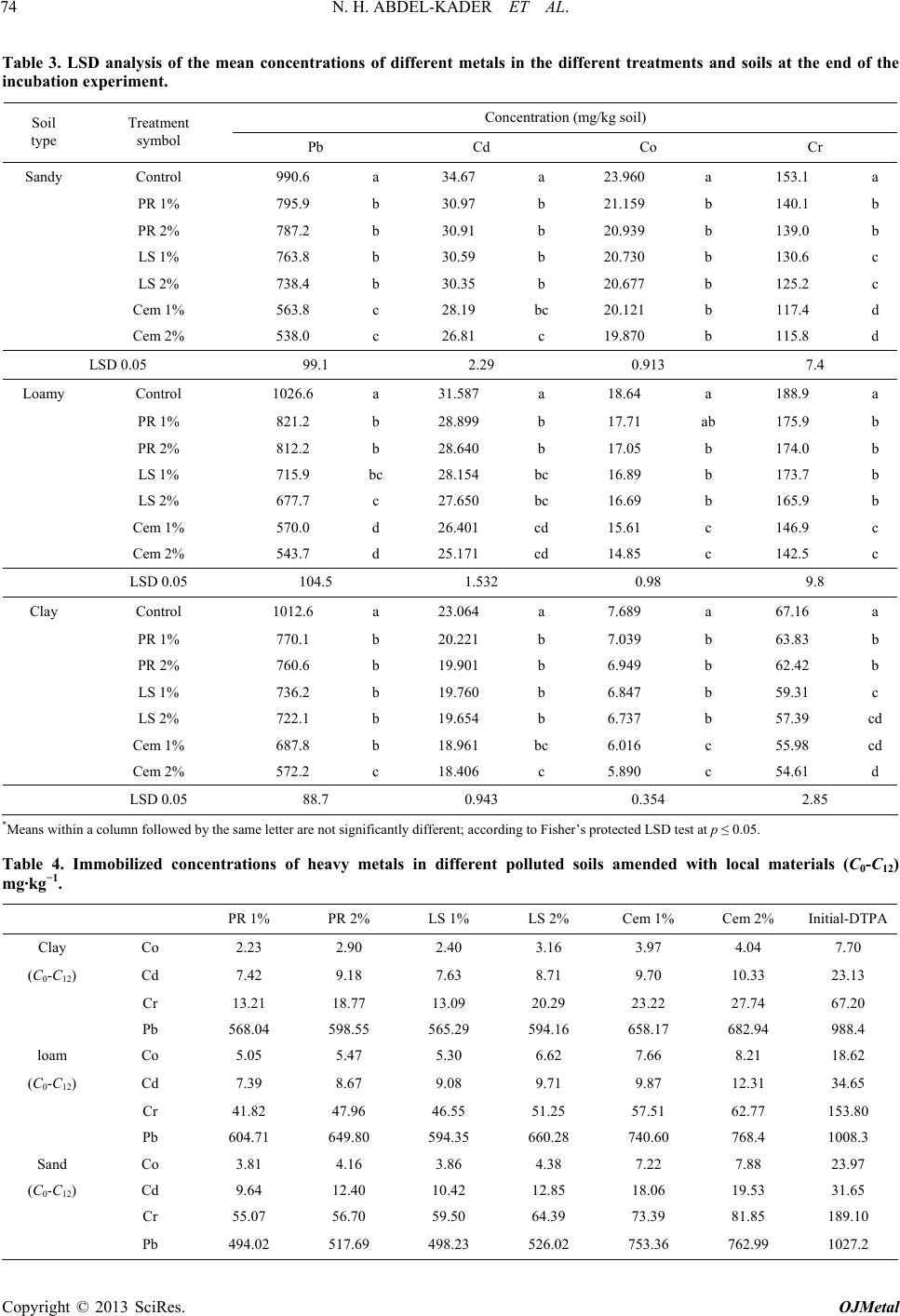 N. H. ABDEL-KADER ET AL. 74 Table 3. LSD analysis of the mean concentrations of different metals in the different treatments and soils at the end of the incubation experiment. Concentration (mg/kg soil) Soil type Treatment symbol Pb Cd Co Cr Sandy Control 990.6 a 34.67 a 23.960 a 153.1 a PR 1% 795.9 b 30.97 b 21.159 b 140.1 b PR 2% 787.2 b 30.91 b 20.939 b 139.0 b LS 1% 763.8 b 30.59 b 20.730 b 130.6 c LS 2% 738.4 b 30.35 b 20.677 b 125.2 c Cem 1% 563.8 c 28.19 bc 20.121 b 117.4 d Cem 2% 538.0 c 26.81 c 19.870 b 115.8 d LSD 0.05 99.1 2.29 0.913 7.4 Loamy Control 1026.6 a 31.587 a 18.64 a 188.9 a PR 1% 821.2 b 28.899 b 17.71 ab 175.9 b PR 2% 812.2 b 28.640 b 17.05 b 174.0 b LS 1% 715.9 bc 28.154 bc 16.89 b 173.7 b LS 2% 677.7 c 27.650 bc 16.69 b 165.9 b Cem 1% 570.0 d 26.401 cd 15.61 c 146.9 c Cem 2% 543.7 d 25.171 cd 14.85 c 142.5 c LSD 0.05 104.5 1.532 0.98 9.8 Clay Control 1012.6 a 23.064 a 7.689 a 67.16 a PR 1% 770.1 b 20.221 b 7.039 b 63.83 b PR 2% 760.6 b 19.901 b 6.949 b 62.42 b LS 1% 736.2 b 19.760 b 6.847 b 59.31 c LS 2% 722.1 b 19.654 b 6.737 b 57.39 cd Cem 1% 687.8 b 18.961 bc 6.016 c 55.98 cd Cem 2% 572.2 c 18.406 c 5.890 c 54.61 d LSD 0.05 88.7 0.943 0.354 2.85 *Means within a column followed by the same letter are not significantly different; according to Fisher’s protected LSD test at p ≤ 0.05. Table 4. Immobilized concentrations of heavy metals in different polluted soils amended with local materials (C0-C12) mg·kg−1. PR 1% PR 2% LS 1% LS 2% Cem 1% Cem 2% Initial-DTPA Clay Co 2.23 2.90 2.40 3.16 3.97 4.04 7.70 (C0-C12) Cd 7.42 9.18 7.63 8.71 9.70 10.33 23.13 Cr 13.21 18.77 13.09 20.29 23.22 27.74 67.20 Pb 568.04 598.55 565.29 594.16 658.17 682.94 988.4 loam Co 5.05 5.47 5.30 6.62 7.66 8.21 18.62 (C0-C12) Cd 7.39 8.67 9.08 9.71 9.87 12.31 34.65 Cr 41.82 47.96 46.55 51.25 57.51 62.77 153.80 Pb 604.71 649.80 594.35 660.28 740.60 768.4 1008.3 Sand Co 3.81 4.16 3.86 4.38 7.22 7.88 23.97 (C0-C12) Cd 9.64 12.40 10.42 12.85 18.06 19.53 31.65 Cr 55.07 56.70 59.50 64.39 73.39 81.85 189.10 Pb 494.02 517.69 498.23 526.02 753.36 762.99 1027.2 Copyright © 2013 SciRes. OJMetal 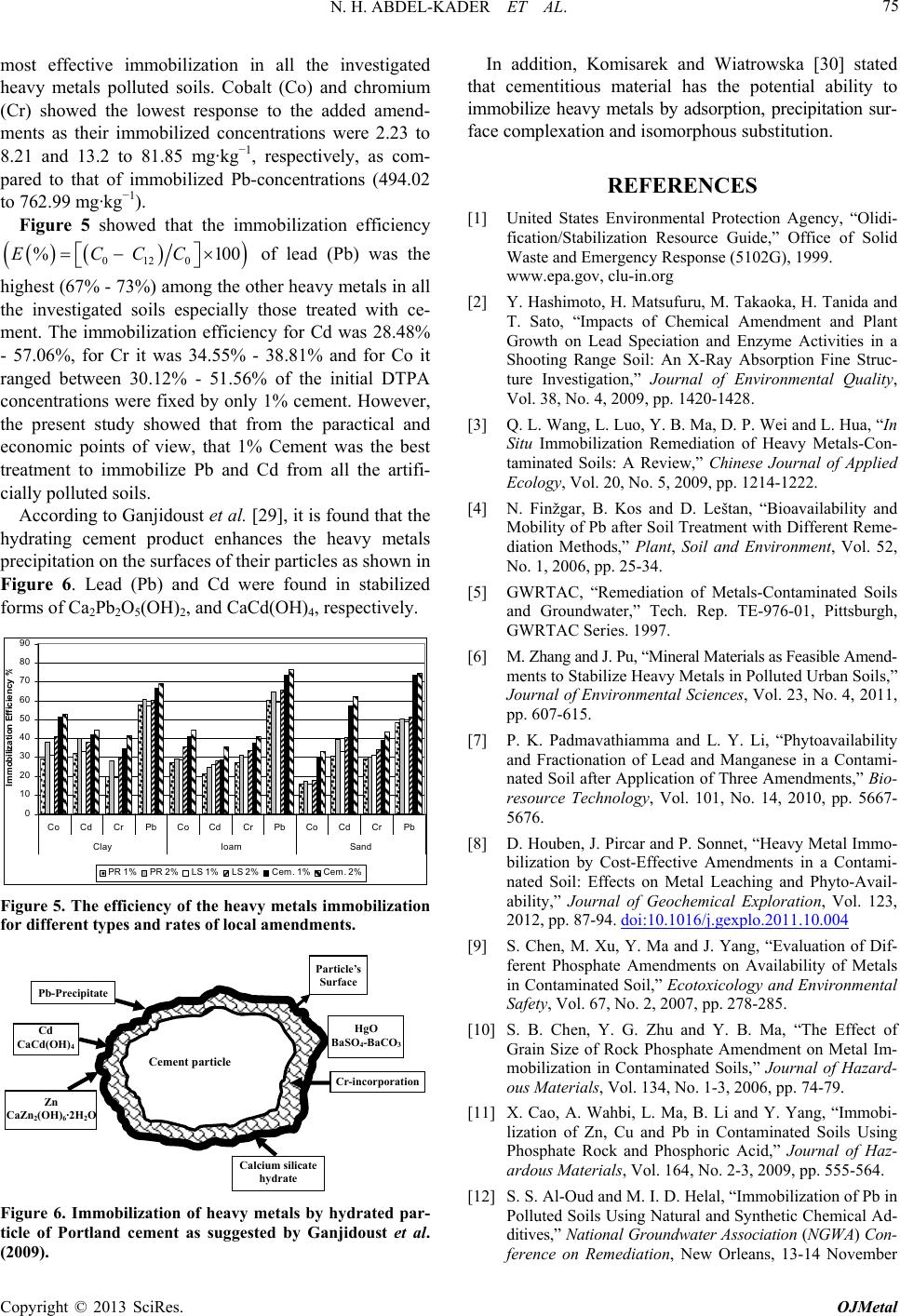 N. H. ABDEL-KADER ET AL. 75 most effective immobilization in all the investigated heavy metals polluted soils. Cobalt (Co) and chromium (Cr) showed the lowest response to the added amend- ments as their immobilized concentrations were 2.23 to 8.21 and 13.2 to 81.85 mg·kg−1, respectively, as com- pared to that of immobilized Pb-concentrations (494.02 to 762.99 mg·kg−1). Figure 5 showed that the immobilization efficiency 0120 % ECCC 100 of lead (Pb) was the highest (67% - 73%) among the other heavy metals in all the investigated soils especially those treated with ce- ment. The immobilization efficiency for Cd was 28.48% - 57.06%, for Cr it was 34.55% - 38.81% and for Co it ranged between 30.12% - 51.56% of the initial DTPA concentrations were fixed by only 1% cement. However, the present study showed that from the paractical and economic points of view, that 1% Cement was the best treatment to immobilize Pb and Cd from all the artifi- cially polluted soils. According to Ganjidoust et al. [29], it is found that the hydrating cement product enhances the heavy metals precipitation on the surfaces of their particles as shown in Figure 6. Lead (Pb) and Cd were found in stabilized forms of Ca2Pb2O5(OH)2, and CaCd(OH)4, respectively. 0 10 20 30 40 50 60 70 80 90 Co CdCrPb CoCdCrPb Co Cd CrPb Clayloam Sand I mmobilizati on E f ficiency PR 1%PR 2%LS 1%LS 2%Cem. 1%Cem. 2% Figure 5. The efficiency of the heavy metals immobilization for different types and rates of local amendments. Cement particle Pb-Precipitate Zn CaZn 2 (OH) 6 ·2H 2 O Cd CaCd(OH) 4 Particle’s Surface Cr-incorpor ation HgO BaSO 4 -BaCO 3 Calcium silicate hydrate Figure 6. Immobilization of heavy metals by hydrated par- ticle of Portland cement as suggested by Ganjidoust et al. (2009). In addition, Komisarek and Wiatrowska [30] stated that cementitious material has the potential ability to immobilize heavy metals by adsorption, precipitation sur- face complexation and isomorphous substitution. REFERENCES [1] United States Environmental Protection Agency, “Olidi- fication/Stabilization Resource Guide,” Office of Solid Waste and Emergency Response (5102G), 1999. www.epa.gov, clu-in.org [2] Y. Hashimoto, H. Matsufuru, M. Takaoka, H. Tanida and T. Sato, “Impacts of Chemical Amendment and Plant Growth on Lead Speciation and Enzyme Activities in a Shooting Range Soil: An X-Ray Absorption Fine Struc- ture Investigation,” Journal of Environmental Quality, Vol. 38, No. 4, 2009, pp. 1420-1428. [3] Q. L. Wang, L. Luo, Y. B. Ma, D. P. Wei and L. Hua, “In Situ Immobilization Remediation of Heavy Metals-Con- taminated Soils: A Review,” Chinese Journal of Applied Ecology, Vol. 20, No. 5, 2009, pp. 1214-1222. [4] N. Finžgar, B. Kos and D. Leštan, “Bioavailability and Mobility of Pb after Soil Treatment with Different Reme- diation Methods,” Plant, Soil and Environment, Vol. 52, No. 1, 2006, pp. 25-34. [5] GWRTAC, “Remediation of Metals-Contaminated Soils and Groundwater,” Tech. Rep. TE-976-01, Pittsburgh, GWRTAC Series. 1997. [6] M. Zhang and J. Pu, “Mineral Materials as Feasible Amend- ments to Stabilize Heavy Metals in Polluted Urban Soils,” Journal of Environmental Sciences, Vol. 23, No. 4, 2011, pp. 607-615. [7] P. K. Padmavathiamma and L. Y. Li, “Phytoavailability and Fractionation of Lead and Manganese in a Contami- nated Soil after Application of Three Amendments,” Bio- resource Technology, Vol. 101, No. 14, 2010, pp. 5667- 5676. [8] D. Houben, J. Pircar and P. Sonnet, “Heavy Metal Immo- bilization by Cost-Effective Amendments in a Contami- nated Soil: Effects on Metal Leaching and Phyto-Avail- ability,” Journal of Geochemical Exploration, Vol. 123, 2012, pp. 87-94. doi:10.1016/j.gexplo.2011.10.004 [9] S. Chen, M. Xu, Y. Ma and J. Yang, “Evaluation of Dif- ferent Phosphate Amendments on Availability of Metals in Contaminated Soil,” Ecotoxicology and Environmental Safety, Vol. 67, No. 2, 2007, pp. 278-285. [10] S. B. Chen, Y. G. Zhu and Y. B. Ma, “The Effect of Grain Size of Rock Phosphate Amendment on Metal Im- mobilization in Contaminated Soils,” Journal of Hazard- ous Materials, Vol. 134, No. 1-3, 2006, pp. 74-79. [11] X. Cao, A. Wahbi, L. Ma, B. Li and Y. Yang, “Immobi- lization of Zn, Cu and Pb in Contaminated Soils Using Phosphate Rock and Phosphoric Acid,” Journal of Haz- ardous Materials, Vol. 164, No. 2-3, 2009, pp. 555-564. [12] S. S. Al-Oud and M. I. D. Helal, “Immobilization of Pb in Polluted Soils Using Natural and Synthetic Chemical Ad- ditives,” National Groundwater Association (NGWA) Con- ference on Remediation, New Orleans, 13-14 November Copyright © 2013 SciRes. OJMetal  N. H. ABDEL-KADER ET AL. 76 2003. [13] B. Alpaslan and M. A. Yukselen, “Remediation of Lead Contaminated Soils by Stabilization/Solidification,” Wa- ter, Air and Soil Pollution, Vol. 133, No. 1-4, 2002, pp. 253-263. [14] D. L. Sparks, “Soil Science Society of America, and Ame- rican Society of Agronomy, Methods of Soil Analysis. Part 3, Chemical Methods,” Soil Science Society of Ame- rica Book Series, No. 5, Madison, 1996. [15] Environmental Protection Agency, “Integrated Risk In- formation System (IRIS),” National center for Environ- mental Assessment, Office of Research and Development, Washington DC, 2001. [16] A. Shanbleh and A. Kharabsheh, “Stabilization of Cd, Ni and Pb in Soil Using Natural Zeolite,” Journal of Haz- ardous Material, Vol. 45, No. 11, 1996, pp. 207-217. [17] C. F. Lin, S. S. Lo, H. Y. Lin and Y. Lee, “Stabilization of Cadmium Contaminated Soil Using Synthesized Zeo- lite,” Journal of Hazardous Material, Vol. 60, No. 10, 1998, pp. 217-226. [18] A. Cottenie, M. Verloo, L. Kiekens, G. Velgh and R. Camerlynch, “Chemical Analysis of Plants and Soils,” Lab. Anal. Agrochem. State Univ. Ghent Belgium, 1982. [19] ISO 11466, “Soil Quality, Extraction of Trace Elements Soluble in Aqua Regia,” International Organization for Standardization, 1995. [20] W. L. Lindsay and W. A. Norwell, “Development of a DTPA Soil Test for Zinc, Iron, Manganese and Copper,” Soil Science Society of America Journal, Vol. 42, No. 3, 1978, pp. 421-428. doi:10.2136/sssaj1978.03615995004200030009x [21] ISO 14870, “Soil Quality—Extraction of Trace Elements by Buffered DTPA Solution,” International Organization for Standardization, 2001. [22] Costat 2.1, “CoHort Software,” 2005. http://www.Cohort.com/Costat.html [23] N. Irhaa, E. Steinnesb, U. Kirsoa and V. Petersellc, “Mo- bility of Cd, Pb, Cu, and Cr in Some Estonian Soil Types,” Estonian Journal of Earth Sciences, Vol. 58, No. 3, 2009, pp. 209-214. [24] L. Yi, Y. Hong, D. Wang and Y. Zhu, “Determination of Free Heavy Metal Ion Concentrations in Soils around a Cadmium Rich Zinc Deposit,” Geochemical Journal, Vol. 41, 2007, pp. 235-240. doi:10.2343/geochemj.41.235 [25] S. Mbarki, N. Labidi, H. Mahmoudi, N. Jedidi and C. Abdelly, “Contracting Effects of Municipal Compost on Alfalfa Growth in Clay and Sandy Soils: N, P, K Content and Heavy Metal Toxicity,” Bioresource Technology, Vol. 99, No. 15, 2008, pp. 6745-6750. [26] H. M. Selim, “Competitive Sorption and Transport of Trace Elements in Soils and Geological Media,” CRC/ Tylor and Francis, Boca Raton, 2012. doi:10.1201/b13041 [27] H. F. W. Taylor, “Cement Chemistry,” 2nd Edition, Aca- demic Press, London, 1997. doi:10.1680/cc.25929 [28] P. W. Brown, “Early Hydration of Tetracalcium Alumi- noferrite in Gypsum and Lime Gypsum Solutions,” Jour- nal of the American Ceramic Society, Vol. 70, No. 7, 1987, pp. 493-496. http://ciks.cbt.nist.gov/garbocz/cell1994/node18.htm doi:10.1111/j.1151-2916.1987.tb05682.x [29] H. Ganjidoust, A. Hassani and A. R. Ashkiki, “Cement- Based Solidification/Stabilization of Heavy Metal Con- taminated Soils with the Objective of Achieving High Compressive Strength for the Final Matrix,” Transaction Civil Engineering, Vol. 16, No. 2, 2009, pp. 107-115. http://www.sid.ir/en/VEWSSID/J_pdf?95520092A05.pdf [30] J. Komisarek and K. Wiatrowska, “Effectiveness of Ox- ide-Amendments in the Stabilization Process of Cu, Pb and Zn in Artificially Contaminated Soil,” Polish Journal of Environmental Studies, Vol. 18, No. 6, 2009, pp. 1029- 1038. http://www.pjoes.com/pdf/18.6/1029-1038.pdf Copyright © 2013 SciRes. OJMetal
|