Paper Menu >>
Journal Menu >>
 American Journal of Plant Sciences, 2010, 1, 55-68 doi:10.4236/ajps.2010.12008 Published Online December 2010 (http://www.SciRP.org/journal/ajps) Copyright © 2010 SciRes. AJPS 55 Biotechnology and Plant Disease Control-Role of RNA Interference Shabir H. Wani1*, Gulzar S. Sanghera2, N. B. Singh3 1Biotechnology Laboratory, Central Institute of Temperate Horticulture, Srinagar, Jammu and Kashmir, India; 2Rice Research & Regional Station (SKUAST-K) Khudwani, Anantnag Jammu and Kashmir, India; 3Department of Plant Breeding and Genetics, COA, Central Agricultural University, Imphal, Manipur, India. Email: *shabirhussainwani@gmail.com Received July 10th, 2010; revised August 24th, 2010; accepted September 3rd, 2010. ABSTRACT Development of crop varieties which are resistant against many economically important diseases is a major challenge for plant biotechnologists worldwide. Although much progress in this area has been achieved through classical genetic approaches, this goal can be achieved in a more selective and robust manner with the success of genetic engineering techniques. In this regard , RNA interference (RNAi) has emerged as a powerful modality for battling some of the most notoriously challenging diseases caused by viruses, fungi and bacteria. RNAi is a mechanism for RNA-guided regula- tion of gene expression in which double-stranded ribonucleic acid (dsRNA) inhibits the expression of genes with com- plementary nucleotid e sequences. The application of tissu e-specific or inducible gene silencing in comb ination with the use of appropriate promo ters to silence several genes simultaneously will result in protection of crops against destruc- tive pathogens. RNAi application has resulted in successful control of many economically important diseases in plants. Keywords: RNAi, Viruses, Fungi, dsRNA, Gene Silencing 1. Introduction Plant diseases are a threat to world agriculture. Signifi- cant yield losses due to the attack of pathogen occur in most of the agricultural and horticultural crop species. More than 70% of all major crop diseases are caused by fungi [1]. Plant diseases are usually handled with appli- cations of chemicals. For some diseases, chemical con- trol is very effective; but it is often non-specific in its effects, killing beneficial organisms as well as pathogens. Chemical control may have undesirable effects on health, safety and cause environmental risks [2]. Traditional plant breeding methods have been used to develop culti- vars resistant to various diseases. However, this process is time-consuming and limited availability of genetic resources for most of the crops has left little room to continued improvement by these means. There are many reasons for the limited genetic resources available for breeding [3]. Two of the most important ones are: 1) loss of gene pools occurring during the domestication and breeding of crop plants [4] and 2) many of the natural gene traits that may be beneficial in one plant tissue such as seeds and fruits, may be deleterious in other plant tis- sues such as vegetative tissues [5,6]. Over the past few decades, breeding possibilities have been broadened by genetic engineering and gene transfer technologies in- cluding gene mapping and identification of the genome sequences of model plants and crops. Modern technolo- gies such as trancriptomics, proteomics, and metabolom- ics are now proved to be important in understanding plant metabolic pathways and the role of key genes asso- ciated with their regulation. This can facilitate new in- sights into the complex metabolite neighborhoods that give rise to a given phenotype and may allow discovery of new target genes to modify a given pathway. Such genes can then be subject to new metabolic engineering efforts and applications. During the last decade, our knowledge repertoire of RNA-mediated functions has been greatly increased with the discovery of small non-coding RNAs which play a central part in a process called RNA silencing. Ironically, the very important phenomenon of co-suppression has recently been recognized as a manifestation of RNA in- terference (RNAi), an endogenous pathway for negative post-transcriptional regulation. RNAi has revolutionized the possibilities for creating custom “knock-downs” of gene activity. RNAi operates in both plants and animals, and uses double stranded RNA (dsRNA) as a trigger that  Biotechnology and Plant Disease Control-Role of RNA Interference Copyright © 2010 SciRes. AJPS 56 targets homologous mRNAs for degradation or inhibiting its transcription or translation [7,8], whereby susceptible genes can be silenced. This RNA-mediated gene control technology has provided new platforms for developing eco-friendly molecular tools for crop improvement by suppressing the specific genes which are responsible for various stresses and improving novel traits in plants in- cluding disease resistance. It has emerged as a method of choice for gene targeting in fungi [9], viruses [10,11], bacteria [12] and plants [13] as it allows the study of the function of hundreds of thousands of genes to be tested [14]. It can silence a gene throughout an organism or in specific tissues [15], offer the versatility to partially si- lence or completely turn off genes, work in both cultured cells and whole organisms and can selectively silence genes at particular stages of the organism’s life cycle [16]. Methods that introduce dsRNA into plant and ani- mal cells have been enormously successful for decreas- ing cognate gene expression in vivo [17,18]. Due to all these elegant and unique features of RNAi, our review specifically focuses on 1) the current knowledge of RNAi concept and 2) its pathways and induction in plants and explore the possibilities for the applications of this technology in the development of disease resistance plants. 2. Mechanism of RNAi ‘RNA interference’ refers collectively to diverse RNA-based processes that all result in sequence-specific inhibition of gene expression at the transcription, mRNA stability or translational levels. It has most likely evolved as a mechanism for cells to eliminate foreign genes. The unifying features of this phenomena are the production of small RNAs (21-26 nucleotides (nt) that act as specific determinants for down-regulating gene expression [17, 19,20] and the requirement for one or more members of the Argonaute family of proteins [21]. RNAi operates by triggering the action of dsRNA intermediates, which are processed into RNA duplexes of 21-24 nucleotides by a ribonuclease III-like enzyme called Dicer [22-24]. Once produced, these small RNA molecules or short interfer- ing RNAs (siRNAs) are incorporated in a multi-subunit complex called RNA induced silencing complex (RISC) [21,25]. RISC is formed by a siRNA and an endonucle- ase among other components. The siRNAs within RISC acts as a guide to target the degradation of complemen- tary messenger RNAs (mRNAs) [21,25]. The host ge- nome codifies for small RNAs called miRNAs that are responsible for endogenous gene silencing. The dsRNAs triggering gene silencing can originate from several sources such as expression of endogenous or transgenic antisense sequences, expression of inverted repeated se- quences or RNA synthesis during viral replication [26]. When dsRNA molecules produced during viral replica- tion trigger gene silencing, the process is called vi- rus-induced gene silencing (VIGS) [27]. One interesting feature of RNA silencing in plants is that once it is trig- gered in a certain cell, a mobile signal is produced and spread through the whole plant causing the entire plant to be silenced [28]. After triggering RNA silencing, the mobile signaling molecules can be relay-amplified by synthesis of dsRNAs on the primary cleavage of product templates or by their cleavage into secondary siRNAs. This amplification leads to the transitory nature of si- lencing reaction that may spread along the mRNA, though initiated by a locally targeted single siRNA [29] and spreads in both the 5´and 3´ directions [25]. This bi-directional transition further have been witnessed by a process where both the 5´ and 3´ cleavage products of the initial target RNA act as aberrant mRNAs to trigger dsRNA synthesis [30], and induce secondary silencing reactions. This silencing process is also enhanced by the enzymatic activity of the RISC complex, mediating mul- tiple turnover reactions [31,25]. Furthermore, production of the secondary siRNAs leads to enrichment of silencing via its spread from the first activated cell to neighboring cells, and systemically through the system [32]. The cell-to-cell spread can be mediated as passive spread of the small RNAs via plasmodesmata or by the silencing signal complex which is between 27 and 54 kDa [33]. The systemic spread in phloem is mediated by the 24 nt siRNAs [32], unloading of the systemic signal appears to be mediated via plasmodesmata, since it does not spread into meristematic cells [26]. The discovery of RNA- binding protein (PSRP1) in the phloem and its ability to bind 25 nt ssRNA species add further to the argument that siRNAs (24-26 nt) are the key components for sys- temic silencing signal. The extent of cell-to-cell move- ment is dependent on the levels of siRNAs produced at the site of silencing initiation, but is independent of the presence of siRNA target transcripts in either source or recipient cells [34]. 3. Methods to Induce RNAi in Plants One of the biggest challenges in RNAi research is the delivery of the active molecules that will trigger the RNAi pathway in plants. In this system, a number of methods for delivery of dsRNA or siRNA into different cells and tissue include transformation with dsRNA- forming vectors for selected gene(s) by an Agrobacte- rium mediated transformations [19,35], delivery cognate dsRNA of uidA GUS (β-glucuronidase) and TaGLP2a: GFP (green fluorescent protein) reporter genes into sin- gle epidermal cells of maize, barley and wheat by parti- cle bombardment [36], introducing a Tobacco rattle virus (TRV)-based vector in tomato plants by infiltration [37],  Biotechnology and Plant Disease Control-Role of RNA Interference Copyright © 2010 SciRes. AJPS 57 delivery of dsRNA into tobacco suspension cells by cationic oligopeptide polyarginine-siRNA complex; in- fecting plants with viral vectors that produce dsRNA [38] and delivery of siRNA into cultured plant cells of rice, cotton and slash pine for gene silencing by nanosense pulsed laser-induced stress wave (LISW) [40]. Among these the most reliable and commonly used approaches for delivery of dsRNA to plants cells are agroinfiltration, micro-bombardment and VIGS. These are discussed in the following sections. 3.1. Agroinfiltration Agroinfiltration is a powerful method to study processes connected with RNAi. The injection of Agrobacterium carrying similar DNA constructs into the intracellular spaces of leaves for triggering RNA silencing is known as agroinoculation or agroinfiltration [41]. In most cases agroinfiltration is used to initiate systemic silencing or to monitor the effect of suppressor genes. In plants, cyto- plasmic RNAi can be induced efficiently by agroinfiltra- tion, similar to a strategy for transient expression of T-DNA vectors after delivery by Agrobacterium tumefa- ciens. The transiently expressed DNA encodes either an ss- or dsRNA, which is typically a hairpin (hp) RNA. The infiltration of hairpin constructs are especially effec- tive, because their dsRNA can be processed directly to siRNAs, while the constructs expressing ssRNA can also be useful to induce silencing [42-45] and for dissecting the mechanism of gene silencing, especially concerned with its suppressors, systemic silencing signal and also for simple protein purification [42-45]. Besides, they provide a rapid, versatile and convenient way for achieving a very high level of gene expression in a dis- tinct and defined zone. 3.2. Micro-Bombardment In this method, a linear or circular template is transferred into the nucleus by micro-bombardment. Synthetic siRNAs are delivered into plants by biolistic pressure to cause silencing of GFP expression. Bombarding cells with particles coated with dsRNA, siRNA or DNA that encode hairpin constructs as well as sense or antisense RNA, activate the RNAi pathway. The silencing effect of RNAi is occasionally detected as early as a day after bombardment, and it continues up to 3 to 4 days of post bombardment. Systemic spread of the silencing occurred 2 weeks later to manifest in the vascular tissues of the non-bombarded leaves of Nicotiana benthamiana that were closest to the bombarded ones. After one month or so, the loss of GFP expression was seen in non-vascular tissues as well. RNA blot hybridization with systemic leaves indicated that the biolistically delivered siRNAs induced due to de novo formation of siRNAs, which ac- cumulated to cause systemic silencing [29]. 3.3. Virus Induced Gene Silencing (VIGS) Modified viruses as RNA silencing triggers are used as a mean for inducing RNA in plants. Different RNA and DNA viruses have been modified to serve as vectors for gene expression [46,47]. Some viruses, such as Tobacco mosaic virus (TMV), Potato virus X (PVX) and TRV, can be used for both protein expression and gene silenc- ing [48-51]. All RNA virus-derived expression vectors will not be useful as silencing vectors because many have potent anti-silencing proteins such as TEV (Tobacco e tch virus), that directly interfere with host silencing machin- ery [48,52]. Similarly, DNA viruses have not been used extensively as expression vectors due to their size con- straints for movement [53]. However, a non-mobile Maize streak Virus (MSV)-derived vector has been suc- cessfully used for long-term production of protein in maize cell cultures [48]. Using viral vectors to silence endogenous plant genes requires cloning homologous gene fragments into the virus without compromising viral replication and movement. This was first demonstrated in RNA viruses by inserting sequences into TMV [54], and then for DNA viruses by replacing the coat protein gene with a homologous sequence [53]. These reports used visible markers for gene silencing phytoene desatu- rase( PDS) and chalcone synthase (CHS), providing a measure of the tissue specificity of silencing as these have been involved in carotenoid metabolic pathway. The PDS gene acts on the antenna complex of the thyla- koid membranes, and protects the chlorophyll from photooxidation. By silencing this gene, a drastic decrease in leaf carotene content resulted into the appearance of photobleaching symptom [55,56]. Similarly, over ex- pression of CHS gene causes an albino phenotype instead of producing the anticipated deep orange color [57]. As a result, their action as a phenotypic marker helps in easy understanding of the mechanism of gene silencing. Table 1 shows some general characteristics for currently avail- able virus-derived gene silencing vectors. Most viruses are plus-strand RNA viruses or satellites, whereas To- mato golden mosaic virus (TGMV) and Cabbage leaf curl virus (CaLCuV) are DNA viruses. Though RNA viruses replicate in the cytoplasm DNA viruses replicate in plant nuclei using the host DNA replication machinery. Both types of viruses induce diffusible, homol- ogy-dependent systemic silencing of endogenous genes. However, the extent of silencing spread and the severity of viral symptoms can vary significantly in different host plants and host/virus combinations. With the variety of viruses and the diversity of infection patterns, transmis- sion vectors, and plant defenses it is not surprising that viruses differ with respect to silencing [58]. Because the  Biotechnology and Plant Disease Control-Role of RNA Interference Copyright © 2010 SciRes. AJPS 58 continuing development of virus-based silencing vectors can extend VIGS to economically important plants, it is useful to consider some of the characteristics of success- ful VIGS vectors. 4. RNAi in Plant Disease Management Despite substantial advances in plant disease manage- ment strategies, our global food supply is still threatened by a multitude of pathogens and pests. This changed scenario warrants us to respond more efficiently and ef- fectively to this problem. The situation demands judi- cious blending of conventional, unconventional and fron- tier technologies. In this sense, RNAi technology has emerged as one of the most potential and promising strategies for enhancing the building of resistance in plants to combat various fungal, bacterial, viral and nematode diseases causing huge losses in important ag- ricultural crops. The nature of this biological phenome- non has been evaluated in a number of host-pathogen systems and effectively used to silence the action of pathogen. Many of the examples listed below illustrate the possibilities for commercial exploitation of this in- herent biological mechanism to generate disease-resistant plants in the future by taking advantage of this approach. 4.1. Management of Plant Pathogenic Fungi RNA-mediated gene silencing (RNA silencing) is used as a reverse tool for gene targeting in fungi. Homology- based gene silencing induced by transgenes (co-suppres- sion), antisense, or dsRNA has been demonstrated in many plant pathogenic fungi, including Cladosporium fulvum [59], Magnaporthae oryzae [60-62], Venturia inaequalis [63], Neurospora crassa [64], Aspergillus nidulans [65], and Fusarium graminearum [9] (Table 1), whether it is suitable for large scale mutagenesis in fun- gal pathogens remains to be tested. Hypermorphic mechanism of RNA interference implies that this tech- nique can also be applicable to all those plant pathogenic fungi, which are polyploid and polykaryotic in nature, and also offers a solution to the problem where frequent lack of multiple marker genes in fungi is experienced. Simultaneous silencing of several unrelated genes by introducing a single chimeric construct has been demon- strated in case of Venturia inaequalis [63]. HCf-1, a gene that codes for a hydrophobin of the tomato pathogen C. fulvum [66], was co-suppressed by ectopic integration of homologous transgenes. Transformation of Cladospo- rium fulvum with DNA containing a truncated copy of the hydrophobin gene HCf-1 caused co-suppression of hydrophobin synthesis in 30% of the transformants. The co-suppressed isolates had a hydrophilic phenotype, lower levels of HCf-1 mRNA than wild type and contain multiple copies of the plasmid integrated as tandem re- peats at ectopic sites in the genome. The transcription rate of HCf-1 in the co-suppressed isolates was higher in the untransformed strains, which suggested that silencing acted at the post-transcriptional level. This was due to ectopic integration of the transgene next to promoters which initiate transcription to form antisense RNA and that this in turn determines down-regulation of HCf-1. But gene silencing was not associated with DNA cyto- sine methylation [59]. Similarly, the silencing of cgl1 and cgl2 genes using the cgl2 hairpin construct in Cladosporium fulvum has also been reported [67], though the effect was possibly restricted to highly homolougous genes (exons of cgl 1 and cgl 2 are 87% identical). However, the less homologus cgl 3 (53% overall identity to cgl 2) was not affected as the target specificity always depends upon the actual sequence alignment and more over, short regions of high density that led to unwanted off-targets effects. Such a strategy could be exploited for protecting the consumable products of vegetables and fruits crops from the post-harvest diseases caused by different plant pathogens in future. Hairpin vector technology resulted in simultaneous high frequency silencing of a green fluorescent protein (GFP) transgene and an endogenous trihydroxynaphtha- lene reductase gene (THN) in Venturia inaequalis [63] GFP transgene, acting as easily detectable visible marker while the trihydroxynaphthalene reductase gene (THN) playing role in melanin biosynthesis. High frequency gene silencing was achieved using hairpin constructs for the GFP or the THN genes transferred by Agrobacterium (71 and 61%, respectively). THN-silenced transformants exhibited a distinctive light brown phenotype and main- tained the ability to infect apple. Silencing of both genes with this construct occurred at a frequency of 51% of all the transformants. All 125 colonies silenced for the GFP gene were also silenced for THN [63]. Similarly, multi- ple gene silencing has been achieved in Cryptococcus Table 1. RNAi effects on targeted region in some fungal plant pathogens. Pathogen Targeted region Result References Magnaporthae oryzae eGFP Sequence specific degradation of mRNA [60] Cladosporium falvum cgl 1 and cgl 2Blocking disease infection spread [67] Venturia inaequalis Multiple inverted repeats - [63] Fusarium graminearum - - [9] Blumeria graminis Mlo Immunity [36]  Biotechnology and Plant Disease Control-Role of RNA Interference Copyright © 2010 SciRes. AJPS 59 neoformans using chimeric hairpin constructs [39] and in plants using partial sense constructs [68]. The first effort towards the systematic silencing of Magnaporthe grisea, a causal organism of rice blast was carried by using the enhanced green florescent protein gene as a model [60]. To assess the ability of RNA species to induce silencing in fungus, plasmid construct expressing sense, antisense and hairpin RNA were introduced into an eGFP-ex- pressing transformants. The fluorescence of eGFP in the transformants was silenced much more efficiently by hairpin RNA of eGFP than by other RNA species. In the silenced transformants, the accumulation of eGFP mRNA was drastically reduced. But not methylation of coding or promoter region was involved. The small in- terfering RNA molecules of 19-23 nucleotides were ob- served in both sense and antisense strands of eGFP gene [60]. Later on a protocol for silencing the mpg1 and polyketide synthase-like genes was also developed [9]. Mpg1 gene is a hydrophobin gene which is essential for pathogenicity as it act as a cellular relay for adhesion and trigger for the development of appressorium [69]. Their work on this host-pathogen system revealed that they were successfully able to silence the above mentioned genes at varying degrees by pSilent-1-based vectors in 70–90% of the resulting transformants. Ten to fifteen percent of the silenced transformants exhibited almost ‘‘null phenotype’’. This vector was also efficiently ap- plicable to silence a GFP reporter in another ascomycete fungus Colletotrichum lagenarium [9]. A novel high- throughput approach for gene function analysis using RNAi, which provides an alternative to the gene knock-out by homologous recombination was also de- scribed [70]. The authors developed an RNA silencing vector, pSilent-Dual1 (pSD1) that carries two convergent dual promoters, the Aspergillus nidulans tryptophan promoter (PtrpC) and the A. nidulans glyceraldehyde-3- phosphate dehydrogenase promoter (Pgpd). Both pro- moters have been used to drive constitutive gene expres- sion in a large number of filamentous fungi. A multi- cloning site (MCS) has been inserted between two pro- moters. The greatest merit of the pSD1 system over oth- ers, such as hpRNA or intron spliced hair-pin RNA (ih- pRNA) silencing system is that it allows a single step cloning for generation of an RNAi construct. To facilitate efficient screening for silenced transformants, gfp gene was incorporated the into pSD1 system [70]. It allows expression of a chimeric RNA and assessment of gene silencing efficiency by utilizing a recipient strain that produces GFP and therefore, fluoresces green when us- ing epifluorescence microscopy. A main bottleneck of this system is its lower silencing efficiency compared with hpRNA or ihpRNA-expressing RNA-silencing vec- tors. Formation of dsRNA in the pSD1 system requires physical annealing of two different RNA molecules in the target cells while that in the hpRNA systems is achieved by self-folding of inverted repeats within RNA molecule. The difference in dsRNA formation between the systems can be a major cause of the different silenc- ing efficiencies. The authors generated a series of knock-down mutants of almost all known calcium related genes in the genome of M. oryzae and examined for phenotypical defects. Gene knock-down requires rela- tively short stretches of sequence information. This is a major advantage for phytopathogens for which there is little sequence information available. As RNAi works at the mRNA level, its efficacy is not compromised by the presence of non-transformed nuclei or multicopy genes due to aneuploidy [71]. RNAi causes only a partial re- duction, but not a complete loss of, in gene expression. Partial gene suppression is considered a main drawback of RNAi. However, it could be a merit where the effect of an essential gene on a phenotype is of interest. Gene knock-down offers a more convenient and effective tool, especially in combination with an inducible promoter that allows gene expression to be diminished at specific stages during development [72]. Another disadvantage of gene knock-down is, as it requires only a short sequence, that genes other than those targeted might be silenced. This causes unexpected changes in gene expression pat- terns (off-target effects). Testing for the possibility of off-target effects is simpler for phytopathogen species for which complete genome sequence data are available but remains elusive for those phytopathogens whose ge- nomes have not been sequenced [71]. In another study, RNA interference (RNAi) strategy was used to specifi- cally knockdown 59 individual rice genes encoding puta- tive LRR-RLKs, and a novel rice blast resistance-related gene (designated as OsBRR1) was identified by screen- ing T0 RNAi population using a weakly virulent isolate of Magnaporthe oryzae, Ken 54-04. Wild-type plants (Oryza sativa L. cv. ‘Nipponbare’) showed intermediate resistance to Ken 54-04, while OsBRR1 suppression plants were susceptible to Ken 54-04 [73]. Furthermore, OsBRR1-overexpressing plants exhibited enhanced re- sistance to some virulent isolates (97-27-2, 99-31-1 and zhong 10-8-14). OsBRR1 expression was low in leaves and undetectable in roots under normal growth condi- tions, while its transcript was significantly induced in leaves infected with the blast fungus (Ken 54-04) and was moderately affected by ABA, JA and SA treatment. Overexpression or RNAi suppression of OsBRR1did not cause visible developmental changes in rice plants. 4.2. Management of Plant Pathogenic Bacteria One of the striking examples of bacterial disease man- agement where RNAi showed a remarkable type of gene 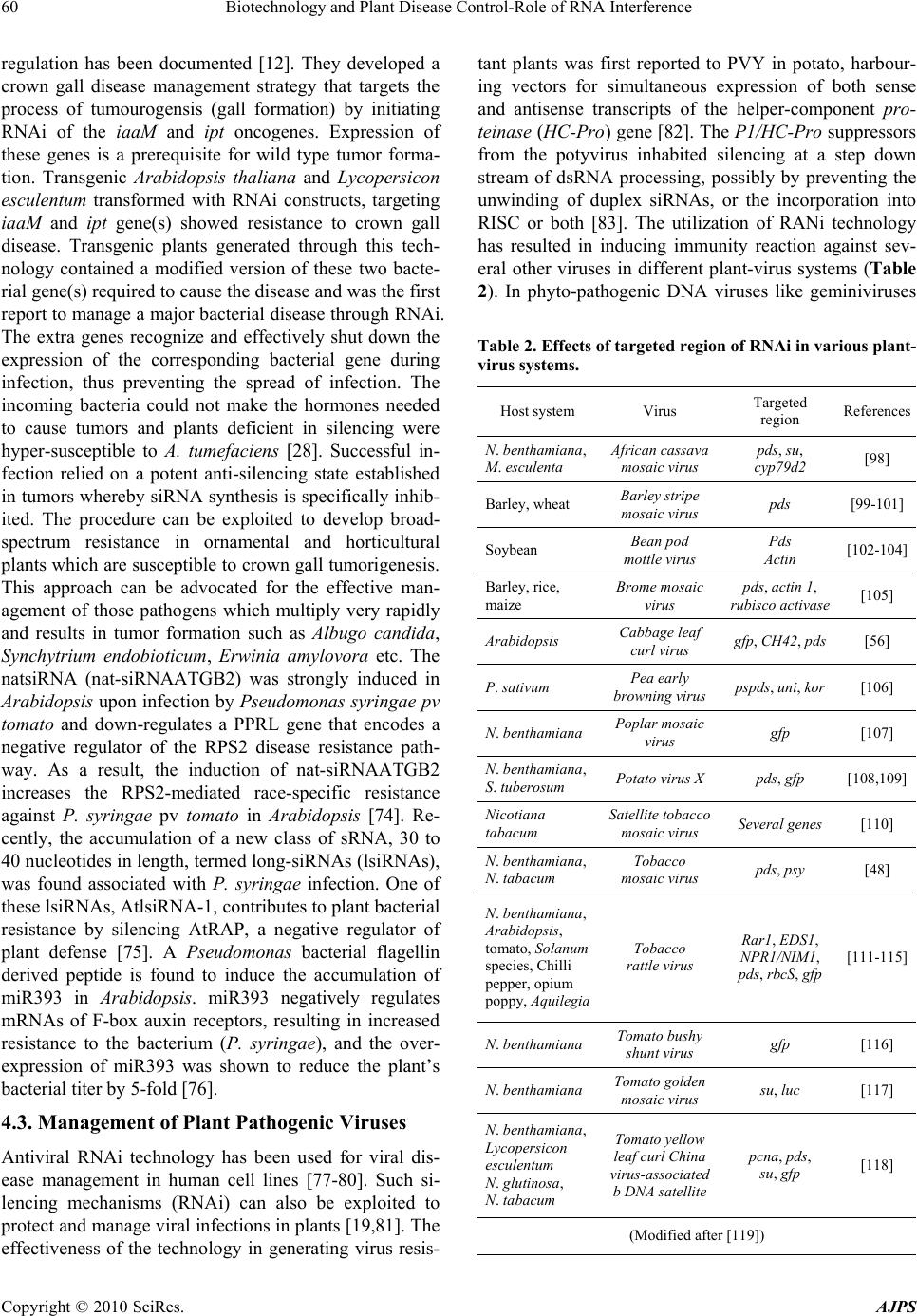 Biotechnology and Plant Disease Control-Role of RNA Interference Copyright © 2010 SciRes. AJPS 60 regulation has been documented [12]. They developed a crown gall disease management strategy that targets the process of tumourogensis (gall formation) by initiating RNAi of the iaaM and ipt oncogenes. Expression of these genes is a prerequisite for wild type tumor forma- tion. Transgenic Arabidopsis thaliana and Lycopersicon esculentum transformed with RNAi constructs, targeting iaaM and ipt gene(s) showed resistance to crown gall disease. Transgenic plants generated through this tech- nology contained a modified version of these two bacte- rial gene(s) required to cause the disease and was the first report to manage a major bacterial disease through RNAi. The extra genes recognize and effectively shut down the expression of the corresponding bacterial gene during infection, thus preventing the spread of infection. The incoming bacteria could not make the hormones needed to cause tumors and plants deficient in silencing were hyper-susceptible to A. tumefaciens [28]. Successful in- fection relied on a potent anti-silencing state established in tumors whereby siRNA synthesis is specifically inhib- ited. The procedure can be exploited to develop broad- spectrum resistance in ornamental and horticultural plants which are susceptible to crown gall tumorigenesis. This approach can be advocated for the effective man- agement of those pathogens which multiply very rapidly and results in tumor formation such as Albugo candida, Synchytrium endobioticum, Erwinia amylovora etc. The natsiRNA (nat-siRNAATGB2) was strongly induced in Arabidopsis upon infection by Pseudomonas syringae pv tomato and down-regulates a PPRL gene that encodes a negative regulator of the RPS2 disease resistance path- way. As a result, the induction of nat-siRNAATGB2 increases the RPS2-mediated race-specific resistance against P. syringae pv tomato in Arabidopsis [74]. Re- cently, the accumulation of a new class of sRNA, 30 to 40 nucleotides in length, termed long-siRNAs (lsiRNAs), was found associated with P. syringae infection. One of these lsiRNAs, AtlsiRNA-1, contributes to plant bacterial resistance by silencing AtRAP, a negative regulator of plant defense [75]. A Pseudomonas bacterial flagellin derived peptide is found to induce the accumulation of miR393 in Arabidopsis. miR393 negatively regulates mRNAs of F-box auxin receptors, resulting in increased resistance to the bacterium (P. syringae), and the over- expression of miR393 was shown to reduce the plant’s bacterial titer by 5-fold [76]. 4.3. Management of Plant Pathogenic Viruses Antiviral RNAi technology has been used for viral dis- ease management in human cell lines [77-80]. Such si- lencing mechanisms (RNAi) can also be exploited to protect and manage viral infections in plants [19,81]. The effectiveness of the technology in generating virus resis- tant plants was first reported to PVY in potato, harbour- ing vectors for simultaneous expression of both sense and antisense transcripts of the helper-component pro- teinase (HC-Pro) gene [82]. The P1/HC-Pro suppressors from the potyvirus inhabited silencing at a step down stream of dsRNA processing, possibly by preventing the unwinding of duplex siRNAs, or the incorporation into RISC or both [83]. The utilization of RANi technology has resulted in inducing immunity reaction against sev- eral other viruses in different plant-virus systems (Table 2). In phyto-pathogenic DNA viruses like geminiviruses Table 2. Effects of targeted region of RNAi in various plant- virus systems. Host system Virus Targeted region References N. benthamiana, M. esculenta African cassava mosaic virus pds, su, cyp79d2 [98] Barley, wheat Barley stripe mosaic virus pds [99-101] Soybean Bean pod mottle virus Pds Actin [102-104] Barley, rice, maize Brome mosaic virus pds, actin 1, rubisco activase[105] Arabidopsis Cabbage leaf curl virus gfp, CH42, pds [56] P. sativum Pea early browning virus pspds, uni, kor [106] N. benthamianaPoplar mosaic virus gfp [107] N. benthamiana, S. tuberosum Potato virus X pds, gfp [108,109] Nicotiana tabacum Satellite tobacco mosaic virus Several genes [110] N. benthamiana, N. tabacum Tobacco mosaic virus pds, psy [48] N. benthamiana, Arabidopsis, tomato, Solanum species, Chilli pepper, opium poppy, Aquilegia Tobacco rattle virus Rar1, EDS1, NPR1/NIM1, pds, rbcS, gfp [111-115] N. benthamianaTomato bushy shunt virus gfp [116] N. benthamianaTomato golden mosaic virus su, luc [117] N. benthamiana, Lycopersicon esculentum N. glutinosa, N. tabacum Tomato yellow leaf curl China virus-associated b DNA satellite pcna, pds, su, gfp [118] (Modified after [119])  Biotechnology and Plant Disease Control-Role of RNA Interference Copyright © 2010 SciRes. AJPS 61 non-coding intergenic region of Mungbean yellow mo- saic India virus (MYMIV) was expressed as hairpin con- struct under the control of the 35S promoter and used as biolistically to inoculate MYMIV-infected black gram plants and showed a complete recovery from infection, which lasted until senescence [84]. RNAi mediated si- lencing of geminiviruses using transient protoplast assay where protoplasts were co-transferred with a siRNA de- signed to replicase (Rep)-coding sequence of African cassava mosaic virus (ACMV) and the genomic DNA of ACMV resulted in 99% reduction in Rep transcripts and 66% reduction in viral DNA [85]. It was observed that siRNA was able to silence a closely related strain of ACMV but not a more distantly related virus. More than 40 viral suppressors have been identified in plant viruses [86]. Results from some of the well-studied virus sup- pressors indicated that suppressors interfere with sys- temic signaling for silencing [44]. During last few years, the p69 encoded by Turnip yellow mosaic viru s has been identified as silencing suppressors that prevented host RDR-dependent secondary dsRNA synthesis [87]. P14 protein encoded by aureus viruses suppressed both virus and transgene-induced silencing by sequestering both long dsRNA and siRNA without size specificity [88]. Multiple suppressors have been reported in Citrus tristeza virus where p20 and coat protein (CP) play im- portant role in suppression of silencing signal and p23 inhibited intracellular silencing [27]. Multiple viral components, viral RNAs and putative RNA replicase proteins were reported for a silencing or suppression of Red clover necrotic mosaic virus [89]. In this case, the RNA silencing machinery deprived of DICER-like en- zymes by the viral replication complexes appears to be the cause of the suppression. Pns10 encoded by Rice dwarf virus suppressed local and systemic S-PTGS but not IR-PTGS suggesting that Pns10 also targets an up- stream step of dsRNA formation in the silencing pathway [90]. A 273-bp (base pair) sequence of the Arabidopsis miR159 a pre-miRNA transcript expressing amiRNAs was used against the viral suppressor genes P69 and HC-Pro to provide resistance against Turnip yellow mo- saic virus and Turnip mosaic virus infection, respectively [91]. In addition, a dimeric construct harboring two unique amiRNAs against both viral suppressors con- ferred resistance against these two viruses in inoculated Arabidopsis plants. Similarly, a different amiRNA vector was used to target the 2 b viral suppressor of the Cu- cumber mosaic virus (CMV), a suppressor that interacted with and blocked the slicer activity of AGO1 had also shown to confer resistance to CMV infection in trans- genic tobacco [92]. A strong correlation between virus resistance and the expression level of the 2 b-specific amiRNA was shown for individual plant lines. It is evi- dent from above-mentioned reports that the RNA com- ponents, such as single strand template RNA, dsRNA and/or siRNA of the silencing pathways are the preferred targets of most viral suppressors. However, plant viruses are known to have evolved a counter-silencing mecha- nism by encoding proteins that can overcome such resis- tance [34,93]. These suppressors of gene silencing are often involved in viral pathogenicity, mediate synergism among plant viruses and result in the induction of more severe disease. Simultaneous silencing of such diverse plant viruses can be achieved by designing hairpin struc- tures that can target a distinct virus in a single construct [93]. Contrarily, the RNAi system may cause an increase in the severity of viral pathogenesis and/or encode pro- teins, which can inactivate essential genes in the RNAi machinery [94] that helps them in their replication in the host genome [17]. Transgenic rice plants expressing DNA encoding ORF IV of Rice tungro bacilliform virus (RTBV), both in sense and in anti-sense orientation, re- sulting in the formation of dsRNA, were generated. Spe- cific degradation of the transgene transcripts and the ac- cumulation of small RNA were observed in transgenic plants. In RTBV-ODs2 line, RTBV DNA levels gradu- ally rose from an initial low to almost 60% of that of the control at 40 days after inoculation [95]. For the effective control of PRSV and Papaya leaf-distortion mosaic virus (PLDMV), an untranslatable chimeric construct contain- ing truncated PRSV YK CP and PLDMV P-TW-WF CP genes has been transferred into papaya (Carica papaya cv. ‘Thailand’) by Agrobacterium-mediated transforma- tion via embryogenic tissues derived from immature zy- gotic embryos of papaya [96]. Based on sequence profile of silencing suppressor protein, HcPro, it was that PRSV-HcPro acts as a suppressor of RNA silencing through micro RNA binding in a dose dependent manner. In planta expression of PRSV-HcPro affects develop- mental biology of plants, suggesting the interference of suppressor protein in micro RNA-directed regulatory pathways of plants. Besides facilitating the establishment of PRSV, it showed strong positive synergism with other heterologous viruses as well [97]. 4.4. Management of Plant Parasitic Nematodes Several major plant parasitic nematodes such as the root- knot (Meloidogyne spp.) and cyst (Heterodera spp.) along with other minor nematodes cause significant damage to important agricultural crops such as legumes, vegetables and cereals in most parts of the world. There- fore, a natural, eco-friendly defense strategy that delivers a cost-effective control of plant parasitic nematodes is needed urgently which is difficult to achieve through conventional approaches. However, the origin of RNAi technology from classical C. elegans studies has shown 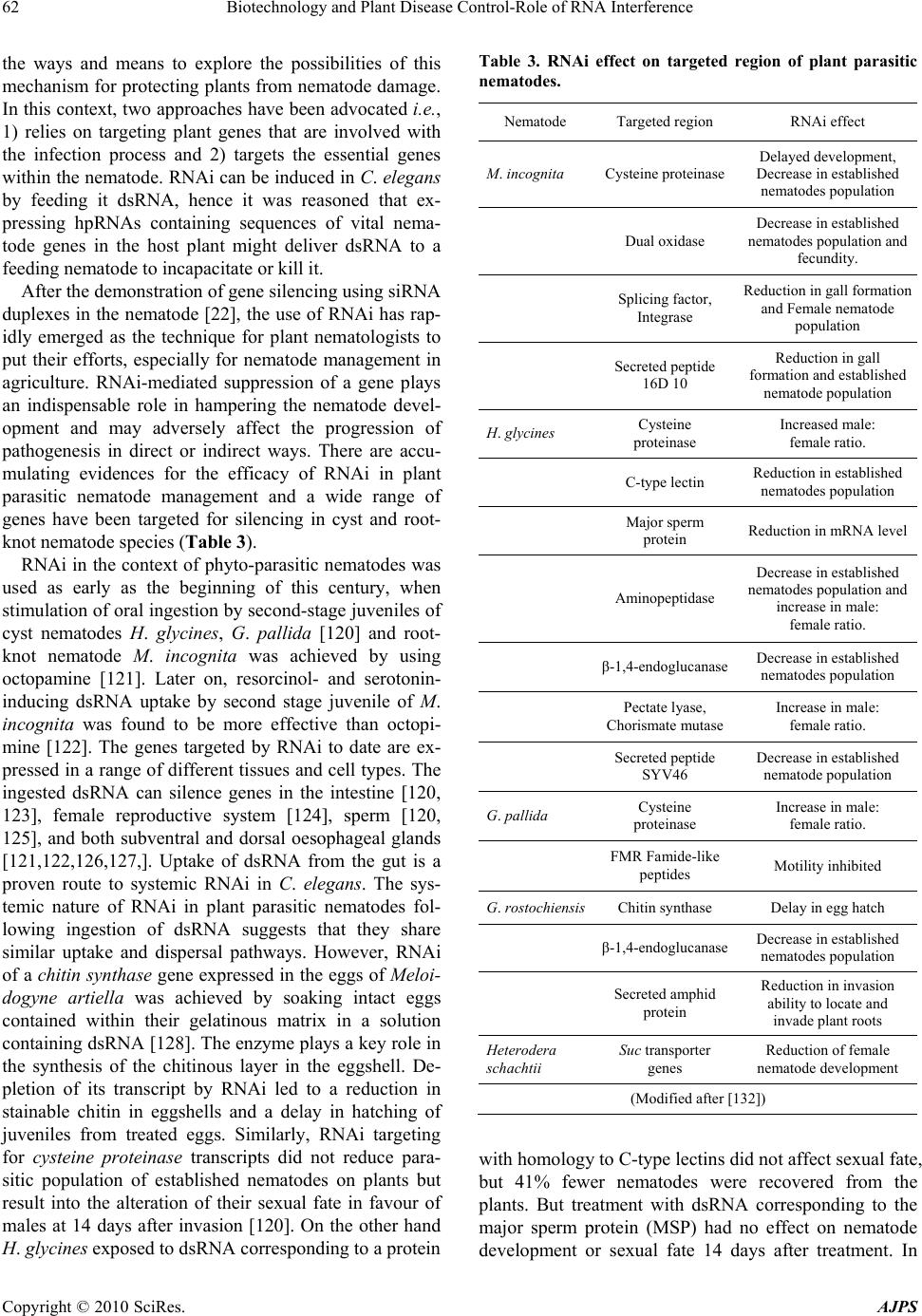 Biotechnology and Plant Disease Control-Role of RNA Interference Copyright © 2010 SciRes. AJPS 62 the ways and means to explore the possibilities of this mechanism for protecting plants from nematode damage. In this context, two approaches have been advocated i.e., 1) relies on targeting plant genes that are involved with the infection process and 2) targets the essential genes within the nematode. RNAi can be induced in C. elegans by feeding it dsRNA, hence it was reasoned that ex- pressing hpRNAs containing sequences of vital nema- tode genes in the host plant might deliver dsRNA to a feeding nematode to incapacitate or kill it. After the demonstration of gene silencing using siRNA duplexes in the nematode [22], the use of RNAi has rap- idly emerged as the technique for plant nematologists to put their efforts, especially for nematode management in agriculture. RNAi-mediated suppression of a gene plays an indispensable role in hampering the nematode devel- opment and may adversely affect the progression of pathogenesis in direct or indirect ways. There are accu- mulating evidences for the efficacy of RNAi in plant parasitic nematode management and a wide range of genes have been targeted for silencing in cyst and root- knot nematode species (Table 3). RNAi in the context of phyto-parasitic nematodes was used as early as the beginning of this century, when stimulation of oral ingestion by second-stage juveniles of cyst nematodes H. glycines, G. pallida [120] and root- knot nematode M. incognita was achieved by using octopamine [121]. Later on, resorcinol- and serotonin- inducing dsRNA uptake by second stage juvenile of M. incognita was found to be more effective than octopi- mine [122]. The genes targeted by RNAi to date are ex- pressed in a range of different tissues and cell types. The ingested dsRNA can silence genes in the intestine [120, 123], female reproductive system [124], sperm [120, 125], and both subventral and dorsal oesophageal glands [121,122,126,127,]. Uptake of dsRNA from the gut is a proven route to systemic RNAi in C. elegans. The sys- temic nature of RNAi in plant parasitic nematodes fol- lowing ingestion of dsRNA suggests that they share similar uptake and dispersal pathways. However, RNAi of a chitin synth ase gene expressed in the eggs of Meloi- dogyne artiella was achieved by soaking intact eggs contained within their gelatinous matrix in a solution containing dsRNA [128]. The enzyme plays a key role in the synthesis of the chitinous layer in the eggshell. De- pletion of its transcript by RNAi led to a reduction in stainable chitin in eggshells and a delay in hatching of juveniles from treated eggs. Similarly, RNAi targeting for cysteine proteinase transcripts did not reduce para- sitic population of established nematodes on plants but result into the alteration of their sexual fate in favour of males at 14 days after invasion [120]. On the other hand H. glycines exposed to dsRNA corresponding to a protein Table 3. RNAi effect on targeted region of plant parasitic nematodes. Nematode Targeted region RNAi effect M. incognita Cysteine proteinase Delayed development, Decrease in established nematodes population Dual oxidase Decrease in established nematodes population and fecundity. Splicing factor, Integrase Reduction in gall formation and Female nematode population Secreted peptide 16D 10 Reduction in gall formation and established nematode population H. glycines Cysteine proteinase Increased male: female ratio. C-type lectin Reduction in established nematodes population Major sperm protein Reduction in mRNA level Aminopeptidase Decrease in established nematodes population and increase in male: female ratio. β-1,4-endoglucanase Decrease in established nematodes population Pectate lyase, Chorismate mutase Increase in male: female ratio. Secreted peptide SYV46 Decrease in established nematode population G. pallida Cysteine proteinase Increase in male: female ratio. FMR Famide-like peptides Motility inhibited G. rostochiensisChitin synthase Delay in egg hatch β-1,4-endoglucanase Decrease in established nematodes population Secreted amphid protein Reduction in invasion ability to locate and invade plant roots Heterodera schachtii Suc transporter genes Reduction of female nematode development (Modified after [132]) with homology to C-type lectins did not affect sexual fate, but 41% fewer nematodes were recovered from the plants. But treatment with dsRNA corresponding to the major sperm protein (MSP) had no effect on nematode development or sexual fate 14 days after treatment. In  Biotechnology and Plant Disease Control-Role of RNA Interference Copyright © 2010 SciRes. AJPS 63 addition to this, reduction in transcript abundance for targeted mRNAs in the infective juvenile and for MSP transcripts when males reached sexual maturity and sperm are produced was observed [120]. In further ex- tension of such types of experiments showed efficient FITC uptake by soaking M. incognita, 90-95% of indi- viduals swallowed the dye when the target was a dual oxidase (an enzyme comprised with a peroxidase domain EF-hands and NADPH oxidase domain and potentially involved in extracellular matrix development). The effect of RNAi was observed when root knot nematode (RKN) juveniles were fed on dual oxidase-derived dsRNA, the reduction in the number and size of established females at 14 and 35 days post- infection with an overall reduc- tion of 70% in egg production was observed [121]. RNAi has also been induced for a chitin synthase gene that is expressed in the eggshells of M. artiella after soaking its developing eggs in a dsRNA [128]. Heterodera schachtii induces syncytial feeding structures in the roots of host plants, and this requires the up-regulation of Suc trans- porter genes to facilitate increased nutrient flow to the developing structure. Targeting these genes and down- regulating them with RNA silencing resulted in a sig- nificant reduction of female nematode development [129]. Indeed, tobacco plants transformed with hpRNA constructs against two such root-knot nematode genes have shown such an effect: the target mRNAs in the plant parasitic nematodes were dramatically reduced, and the plants showed effective resistance against the parasite [130]. In another study mRNA abundances of targeted nematode genes were specifically reduced in nematodes feeding on plants expressing corresponding RNAi con- structs. Furthermore, this host-induced RNAi of all four nematode parasitism genes led to a reduction in the number of mature nematode females. Although no com- plete resistance was observed, the reduction of develop- ing females ranged from 23% to 64% in different RNAi lines [131]. These observations demonstrate the rele- vance of the targeted parasitism genes during the nema- tode life cycle and more importantly, suggest that a vi- able level of resistance in crop plants may be accom- plished in the future by using RNAi technology against cyst nematodes. 5. Conclusions and Future Prospects RNAi and miRNA technologies of gene silencing are newly developed genomics tools that have great advan- tages over antisense and co-suppression due to their higher silencing efficiency and shorter time requirements for screening. These technologies are particularly useful in conjunction with the practice of gene or pathway dis- coveries through nutritional genomics, trancriptomics, proteomics and metabolomics in plants to improve hu- man health. The RNA silencing has ability to reduce gene expression in a manner that is highly sequence spe- cific as well as technologically facile and economical. Therefore, this technique has great potential in agricul- ture specifically for nutritional improvement of plants and the management of mascotous plant diseases. How- ever, the major obstacles hindering its immediate appli- cations include selection of targeting sequences and in the delivery of siRNA. The key issues are: 1) how to select silencing targets for a particular disease, and 2) how to efficiently deliver siRNAs into specific cell types in vivo. Tissue or organ-specific RNAi vectors have al- ready been proven to be useful for targeted gene silenc- ing in specific plant tissues and organs with minimal in- terference with the normal plant life cycle. New genera- tion RNAi and miRNA vectors have been developed with high silencing accuracy and fewer side effects in plants. Genetic engineering of highly nutritional food crops requires both gene silencing and counter-silencing technologies. Besides, RNAi technology can be consid- ered an eco-friendly, biosafe and ever green technology as it eliminates even certain risks associated with devel- opment of transgenic plants carrying first generation constructs (binary vectors and sense and antisense genes). As witnessed from earlier strategies for obtaining viral resistant plants, the expression of protein product from the transgene of interest risked hetero-encapsidation through protein-protein interactions between target and non-target viral gene product, resulted in the develop- ment of a non-aphid transmissible strain of Zucchini yel- low mosaic virus to aphid-transmissible strain from a transgene expressing a plum pox capsid protein. Since RNAi triggers the formation of dsRNA molecules that target and facilitate the degradation of the gene of inter- est as well as the transgene itself to avoid problems aris- ing from the synthesis of gene sequences as well as non- coding regions of gene, thus limiting undesirable recom- bination events. Keeping in view the potentialities of RNAi technology this technology has emerged to combat plant pathogens in the near future as it has already added new dimensions in the chapter of plant disease manage- ment. Further, development of vectors that can suppress the RNAi pathway but overexpress transgenes in a tis- sue-specific manner will revolutionize this field. Such vectors could be based on various viral RNA silencing suppressors and their derivatives. Future directions will focus on developing finely tuned RNAi-based gene si- lencing vectors that are able to operate in a temporally and spatially controlled manner. However, a better and comprehensive understanding of RNAi would allow the researchers to work effectively and efficiently in order to improve crop plants nutritionally and manage various mascotous intruders of crop plants.  Biotechnology and Plant Disease Control-Role of RNA Interference Copyright © 2010 SciRes. AJPS 64 REFERENCES [1] G. W. Chang, H.-W. Lin and S.-K. Chen, “Modeling Characteristics of Harmonic Currents Generated by High- Speed Railway Traction Drive Converters,” Transactions on Power Delivery, IEEE, Vol. 19, No. 2, April 2004, pp. 766-773. [2] L. Manczinger, Z. Antal and L. Kredics, “Ecophysiology and Breeding of Mycoparasitic Trichoderma Strains (a Review),” Acta Microbiologica et Immunologica Hunga- rica, Vol. 49, 2002, pp. 1-14. [3] D. Hoisington, M. Khairallah, T. Reeves, J. M. Ribaut, B. Skovmand, S. Taba and M. Warburton, “Plant Genetic Resources: What can they Contribute toward Increased Crop Productivity,” Proceedings of the National Academy of Sciences of the USA, Vol. 96, 1999, pp. 5937-5943. [4] M. Lee, “Genome Projects and Gene Pools: New Germ- plasm for Plant Breeding,” Proceedings of the National Academy of Sciences of the USA, Vol. 95, 1998, pp. 2001-2004. [5] V. Frankard, M. Ghislain and M. Jacobs, “Two Feed- back-Insensitive Enzymes of the Aspartate Pathway in Nicotiana sylvestris,” Plant Physiology, Vol. 99, 1992, pp. 1285-1293. [6] G. Galili, S. Galili, E. Lewinsohn and Y. Tadmor, “Ge- netic, Molecular and Genomic Approaches to Improve the Value of Plant Foods and Feeds,” Critical Reviews in Plant Sciences, Vol. 21, 2002, pp. 167-204. [7] M. D. de Bakker, M. Raponi and G. M. Arndr, “RNA-Meditaed Gene Silencing in Non-Pathogenic and Pathogenic Fungi,” Current Opinion in Microbiology, Vol. 5, 2002, pp. 323-329. [8] R, Almeida and R. C. Allshire, “RNA Silencing and Ge- nome Regulation,” Trends in Cell Biology, Vol. 15, 2005, pp. 251-258. [9] H. Nakayashiki, “RNA Silencing in Fungi: Mechanisms and Applications,” Federation of European Biochemical Societies Letters, Vol. 579, 2005, pp. 5950-5970. [10] D. C. Baulcombe, “RNA silencing in Plants,” Nature, Vol. 431, 2004, pp. 356-363. [11] S. H. Wani and G. S. Sanghera, “Genetic Engineering for Viral Disease Management in Plants,” Notulae Scientia Biologicae, Vol. 2, 2010, pp. 20-28. [12] M. A. Escobar, E. L. Civerolo, K. R. Summerfelt and A.M Dandekar, “RNAi-Mediated Oncogene Silencing Confers Ressitance to Crown Gall Tumorigenesis,” Pro- ceedings of the National Academy of Sciences USA, Vol. 98, 2001, pp. 13437-13442. [13] P. Brodersen and O. Voinnet, “The Diversity of RNA Silencing Pathways in Plants,” Trends in Genetics, Vol. 22, 2006, pp. 268-280. [14] M. R. Godge, A. Purkayastha, I. Dasgupta and P. P. Kumar, “Virus-Induced Gene Silencing for Functional Analysis of Selected Genes,” Plant Cell Reporter, Vol. 27, 2008, pp. 209-219. [15] S. M. Shahinul Islam, T. Miyazaki, F. Tanno and K. Itoh, “Dissection of Gene Function by RNA Silencing,” Plant Biotechnology, Vol. 22, 2005, pp. 443-446. [16] O. Milhavet, D. S. Gary and M. P. Mattson “RNA Inter- ference in Biology and Medicine,” Pharmacological Re- views, Vol. 55, 2003, pp. 629-648. [17] G. J. Hannon, “RNA Interference,” Nature, Vol. 418, 2002, pp. 244-251. [18] H. Vaucheret, F. Vazquez, P. Crete, and D. P. Bartel, “The Action of ARGONAUTE1 in the miRNA Pathway and Its Regulation by the miRNA Pathway are Crucial for Plant Development,” Genes and Development, Vol. 18, 2004, pp. 187-1197. [19] P. M. Waterhouse, M. B. Wang and T. Lough, “Gene Silencing as an Adaptive Defense against Viruses,” Na- ture, Vol. 411, 2001, pp. 834-842. [20] A. S. Pickford and C. Cogoni, “RNA-Mediated Gene Silencing,” Cellular and Molecular Life Science, Vol. 60, 2003, pp. 871-882. [21] S. M. Hammond, E. Bernstein, D. Beach and G. J. Han- non, “An RNA-Directed Nuclease Mediates Post-Tran- scriptional Gene Silencing in Drosophila Cells,” Nature, Vol. 404, 2000, pp. 293-296. [22] A. Fire, S. Xu, M. K. Montgomery, S. A. Kostas, S. E .Driver and C. C. Mello, “Potent and Specific Genetic Interference by Double-Stranded RNA in Caenorhabditis elegans,” Nature, Vol. 391, 1998, pp. 806-811. [23] E. Bernstein, A. A. Caudy, S. M. Hammond and G. J. Hannon, “Role for a Bidentate Ribonuclease in the Initia- tion Step of RNA Interference,” Nature, Vol. 409, 2001, pp. 363-366. [24] S. V. Wesley, C. A. Helliwell, N. A. Smith, M. B. Wang, D. T. Rouse, Q. Liu, P. S. Gooding, S. P. Singh, D. Ab- bott, P. A. Stoutjesdijk, S. P. Robinson, A. P. Gleave, A. G. Green, and P. Waterhouse, “Construct Design for Effi- cient, Effective and High-Throughput Gene Silencing in Plants,” Plant Journal, Vol. 27, 2001, pp. 581-590. [25] G. Tang, B. J. Reinhart, D. Bartel and P. D. Zamore, “A Biochemical Framework for RNA Silencing in Plants,” Genes and Development, Vol.17, 2003, pp. 49-63. [26] O. Voinnet, “Non-Cell Autonomous RNA Silencing,” Federation of European Biochemical Societies Letters, Vol. 579, 2005, pp. 5858-5871. [27] R. Lu, A. M. Martin-Hernandez, J. R. Peart, I. Malcuit and D. C. Baulcombe, “Virus Induced Gene Silencing in Plants,” Methods, Vol. 30, 2003, pp. 296-303. [28] P. Dunoyer, C. Himber, V. Ruiz-Ferrer, A. Alioua and O. Voinnet, “Intra- and Intercellular RNA Interference in Arabidopsis thaliana Requires Components of the Mi- croRNA and Heterochromatic Silencing Pathways,” Na- ture Genetics, Vol. 39, 2007, pp. 848-856. [29] U. Klahre, P. Crete, S. A. Leuenberger, V. A. Iglesias and F. Meins, “High Molecular Weight RNAs and Small In- terfering RNAs Induce Systemic Post Transcriptional Gene Silencing in Plants,” Proceedings of the National Academy of Sciences of the USA, Vol. 99, 2002, pp. 11981-11986. [30] A. J. Herr, A. Molnar, A. Jones and D. C. Baulcombe,  Biotechnology and Plant Disease Control-Role of RNA Interference Copyright © 2010 SciRes. AJPS 65 “Defective RNA Processing Enhances RNA Silencing and Influences Flowering of Arabidopsis,” Proceedings of the National Academy of Sciences of the USA, Vol. 103, 2006, pp. 14994-15001. [31] G. Hutvagner and P. D. Zamore, “A microRNA in a Multiple-Turnover RNAi Enzyme Complex,” Science, Vol. 297, 2002, pp. 2056-2060. [32] C. Himber, P. Dunoyer, G. Moissiard, C. Ritzenthaler and O. Voinnet, “Transitivity Dependent and Independent Cell-to-Cell Movement of RNA Silencing,” The EMBO Journal, Vol. 22, 2003, pp. 4523-4533 [33] K. Kobayashi and P. Zambryski, “RNA Silencing and Its Cell-to-Cell Spread during is Embryogenesis,” The Plant Journal, Vol. 50, 2007, pp. 597-604. [34] F. Li, and S. W. Ding, “Virus Counter Defense: Diverse Strategies for Evading the RNA Silencing Immunity,” Annual Review of Microbiology, Vol. 60, 2006, pp. 503- 531. [35] C. F. Chuang and E. M. Meyerowtiz, “Specific and Heri- table Genetic Interference by Double-Stranded RNA in Arabidopsis thaliana,” Proceedings of the National Academy of Sciences of the USA, Vol. 97, 2000, pp. 985- 4990. [36] P. Schweizer, J. Pokorny, P. Schulze-Lefert and R Dudler, “Double Stranded RNA Interference with Gene Functions at the Single Cell in Cereals,” The Plant Journal, Vol. 24, 2000, pp. 895-903. [37] Y. L. Liu, M. Schiff and S. P. Dinesh-Kumar, “Virus Induced Gene Silencing in Tomato,” The Plant Journal, Vol. 31, 2002a, pp. 777-786. [38] T. Dalmay, A. J. Hamilton, E. Mueller and D. C. Baul- combe, “Potato Virus X Amplicons in Arabidopsis Medi- ate Genetic and Epigenetic Gene Silencing,” The Plant Cell, Vol. 12, 2000, pp. 369-380. [39] H. Liu, T. R. Cottrell, L. M. Pierini, W. E. Goldman and T. L. Doering, “RNA Interference in the Pathogenic Fun- gus Cryptococcus neoformans,” Genetics, Vol. 160, 2002b, pp. 463-470. [40] W. Tang, D. A. Weidner, B. Y. Hu, R. J. Newton and X. Hu, “Efficient Delivery of Small Interfering RNA to Plant Cells by a Nanosecond Pulsed Laser-Induced Wave for Post Transcriptional Gene Silencing,” Plant Science, Vol. 171, 2006, pp. 375-81. [41] J. M. Hilly, and Z. Liu, “An Overview of Small RNAs,” In: C. L. Bassett, Ed., Regulation of Gene Expression in Plants, Springer-Verlag, Berlin, 2007, pp. 123-147. [42] L. K. Johansen, and J. C. Carrington, “Silencing on the Spot. Induction and Suppression of RNA Silencing in the Agrobacterium-Mediated Transient Expression System,” Plant Physiology, Vol. 126, 2001, pp. 930-938. [43] O. Voinnet, “RNA Silencing as a Plant Immune System against Viruses,” Trends in Genetics, Vol. 17, 2001, pp. 449-459. [44] S. Mlotshwas, O. Voinnet, M. F. Mette, M. Matzke, H. Vaucheret, S. W. Ding, G. Pruss and G. B. Vance, “RNA Silencing and Its Mobile Silencing Signal,” The Plant Cell, Vol. 14, 2002, pp. 289-301. [45] F. Tenllado, B. Martinez-Garcia, M. Vargas and J. R. Diaz-Ruiz, “Crude Extracts of Bacterially Expressed dsRNA can be Used to Protect Plants against Virus Infec- tion,” BMC Biotechnology, Vol. 3, 2003, pp. 3-14. [46] M. Timmermans, O. Das and J. Messing, “Geminivirus and Their Uses as Extrachromosomeal Replicons,” An- nual Review of Plant Physiology, Vol. 45, 1994, pp. 79- 112. [47] G. P. Pogue, J. A. Lindbo, S. J. Garger and W. P. Fitz- maurice, “Making an Ally from an Enemy: Plant Virol- ogy and the New Agriculture,” Annual Review of Phyto- pathology, Vol. 40, 2002, pp. 45-74. [48] M. H. Kumagai, J. Donson, G. della-Cioppa, D. Harvey, K. Hanley and L. K. Grill, “Cytoplasmic Inhibition of Carotenoid Biosynthesis with Virus-Derived RNA,” Pro- ceedings of the National Academy of Sciences of the USA, Vol. 92, 1995, pp. 1679-1683. [49] S. M. Angell and D. C. Baulcombe, “Technical Advance: Potato Virus X Amplicon Mediated Silencing of Nuclear Genes,” The Plant Journal, Vol. 20, 1999, pp. 357-362. [50] S. A. MacFarlane, and A. H. Popovich, “Efficient Ex- pression of Foreign Proteins in Roots from Tobravirus Vectors,” Virology, Vol. 267, 2000, pp. 29-35. [51] A. C. Mallory, G. Parks, M. W. Endres, D. Baulcombe and L. H. Bowman, “The Amplicon-Plus System for High- Level Expression of Transgenes in Plants,” Nature Biotechnogy, Vol. 20, 2002, pp. 622-625. [52] K. E. Palmer and E. P. Rybicki, “Investigation of the potential of maize streak virus to act as an infectious gene vector in maize plants,” Archives of Virology, Vol. 146, 2001, pp. 1089-1104. [53] S. Kjemtrup, K. S. Sampson, C. G. Peele, L. V. Nguyen and M. A. Conkling, “Gene Silencing from Plant DNA Carried by a Geminivirus,” Plant Journal, Vol. 14, 1998, pp. 91-100. [54] M. J. Dallwitz and E. J. Zurcher, “Plant Viruses Online,” In: A. A. Brunt, K. Crabtree, M. J. Dallwitz, A. J. Gibbs, L. Watson and E. J. Zurcher, Ed., Descriptions and Lists from the VIDE Database, CAB International, UK, 1996, pp. 1484. [55] Q. Liu, S. P. Singh, and A. G. Green, “High-Stearic and High-Oleic Cottonseed Oils Produced by Hairpin RNA- Mediated Post-Transcriptional Gene Silencing,” Plant Physiology, Vol. 129, 2002c, pp. 1732-1743. [56] M. A. Turnage, N. Muangsan, C. G. Peele and D. Robertson, “Geminivirus-Based Vectors for Gene Si- lencing in Arabidopsis,” The Plant Journal, Vol. 30, 2002, pp. 107-117. [57] C. Cogoni, N. Romano and G. Macino, “Suppression of Gene Expression by Homologous Transgenes,” Antonie Leeuwenhoek International Journal of General Molecular Microbiology, Vol. 65, 1994, pp. 205-209. [58] P. Y. Teycheney and M Tepfer, “Virus Specific Spatial Differences in the Interference with Silencing of the chs-A Gene in Non-Transgenic Petunia,” Journal of Gen-  Biotechnology and Plant Disease Control-Role of RNA Interference Copyright © 2010 SciRes. AJPS 66 eral Virology, Vol. 82, 2001, pp. 1239-1243. [59] W. Hamada and P. D. Spanu, “Co-Suppression of the Hydrophobin Gene Hcf-1 is Correlated with Antisense RNA Biosynthesis in Cladosporium fulvum,” Molecular and General Genetics, Vol. 259, 1998, pp. 630-638. [60] N. Kadotani, H. Nakayashiki, Y. Tosa and S. Mayama, “RNA Silencing in the Pathogenic Fungus Magnaporthe oryzae,” Molecular Plant-Microbe Interaction, Vol. 16, 2003, pp. 769-776. [61] J. A. Kim, K. Cho, R. Singh, Y. H. Jung, S. H. Jeong, S. H. Kim, J. E. Lee, Y. S. Cho, G. K. Agrawal, R. Rakwal, S. Tamogami, B. Kersten, J. S. Jeon, G. An and N. S. Jwa, “Rice OsACDR1 (Oryza sativa Accelerated Cell Death and Resistance 1) is a Potential Positive Regulator of Fungal Disease Resistance,” Molecules and Cells, Vol. 30, 2009, pp. 431-439. [62] L. Chen, K. Shiotani, T. Togashi, D. Miki, M. Aoyama, H. L. Wong, T. Kawasaki and K. Shimamoto, “Analysis of the Rac/Rop Small GTPase Family in Rice: Expression, Subcellular Localization and Role in Disease Resistance,” Plant and Cell Physiology, Vol. 51, 2010, pp. 585-595. [63] A. Fitzgerald, J. A. Van Kha and K. M. Plummer, “Si- multaneous Silencing of Multiple Genes in the Apple Scab Fungus Venturia inaequalis, by Expression of RNA with Chimeric Inverted Repeats,” Fungal Genetics and Biology, Vol. 41, 2004, pp. 963-971. [64] M. Goldoni, G. Azzalin, G. Macino and C. Cogoni, “Ef- ficient Gene Silencing by Expression of Double Stranded RNA in Neurospora crassa,” Fungal Genetics and Biol- ogy, Vol. 41, 2004, pp. 1016-1024. [65] T. M. Hammond and N. P. Keller, “RNA Silencing in Aspergillus nidulans is Independent of RNA-Dependent RNA polymerase,” Genetics, Vol. 169, 2005, pp. 607-617. [66] P. Spanu, “HCf-1, a Hydrophobin from the Tomato PathoGen Cladosporium fulvum,” Gene, Vol. 93, 1997, pp. 89-96. [67] G. C. Segers, W. Hamada, R. P. Oliver and P. D. Spanu, “Isolation and Characteristaion of Five Different Hydrophobin-Encoding cDNA from the Fungal Tomato Pathogen Cladosporium fulvum,” Molecular and General Genetics, Vol. 261, 1999, pp. 644-652. [68] J. C. Abbott, A. Barakate, G. Pincon, M. Legrand, C. Lapierre, I. Mila, W. Schuch and C. Halpin, “Simultane- ous Suppression of Multiple Genes by Single Transgenes. Down-Regulation of Three Unrelated Lignin Biosynthetic Genes in Tobacco,” Plant Physiology, Vol. 128, 2002, pp. 844-853. [69] N. J. Talbot, M. J. Kershaw, G. E. Wakley, O. M. H. de Vries, J. G. H. Wessels and J. E. Hamer, “MPG1 Encodes a Fungal Hydrophobin Involved in Surface Interactions during Infection-Related Development of Magnaporthe grisea,” Plant Cell, Vol. 8, 1996, pp. 985-999. [70] Q. B. Nguyen, N. Kadotani, S. Kasahara, Y. Tosa, S. Mayama and H. Nakayashiki, “Systematic Functional Analysis of Calcium-Signalling Proteins in the Genome of the Rice-Blast Fungus, Magnaporthe oryzae, Using a High-Throughput RNA-Silencing System,” Molecular Microbiology, Vol. 68, 2008, pp. 1348-1365. [71] R. J. Weld, K. M. Plummer, M. A. Carpenter, and H. J. Ridgway, “Approaches to Functional Genomics in Fila- mentous Fungi,” Cell Research, Vol. 16, 2006, pp. 31-44. [72] H. Nakayashiki and Q. B. Nguyen, “RNA Interference: Roles in Fungal Biology,” Current Opinion in Microbi- ology, Vol. 11, 2008, pp. 1-9. [73] H. Peng, Q. Zhang, Y. Li, C. Lei, Y. Zhai, X. Sun, D. Sun, Y. Sun and T. Lu, “A Putative Leucine-Rich Repeat Re- ceptor Kinase, OsBRR1, is Involved in Rice Blast Resis- tance,” Planta, Vol. 230, 2009, pp. 377-385. [74] S. Katiyar-Agarwal, R. Morgan, D. Dahlbeck, O. Borsani, J. A. Villegas, J. Zhu, B. J. Staskawicz and H. Jin, “A Pathogen-Inducible Endogenous siRNA in Plant Immu- nity,” Proceedings of the National Academy of Sciences of the USA, Vol. 103, 2006, pp. 47-52. [75] S. Katiyar-Agarwal, S. Gao, A. Vivian-Smith and H. Jin, “A Novel Class of Bacteria-Induced Small RNAs in Arabidopsis,” Genes and Development, Vol. 21, 2007, pp. 3123-3134. [76] L. Navarro, P. Dunoyer, F. Jay, B. Arnold, N. Dharmasini, M. Estelle, O. Vionnet and J. D. Jones, “A Plant miRNA Contributes to Antibacterial Resistance by Repressing Auxin Signaling,” Science, Vol. 312, 2006, pp. 436-439. [77] V. Bitko and S. Barik, “Phenotypic Silencing of Cyto- plasmic Genes with Sequence Specific Double Stranded Short Interfering RNA and Its Applications in the Re- verse Genetics of Wild Type Negative Strand RNA Vi- rus,” BMC Microbiology, Vol. 1, 2001, pp. 34-44. [78] L. Gitlin, S. Karelsky, and R. Andino, “Short Interference Confers Intracellular Antiviral Immunity in Human Cells,” Nature, Vol. 4, 2002, pp. 418-430. [79] J. M. Jacque, K. Triques and M. Stevenson, “Modulation of HIV-1 Replication by RNA Interference,” Nature, Vol. 418, 2002, pp. 435-438. [80] C. D. Novina, M. F Murray, D. M. Dykxhoorn, P. J. Beresford, J. Riess, S. K. Lee, R. G. Collman, J. Lieber- man, P. Shanker and P. A. Sharp, “siRNA-Directed Inhi- bition of HIV-1 Infection,” Nature Mediterranean, Vol. 8, 2002, pp. 681-686. [81] E. Ullu, A. Djikeng, H. Shi, and C. Tschudi, “RNA Inter- ference: Advances and Questions,” Philosophical Trans- actions of the Royal Society of London British Biological Science, Vol. 29, 2002, pp. 65-70. [82] P. M. Waterhouse, M. W. Graham and M. B. Wang, “Vi- rus Resistance and Gene Silencing in Plants can be In- duced by Simultaneous Expression of Sense and An- tisense RNA,” Proceedings of the National Academy of Sciences of the USA, Vol. 95, 1998, pp. 13959-13964. [83] E. J. Chapman, A. I. Prokhnevsky, K. Gopinath, V. V. Dolja and J. C. Carrington, “Viral RNA Silencing Sup- pressors Inhibit the micro-RNA Pathway at an Interphase Step,” Genes and Development, Vol. 18, 2004, pp. 1179- 1186. [84] M. Pooggin, P. V. Shivaprasad, K. Veluthambi and T. Hohn, “RNAi Targetting of DNA Viruses,” Nature Bio-  Biotechnology and Plant Disease Control-Role of RNA Interference Copyright © 2010 SciRes. AJPS 67 technology, Vol. 21, 2003, pp. 131-32. [85] R. Vanitharani, P. Chellappan and C. M. Fauquet, “Short Interfering RNA-Mediated Interference of Gene Expres- sion and Viral DNA Accumulation in Cultured Plant Cells,” Proceedings of the National Academy of Sciences of the USA, Vol. 100, 2003, pp. 9632-9636. [86] V. Ruiz-Ferrer and O. Voinnet, “Viral Suppression of RNA Silencing: 2b Wins the Golden Fleece by Defeating Argonaute,” Bioassays, Vol. 29, 2007, pp. 319-323. [87] J. Chen, W. X. Li, D. Xie, J. R. Peng, and S. W. Ding, “Viral Virulence Protein Suppresses RNA Silenc- ing-Mediated Defense but Upregulates the Role of mi- croRNA in Host Gene Regulation,” Plant Cell, Vol. 16, No. 5, 2004, pp. 1302-1313. [88] Z. Merai, Z. Kerenyi, A. Molnar, E. Barta, A. Valcozi, G. Bistray, Z. Havelda, J. Burgyan and D. Silhavy, “Aureus- virus P14 is an Efficient RNA Silencing Suppressor that Binds Double Stranded RNAs without Size specificity,” Journal of Virology, Vol. 79, 2005, pp. 7217- 7226. [89] A. Takeda, M. Tsukuda, H. Mizumoto, K. Okamoto, M. Kaido, K. Mise and T. Okuno, “A Plant RNA Virus Sup- pressor RNA Silencing through RNA Replication,” The EMBO Journal, Vol. 24, 2005 pp. 3147-3157. [90] X. Cao, P. Zhou, X. Zhang, S. Zhu, X. Zhong, Q. Xiao, B. Ding and Y. Li, “Identification of an RNA Silencing Sup- pressors from a Plant Double Stranded RNA Virus,” Journal of Virology, Vol. 79, 2005, pp. 13018-13027. [91] Q. W. Niu, S. S. Lin, J. L. Reyes, K. C. Chen, H. W. Wu, S. D. Yeh and N. H. Chua, “Expression of Artificial mi- croRNAs in Transgenic Arabidopsis thaliana Confers Virus Resistance,” Nature Biotechnology, Vol. 24, 2006, pp. 1420-1428. [92] J. Qu, J. Ye and R. X. Fang, “Artificial microRNA-Me- diated Virus Resistance in Plants,” Journal of Virology, Vol. 81, 2007, pp. 6690-6699. [93] J. A. Díaz-Pendón and S. W. Ding, “Direct and Indirect Roles of Viral Suppressors of RNA Silencing in Patho- genesis,” Annual Review of Phytopathology, Vol. 46, 2008, pp. 303-326. [94] S. M. Elbashir, W. Lendeckel, and T. Tuschl, “RNA In- terference is Mediated by 21 and 22-Nucleotide RNAs,” Genes and Development, Vol. 15, 2001, pp. 188-200. [95] H. Tyagi, S. Rajasubramaniam, M. V. Rajam and I. Das- gupta, “RNA Interference in Rice against Rice Tungro Bacilliform Virus Results in Its Decreased Accumulation in Inoculated Rice Plants,” Transgenic Research, Vol. 17, 2008, pp. 897-904. [96] Y. J. Kung, T. A. Yu, C. H. Huang, H. C. Wang, S. L. Wang and S. D. Yeh, “Generation of Hermaphrodite Transgenic Papaya Lines with Virus Resistance via Transformation of Somatic Embryos Derived from Ad- ventitious Roots of in vitro shoots,” Transgenic Research, 2009, in press. [97] S. K. Mangrauthia, P. Singh and S. Praveen, “Genomics of Helper Component Proteinase Reveals Effective Strat- egy for Papaya Ringspot Virus Resistance,” Molecular Biotechnology, Vol. 44, 2010, pp. 22-29. [98] I. B. Fofana, A. Sangare, R. Collier, C. Taylor and C. M. Fauquet, “A Geminivirus-Induced Gene Silencing System for Gene Function Validation in Cassava,” Plant Mo- lecular Biology, Vol. 56, 2004, pp. 613-624. [99] S. Holzberg, P. Brosio, C. Gross and G. P. Pogue, “Bar- ley Stripe Mosaic Virus-Induced Gene Silencing in a Monocot Plant,” Plant Journal, Vol. 30, 2002, pp. 315- 327. [100] S. R. Scofield, L. Huang, A. S. Brandt and B. S. Gill, “Development of a Virus-Induced Gene Silencing System for Hexaploid Wheat and Its Use in Functional Analysis of the Lr21-Mediated Leaf Rust Resistance Pathway,” Plant Physiology, Vol. 138, 2005, pp. 2165-2173. [101] C. Cakir and M. Tör, “Factors Influencing Barley Stripe Mosaic Virus-Mediated Gene Silencing in Wheat,” Physi- ological and Molecular Plant Pathology, Vol. 74, 2010, pp. 246-253. [102] C. Zhang and S. A. Ghabrial, “Development of Bean Pod Mottle Virus-Based Vectors for Stable Protein Expression and Sequence-Specific Virus-Induced Gene Silencing in Soybean,” Virology, Vol. 344, 2006, pp. 401-411. [103] C. Zhang, C. Yang, S. A. Whitham and J. H. Hill “De- velopment and Use of an Efficient DNA-Based Viral Gene Silencing Vector for Soybean,” Molecular Plant Microbe Interaction, Vol. 22, 2009, pp. 123-131. [104] C. Zhang, J. D. Bradshaw, S. A. Whitham and J. H. Hill “The Development of an Efficient Multi-Purpose BPMV Viral Vector Set for Foreign Gene Expression and RNA Silencing,” Plant Physiology, 2010, in press. [105] X. S. Ding, W. L. Schneider, S. R. Chaluvadi, R. M. Rouf Mian and R. S. Nelson, “Characterization of a Brome Mosaic Virus Strain and Its Use as a Vector for Gene Si- lencing in Monocotyledonous Hosts,” Molecular Plant Microbe Interaction, Vol. 19, 2006, pp. 1229-1239. [106] G. D. Constantin, B. N. Krath, S. A. MacFarlane, M. Nicolaisen, I. E. Johansen and O. S. Lund, “Vi- rus-Induced Gene Silencing as a Tool for Functional Ge- nomics in a Legume Species,” Plant Journal, Vol. 40, 2004, pp. 622-631. [107] M. Naylor, J. Reeves, J. I. Cooper, M. L. Edwards and H. Wang, “Construction and Properties of a Gene Silencing Vector Based on Poplar Mosaic Virus (Genus Carlavi- rus),” Journal of Virology Methods, Vol. 124, 2005, pp. 27-36. [108] M. T. Ruiz, O. Voinnet and D. C. Baulcombe, “Initiation and Maintenance of Virus-Induced Gene Silencing,” Plant Cell, Vol. 10, 1998, pp. 937-946. [109] O. Faivre-Rampant, E. M. Gilroy, K. Hrubikova, I. Hein, S. Millam, G. J. Loake, P. Birch, M. Taylor and C. La- comme, “Potato Virus X-Induced Gene Silencing in Leaves and Tubers of Potato,” Plant Physiology, Vol. 134, 2004, pp. 1308-1316. [110] V. V. Gossele, I. I. Fache, F. Meulewaeter, M. Cornelis- sen and M. Metzlaff, “SVISS-a Novel Transient Gene Si- lencing System for Gene Function Discovery and Valida- tion in Tobacco,” The Plant Journal, Vol. 32, 2002, pp.  Biotechnology and Plant Disease Control-Role of RNA Interference Copyright © 2010 SciRes. AJPS 68 859- 866. [111] F. Ratcliff, A. M. Martin-Hernandez and D. C. Baul- combe, “Tobacco Rattle Virus as a Vector for Analysis of gene Functions by Silencing,” Plant Journal, Vol. 25, 2001, pp. 237-245. [112] G. Brigneti, A. M. Martin-Hernandez, H. Jin, J. Chen, D. C. Baulcombe, B. Baker, and J. D. Jones, “Virus-Induced Gene Silencing in Solanum Species,” The Plant Journal, Vol. 39, 2004, pp. 264-272. [113] E. Chung, E. Seong, Y. C. Kim, E. J. Chung, S. K. Oh, S. Lee, J. M. Park, Y. H. Joung and D. Choi, “A Method of High Frequency Virus Induced Gene Silencing in Chili Pepper Capsicum annuum L. cv. Bukang),” Molecular Cell, Vol. 17, 2004, pp. 377-380. [114] L. C. Hileman, S. Drea, G. Martino, A. Litt and V. F. Irish, “Virus Induced Gene Silencing is an Effective Tool for Assaying Gene Function in the Basal Eudicot Species Papaver somniferum (Opium Poppy),” The Plant Journal, Vol. 44, 2005, pp.334-341. [115] B. Gould and E. M. Kramer, “Virus-Induced Gene Si- lencing as a Tool for Functional Analyses in the Emerg- ing Model Plant Aquilegia (columbine, Ranunculaceae),” BMC Plant Methods, Vol. 12, 2007, pp. 6. [116] H. Hou and W. Qiu, “A Novel Co-Delivery System Con- sisting of a Tomato Bushy Stunt Virus and a Defective Interfering RNA for Studying Gene Silencing,” Journal of Virology Methods, Vol. 111, 2003, pp. 37-42. [117] C. Peele, C.V. Jordan, N. Muangsan, M. Turnage, E. Egelkrout, P. Eagle, L. Hanley-Bowdoin and D. Robert- son, “Silencing of a Meristematic Gene Using Gemi- nivirus-Derived Vectors,” Plant Journal, Vol. 27, 2001, pp. 357-366. [118] X. Tao and X. Zhou, “A Modified Viral Satellite DNA that Suppresses Gene Expression in Plants,” The Plant Journal, Vol. 38, 2004, pp. 850-860. [119] M. R. Godge, A. Purkayastha, I. Dasgupta and P. P. Kumar, “Virus-Induced Gene Silencing for Functional Analysis of Selected Genes,” Plant Cell Reporter, Vol. 27, 2008, pp. 209-219. [120] P. E. Urwin, C. J. Lilley and H. J. Atkinson, “Ingestion of Double-Stranded RNA by Pre-Parasitic Juvenile Cyst Nematodes Leads to RNA Interference,” Molecular Plant-Microbe Interactions, Vol. 15, 2002, pp. 747-752. [121] M. Bakhetia, W. Charlton, H. J. Atkinson and M. J. McPherson, “RNA Interference of Dual Oxidase in the Plant Nematode Meloidogyne incognita,” Molecular Pla n t - Microbe Interaction, Vol. 18, 2005, pp. 1099-1106. [122] M. N. Rosso, M. P. Dubrana, N. Cimbolini, S. Jaubert and P. Abad, “Application of RNA Interference to Root- Knot Nematode Genes Encoding Esophageal Gland Pro- teins,” Molecular Plant-Microbe Interactions, Vol. 18, 2005, pp. 615-620. [123] J. Shingles, C. J. Lilley, H. J. Atkinson and P. E. Urwin, “Meloidogyne incognita: Molecular and Biochemical Characterization of a Cathepsin L Cysteine Proteinase and the Effect on Parasitism Following RNAi,” Experi- mental Parasitology, Vol. 115, 2007, pp. 114-120. [124] C. J. Lilley, S. A. Goodchild, H. J. Atkinson and P. E. Urwin, “Cloning and Characterization of a Heterodera glycines Minopeptidase cDNA,” International Journal of Parasitology, Vol. 35, 2005, pp. 1577-1585. [125] R. M. Steeves, T. C. Todd, J. S. Essig and H. N. Trick, “Transgenic Soybeans Expressing siRNAs Specific to a Major Sperm Protein Gene Suppress Heterodera glycines Reproduction,” Functional Plant Biology, Vol. 33, 2006, pp. 991-999. [126] Q. Chen, S. Rehman, G. Smant and J. T. Jones, “Func- tional Analysis of Pathogenicity Proteins of the Potato Cyst Nematode Globodera rostochiensis Using RNAi,” Molecular Plant-Microbe Interactions, Vol. 18, 2005, pp. 621-625. [127] G. Huang, R. Allen, E. L. Davis, T. J. Baum and R. S. Hussey, “Engineering Broad Root-Knot Resistance in Transgenic Plants by RNAi Silencing of a Conserved and Essential Root-Knot Nematode Parasitism Gene,” Pro- ceedings of the National Academy of Sciences of the USA, Vol. 103, 2006, pp. 4302-14306. [128] E. Fanelli, M. Di Vito, J. T. Jones and C. De Giorgi, “Analysis of Chitin Synthase Function in a Plant Parasitic Nematode, Meloidogyne artiellia, Using RNAi,” Gene, Vol. 349, 2005, pp. 87-95. [129] Y. Hoffman, C. Aflalo, A. Zarka, J. Gutman, T.Y. James, and S. Boussiba, “Isolation and characterization of a novel chytrid species (phylum Blastocladiomycota), para- sitic on the green alga Haematococcus,” Mycological Research, Vol. 112, 2008, pp. 70-81. [130] D. J. Fairbairn, A.S. Cavalloro, M. Bernard, J. Mahal- inga-Iyer, M. W. Graham and J. R. Botella, “Host-Deliv- ered RNAi: An Effective Strategy to Silence Genes in Plant Parasite Nematodes,” Planta, Vol. 226, 2007, pp. 1525-1533. [131] S. Sindhu, T R. Maier, M. G. Mitchum, R. S. Hussey, E. L. Davis and T. J. Baum “Effective and Specific in Planta RNAi in Cyst Nematodes: Expression Interference of Four Parasitism Genes Reduces Parasitic Success,” Jour- nal of Experimental Botany, Vol. 60, 2009, pp. 315-324. [132] M. Karakas, “RNA Interference in Plant Parasitic Nema- todes,” African Journal of Biotechnology, Vol. 7, 2008, pp. 2530-2534. |

