Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio ns, 2010, 1, 369-372 doi:10.4236/msa.2010.16053 Published Online December 2010 (http://www.scirp.org/journal/msa) Copyright © 2010 SciRes. MSA 369 Influence of Ni and Cr Content on Corrosion Resistance of Ni-Cr-Mo Alloys for Fixed Dental Prostheses in 0.05% NaF Aqueous Solution Nilo A. S. Sampaio, José W. J. Silva, Heloisa A. Acciari, Roberto Z. Nakazato, Eduardo N. Codaro, Hamilton de Felipe Chemistry and Physics Departament, Universidade Estadual Paulista, Guaratinguetá, Brazil. E-mail: jwjsilva@feg.unesp.br Received August 13th, 2010; revised October 28th, 2010; accepted November 26th, 2010. ABSTRACT The Ni-Cr-Mo alloys have been used as dental prostheses because they own a good mechanical strength, high corro- sion resistance and even to be econom ica lly viab le. Th ese alloys corro sion p ro tection again st in sa lt solution s typ ical o f physiological med ia is due to passivatio n phenomenon with an oxide surface layer forma tion, mainly chromium oxid es. This protective film, subjected to a mechanical stress in a corrosive environment, can partially dissolve by releasing ions, which have deleterious effects in a human body. Fluoride ions, existing in hygiene products, change the buccal environment and their presence may enable a localized corrosion process initiation. The aim of this work has been to investigate the chemical composition influence of three alloys in corrosion resistance to: A (Ni-73% Cr-14% Mo-8.5% Be-1.8% Al-1.8%), B (Ni-61% Cr-25% Mo-10.5% Si-1.5%) and C (Ni-65% Cr-22.5% Mo-9.5%) in media containing fluorides that simulate mouthwashes solution. The study has been done in a 0.05% NaF solution, pH 6, at 37˚C using electrochemical techniques. The alloy with the highest nickel and the lowest chromium content is not passive in the middle studied, showing a continuous increase in current density with the potential increasing, while the other alloys show passivation range of 600 mV and passive current density o f about 10-6 A/cm2. Keywords: Ni-Cr-Mo Alloys, Dental Prostheses, Corrosion 1. Introduction A large number of metal alloys are used in dentistry for manufacturing of fixed and removable prostheses. In the oral cavity, these structures are exposed to a chemically adverse environment, with saliva being the most corro- sive ag ent, due to th e high concentration of chloride ions that are causing localized corrosion. Another important factor is the pH, which can range from 2 to 11, depend- ing on the ingested food. One of the most important fac- tors determining the use of a metal alloy for making prosthesis is its resistance to corrosion [1,2]. Non-pre- cious metal alloys are being used due to their low cost and adequate mechanical properties. In the '60s, basic alloys such as nickel-chromium were developed. These alloys are basically composed of Ni (68% to 80%) and Cr (12% to 27%) [3], but it is necessary to add other elements to ensure high mechanical strength achieve- ment and adequate corrosion resistance, besides the shaping facility and bonding with porcelains, molybde- num, iron, aluminum, silicon, beryllium, cobalt, niobium, titanium which are the most frequently used alloys ele- ments [3,4]. Chromium presence improves the alloys corrosion resistance in typical salt solutions of physio- logical media with an oxide surface layer formation, mainly chromium oxides [5,6]. This layer thickness is typical of a few nanometers, which is sufficient to act as a protective barrier between the metal surface and the aggressive medium. The passive film slows the metals dissolution rate, making difficult the ions transfer from metal to solution. The human tissue reaction for dis- solved ionic species can vary from a simple allergy to a severe disruption in the region adjacent to the prosthesis [7]. Since the chemical alloys composition can influence the corrosion behavior, studies about corrosion resistance in aggressive media, which simulate the oral environ- ment, may provide information about metals or alloys to be used in dental applications. Sodium Fluoride (NaF) and other fluorides are often used as prophylactic agents  Influence of Ni and Cr Content on Corrosion Resistance of Ni-Cr-Mo Alloys for Fixed Dental 370 Prostheses in 0.05% NaF Aqueous Solution in dental treatments to prevent dental plaque formation and caries development, however, the prophylactic ac- tion may have a side effect of metal materials corrosion caused by this ion. Saliva infiltration containing fluoride in the structure that holds implant and the contact with crowns and bridges can be the corrosive attack cause. Fluorides usually attack reactive metals such as titanium, especially in acidic medium, causing corrosion due to their passivity destruction and mechanical properties decrease. To date, a few researches have been done to analyze the fluorine-containing composites influence in dental alloys corrosion resistance. N. Chiff et al. 2004 [8] studied the fluoridated mouthwashes influence on tita- nium alloys corrosion resistance used in orthodontic wires manufacture, using electrochemical techniques that indicated correlation between corrosion and mouth- washes composition. Mayont, A. M. et al. 2004 [9] ana- lyzing the titanium alloys corrosion behavior found high corrosion rates in neutral solutions with lesser fluoride contents and in acid solutions with low fluoride levels. According to the authors, the fluoride concentration in- crease leads to a decrease in thickness and increase in porosity on oxide layer, which reduces its corrosion pro- tection. Rezende, A, B, C. and other [10] evaluated effect of three commercial mouthwashes on corrosion resis- tance of a Ti-10 Mo alloy developed in their lab, com- paring it with the commercially pure titanium behavior. Through electrochemical techniques, they concluded that corrosion resistance depends predominantly on the mouthwash composition. With respect to Ni-Cr, it has only been found in literature one non-conclusive quote that prophylactic fluoride solution (pH = 6.5) is a reac- tive agent and can cause damage to metal restorations [11]. Theref ore, it is impor tant to probe know ledge abou t the Ni-Cr-Mo reactivity in media containing fluoride, which is this work aim. 2. Materials and Methods The three Ni-Cr-Mo alloys corrosion resistance has been determined in naturally aerated 0.05% NaF solution, pH 6.0 at 37˚C using electrochemical techniques. The alloys have been acquired in the national market, being com- mercialized in a billet form in the as-cast condition. Ta- ble 1 shows the chemical compositions provided by the manufacturers, being named in this work by the letters A, B and C. For electrochemical measurements, the alloys, as re- ceived, have been embedded in polyester resin, leaving an exposed area in the flat disk form of about 1 cm2. Be- fore each experiment, the electrode surfaces have been mechanically polished, degreased with acetone, washed with distilled water and air-dried. Measures have been Table 1. Alloys mass percentage composition. AlloyNi Cr Mo Be Si Al A 73 14 8.5 1.8 --------- 1.8 B 61 25 10.5 ------- 1.5 -------- C 65 22.5 9.5 ------- ------------------ taken at 37˚C using a conventional thermostated electro- chemical cell containing 0.05% NaF solution used as elec- trolyte and prepared using NaF and distilled water. The electrochemical measurements have been carried out using a potentiostat/galvanostat EG & PAR 283 and a Frequency Response Analyzer, EG & PAR 1025 (Perkin Elmer Instruments Inc., USA), both interfaced to a computer for collecting and analyzing data. The work- ing electrodes potentials have been determined using a saturated calomel electrode as reference, and used a platinum ring as counter electrode. Op en circuit potential (OCP) values have been recorded during 400 minutes of immersion. Potentiodynamic polarization curves have been obtained immediately after obtaining the OCP curves, with a scan rate of 20 mV min-1 towards electro- positive. The experiments have been performed in tripli- cate to observe repeatability. The cyclic voltammetry tests have been carried out with a 10 mV/s sp eed starting at –600 mV, advancing towards the cathode up to the potential value where the metals dissolution process starts and returning to the initial poten tial. 3. Results and Discussion 3.1. OCP Measurements At Figure 1, OCP curves for alloys A, B and C in 0.05% NaF are illustrated. An upward trend at the very initial immersion stage has been common for the three studied alloys, but a significant decrease in the OCP values has been observed, specifically for curve A and potential val- ues obtained have been around 200 to 250 mV more nega- tive than those observed for B and C. Fluoride ions pres- ence in solution can alter the oxide la yer structure making it more porous, thus leading to a decrease in corrosion resistance in the considered medium. These observations seem to be consistent with the differences in the alloys compositions, since alloy A, by having a smaller chro- mium amount in its composition, becomes more suscepti- ble to corrosion in me di um cont ai ni ng fluoride. Chromi um is responsible for corrosion resistance and for an oxide film formation commonly called passive film [11]. It takes at least 12% by weight for thi s film formation. In laboratory studies, concen trations of 16%-27% tend to form a more uniform oxide layer of Cr2O3 and Cr(OH)3 with higher corrosion resistance [3]. The chro- mium oxide layer is associated with the aluminum from Copyright © 2010 SciRes. MSA 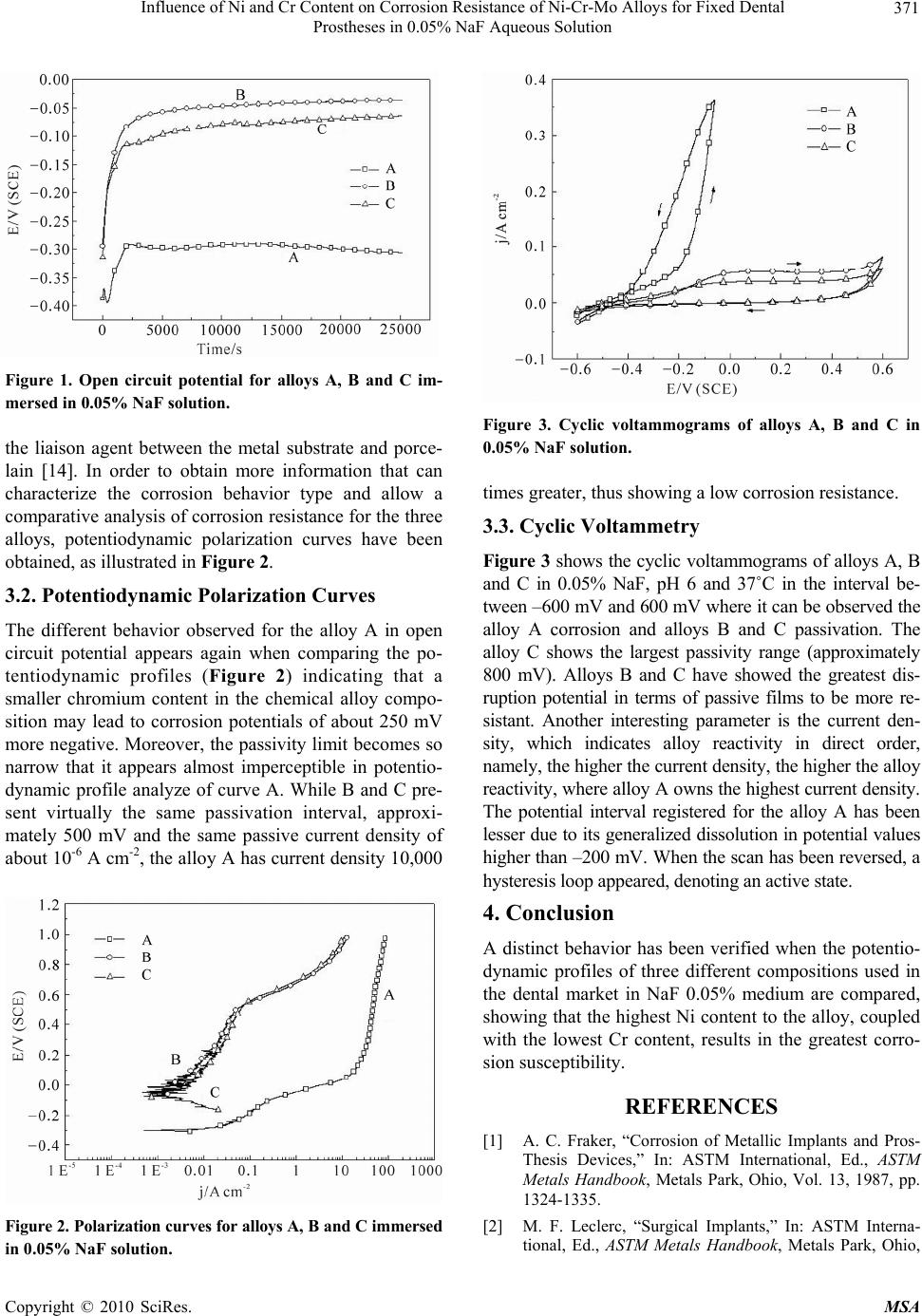 Influence of Ni and Cr Content on Corrosion Resistance of Ni-Cr-Mo Alloys for Fixed Dental 371 Prostheses in 0.05% NaF Aqueous Solution Figure 1. Open circuit potential for alloys A, B and C im- mersed in 0.05% NaF solution. the liaison agent between the metal substrate and porce- lain [14]. In order to obtain more information that can characterize the corrosion behavior type and allow a comparative analysis of corrosion resistance for the three alloys, potentiodynamic polarization curves have been obtained, as illustrated in Figure 2. 3.2. Potentiodynamic Polarization Curves The different behavior observed for the alloy A in open circuit potential appears again when comparing the po- tentiodynamic profiles (Figure 2) indicating that a smaller chromium content in the chemical alloy compo- sition may lead to corrosion potentials of about 250 mV more negative. Moreover, the passivity limit becomes so narrow that it appears almost imperceptible in potentio- dynamic profile analyze of curve A. While B and C pre- sent virtually the same passivation interval, approxi- mately 500 mV and the same passive current density of about 10-6 A cm-2, th e alloy A has curr ent density 10,000 Figure 2. Polarization curves for alloys A, B and C immersed in 0.05% NaF solution. Figure 3. Cyclic voltammograms of alloys A, B and C in 0.05% NaF solution. times greater, thus showing a low corrosion resistance. 3.3. Cyclic Voltammetry Figure 3 shows the cyclic voltammograms of alloys A, B and C in 0.05% NaF, pH 6 and 37˚C in the interval be- tween –600 mV and 600 mV where it can be observed the alloy A corrosion and alloys B and C passivation. The alloy C shows the largest passivity range (approximately 800 mV). Alloys B and C have showed the greatest dis- ruption potential in terms of passive films to be more re- sistant. Another interesting parameter is the current den- sity, which indicates alloy reactivity in direct order, namely, the higher the current density, the higher the alloy reactivity, where alloy A owns the highest current density. The potential interval registered for the alloy A has been lesser due to its generalized dissolution in potential values higher than –200 mV. When the scan has been reversed, a hysteresis loop appeared, denoting an acti ve state. 4. Conclusion A distinct behavior has been verified when the potentio- dynamic profiles of three different compositions used in the dental market in NaF 0.05% medium are compared, showing that the highest Ni content to the alloy, coupled with the lowest Cr content, results in the greatest corro- sion susceptibility. REFERENCES [1] A. C. Fraker, “Corrosion of Metallic Implants and Pros- Thesis Devices,” In: ASTM International, Ed., ASTM Metals Handbook, Metals Park, Ohio, Vol. 13, 1987, pp. 1324-1335. [2] M. F. Leclerc, “Surgical Implants,” In: ASTM Interna- tional, Ed., ASTM Metals Handbook, Metals Park, Ohio, Copyright © 2010 SciRes. MSA  Influence of Ni and Cr Content on Corrosion Resistance of Ni-Cr-Mo Alloys for Fixed Dental Prostheses in 0.05% NaF Aqueous Solution Copyright © 2010 SciRes. MSA 372 Vol. 02, 1987, pp.164-180. [3] G. R. Baran, “The Metallurgy of Ni-Cr Alloys for Fixed Pros-Thodontics,” Journal of Prosthetics Dentistry, Vol. 50, No. 5, 1983, pp. 639-650. [4] American Society of Metals, “Properties and Selection: Nonferrous Alloys and Special-Purpose Materials,” In: ASTM International, Ed., ASTM Metals Handbook, Met- als Park, Ohio, Vol. 02, 1990. [5] J. W. Schultze and M. M. Lohrengel, “Stability, Reactiv- ity and Breakdown of Passive Films. Problems of Recent and Future Research,” Electrochimica Acta, 2000, Vol. 45, pp. 2499-2513. [6] P. Schmuki, “From Bacon to Barriers: A Review on the Passivity of Metals and Alloys,” Journal of Solid State Eletrochemistry, Vol. 6, 2002, pp. 145-164. [7] I. Milosev and H.-H. Strehblow, “The Behavior of Stainless Steels in Physiological Solution Containing Complexing Agent Studied by X-Ray Photoelectron Spectroscopy,” Journal of Biomedical Materials Re- search, Vol. 52, 2000, pp. 404-412. [8] N. Chiff, B. Grosgogeat, M. Lissac and F. Dalard, “In fluence of Fluoridated Mouthwashes on Corrosion Resis- tance of Orthodontics Wires,” Biomaterials, Vol. 25, 2004, pp. 4335-4544. [9] A. M. Al-Mayouf, A. A. Al-Swayih, N. A. Al-Mobarak and A. S. Al-Jabab “Corrosion Behavior of a New Tita- nium Alloy for Dental Implant Applications in Fluoride Media,” Materials Chemistry And Physics, Vol. 86, May 2004, pp. 320-329. [10] M. C. R A. Rezende, A. P. R. Alves, E. N. Codaro and C. A M. Dutra, “Effect of Commercial Mouthwashes on the Corrosion Resistance of Ti-10Mo Experimental Alloy,” Mater.Science: Materials in Medicine, Vol. 18, 2007, pp. 149-154. [11] H. H. Huang, “Surface Characterization of Passive Film on NiCr-Based Dental Casting Alloys,” Biomaterials, April 2003, Vol. 24, No. 9, pp. 1575-1582. [12] L. Reclaru and J.-M. Meyer, “Study of Corrosion be- tween a Titanium Implant and Dental Alloys,” J Dent, Vol. 22, March 1994, No. 3, pp. 159-168. [13] S. P. Kedici, A. A. Aksüt, M. Ali Kílíçarsla n, G. Bayramo g Lu and K. Gökdemir, “Corrosion Behavior of Dental Metals and Alloys in different Media,” Journal of Oral Rehabilitation, October 1998, Vol. 25, pp. 800-808. [14] K. J. Anusavice, Phillips materiais dentários, 10th Edition, Guanabara Koogan, Rio de Janeiro, 1998. |

