Neurotensin Receptor 1 (NTSR1) Overexpression in Breast Carcinomas Is Common and Independent
of ER/PR/Her2 Expression
16
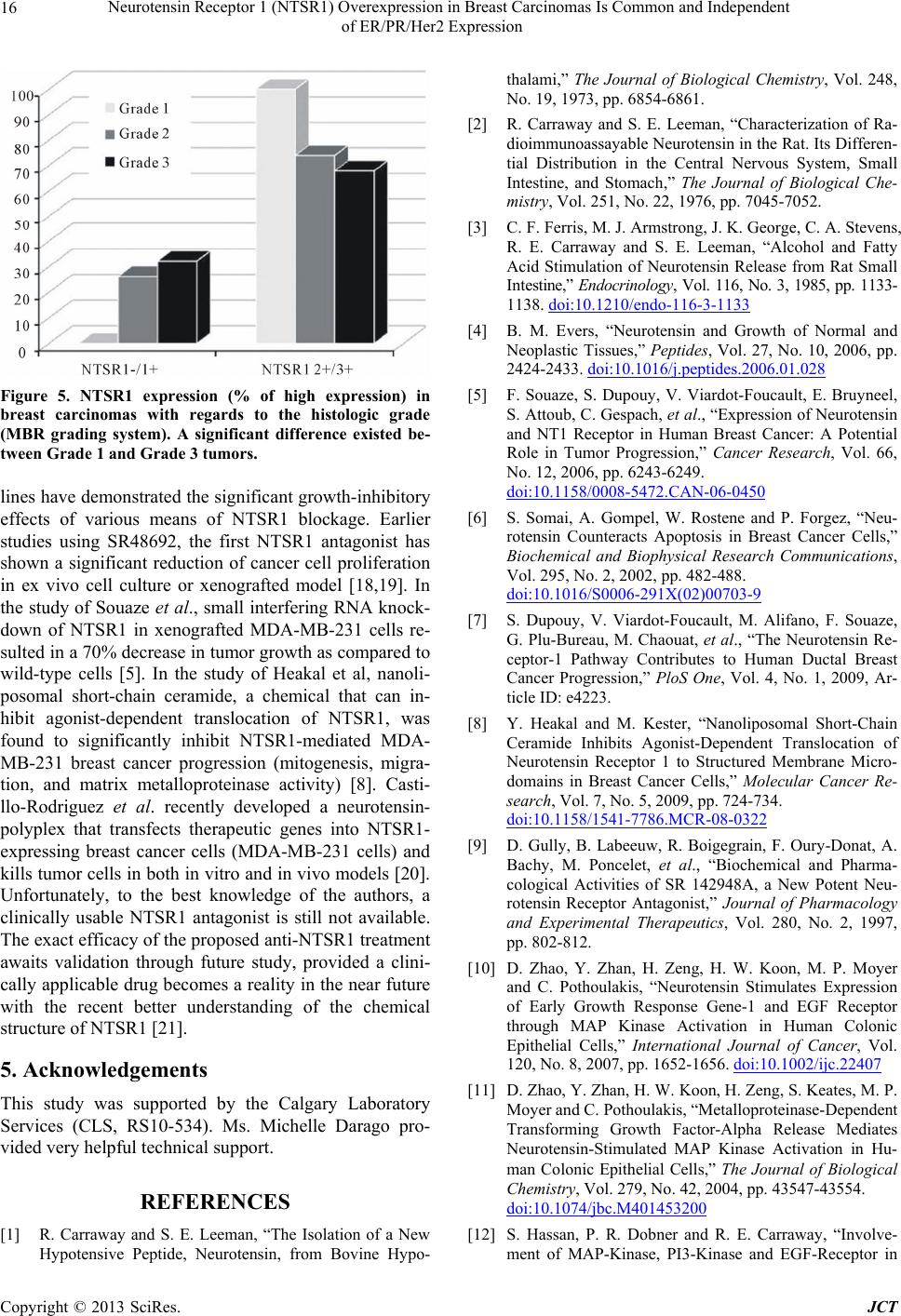
Figure 5. NTSR1 expression (% of high expression) in
breast carcinomas with regards to the histologic grade
(MBR grading system). A significant difference existed be-
tween Grade 1 and Grade 3 tumors.
lines have demonstrated the significant growth-inhibitory
effects of various means of NTSR1 blockage. Earlier
studies using SR48692, the first NTSR1 antagonist has
shown a significant reduction of cancer cell proliferation
in ex vivo cell culture or xenografted model [18,19]. In
the study of Souaze et al., small interfering RNA knock-
down of NTSR1 in xenografted MDA-MB-231 cells re-
sulted in a 70% decrease in tumor growth as compared to
wild-type cells [5]. In the study of Heakal et al, nanoli-
posomal short-chain ceramide, a chemical that can in-
hibit agonist-dependent translocation of NTSR1, was
found to significantly inhibit NTSR1-mediated MDA-
MB-231 breast cancer progression (mitogenesis, migra-
tion, and matrix metalloproteinase activity) [8]. Casti-
llo-Rodriguez et al. recently developed a neurotensin-
polyplex that transfects therapeutic genes into NTSR1-
expressing breast cancer cells (MDA-MB-231 cells) and
kills tumor cells in both in vitro and in vivo models [20].
Unfortunately, to the best knowledge of the authors, a
clinically usable NTSR1 antagonist is still not available.
The exact efficacy of the proposed anti-NTSR1 treatment
awaits validation through future study, provided a clini-
cally applicable drug becomes a reality in the near future
with the recent better understanding of the chemical
structure of NTSR1 [21].
5. Acknowledgements
This study was supported by the Calgary Laboratory
Services (CLS, RS10-534). Ms. Michelle Darago pro-
vided very helpful technical support.
REFERENCES
[1] R. Carraway and S. E. Leeman, “The Isolation of a New
Hypotensive Peptide, Neurotensin, from Bovine Hypo-
thalami,” The Journal of Biological Chemistry, Vol. 248,
No. 19, 1973, pp. 6854-6861.
[2] R. Carraway and S. E. Leeman, “Characterization of Ra-
dioimmunoassayable Neurotensin in the Rat. Its Differen-
tial Distribution in the Central Nervous System, Small
Intestine, and Stomach,” The Journal of Biological Che-
mistry, Vol. 251, No. 22, 1976, pp. 7045-7052.
[3] C. F. Ferris, M. J. Armstrong, J. K. George, C. A. Stevens,
R. E. Carraway and S. E. Leeman, “Alcohol and Fatty
Acid Stimulation of Neurotensin Release from Rat Small
Intestine,” Endocrinology, Vol. 116, No. 3, 1985, pp. 1133-
1138. doi:10.1210/endo-116-3-1133
[4] B. M. Evers, “Neurotensin and Growth of Normal and
Neoplastic Tissues,” Peptides, Vol. 27, No. 10, 2006, pp.
2424-2433. doi:10.1016/j.peptides.2006.01.028
[5] F. Souaze, S. Dupouy, V. Viardot-Foucault, E. Bruyneel,
S. Attoub, C. Gespach, et al., “Expression of Neurotensin
and NT1 Receptor in Human Breast Cancer: A Potential
Role in Tumor Progression,” Cancer Research, Vol. 66,
No. 12, 2006, pp. 6243-6249.
doi:10.1158/0008-5472.CAN-06-0450
[6] S. Somai, A. Gompel, W. Rostene and P. Forgez, “Neu-
rotensin Counteracts Apoptosis in Breast Cancer Cells,”
Biochemical and Biophysical Research Communications,
Vol. 295, No. 2, 2002, pp. 482-488.
doi:10.1016/S0006-291X(02)00703-9
[7] S. Dupouy, V. Viardot-Foucault, M. Alifano, F. Souaze,
G. Plu-Bureau, M. Chaouat, et al., “The Neurotensin Re-
ceptor-1 Pathway Contributes to Human Ductal Breast
Cancer Progression,” PloS One, Vol. 4, No. 1, 2009, Ar-
ticle ID: e4223.
[8] Y. Heakal and M. Kester, “Nanoliposomal Short-Chain
Ceramide Inhibits Agonist-Dependent Translocation of
Neurotensin Receptor 1 to Structured Membrane Micro-
domains in Breast Cancer Cells,” Molecular Cancer Re-
search, Vol. 7, No. 5, 2009, pp. 724-734.
doi:10.1158/1541-7786.MCR-08-0322
[9] D. Gully, B. Labeeuw, R. Boigegrain, F. Oury-Donat, A.
Bachy, M. Poncelet, et al., “Biochemical and Pharma-
cological Activities of SR 142948A, a New Potent Neu-
rotensin Receptor Antagonist,” Journal of Pharmacology
and Experimental Therapeutics, Vol. 280, No. 2, 1997,
pp. 802-812.
[10] D. Zhao, Y. Zhan, H. Zeng, H. W. Koon, M. P. Moyer
and C. Pothoulakis, “Neurotensin Stimulates Expression
of Early Growth Response Gene-1 and EGF Receptor
through MAP Kinase Activation in Human Colonic
Epithelial Cells,” International Journal of Cancer, Vol.
120, No. 8, 2007, pp. 1652-1656. doi:10.1002/ijc.22407
[11] D. Zhao, Y. Zhan, H. W. Koon, H. Zeng, S. Keates, M. P.
Moyer and C. Pothoulakis, “Metalloproteinase-Dependent
Transforming Growth Factor-Alpha Release Mediates
Neurotensin-Stimulated MAP Kinase Activation in Hu-
man Colonic Epithelial Cells,” The Journal of Biological
Chemistry, Vol. 279, No. 42, 2004, pp. 43547-43554.
doi:10.1074/jbc.M401453200
[12] S. Hassan, P. R. Dobner and R. E. Carraway, “Involve-
ment of MAP-Kinase, PI3-Kinase and EGF-Receptor in
Copyright © 2013 SciRes. JCT