Paper Menu >>
Journal Menu >>
 Neuroscience & Medicine, 2010, 1, 43-49 doi:10.4236/nm.2010.12007 Published Online December 2010 (http://www.SciRP.org/journal/nm) Copyright © 2010 SciRes. NM 43 Serum AChE Activities Predict Exercise Heart Rate Parameters of Asymptomatic Individuals Jonathan Canaani1, Shani Shenhar-Tsarfaty1, Nir Weiskopf2, Reut Yakobi1, Einor Ben Assayag1, Shlomo Berliner1, Hermona Soreq2‡ J. Canaani and S. Shenhar-Tsarfaty contributed equally to this work 1The Tel Aviv Sourasky Medical Center, Israel; 2The Life Sciences Institute, The Hebrew University, Jerusalem, Israel. ‡To whom correspondence should be addressed at the above address: Hermona Soreq, PhD, Biological Chemistry Department, The Hebrew University of Jerusalem, Givat Ram, Jerusalem, Israel. Email: soreq@cc.huji.ac.il Received August 21st, 2010; revised September 15th, 2010; accepted September 17th, 2010 ABSTRACT Background specific hea rt rate parameters notably associate with variab le risks of cardiovascular disease and mortal- ity, however, to date there are no readily available blood tests associated with these parameters. Because of the estab- lished parasympathetic contributions towards cardiac regulation, we challenged the working hypothesis that serum acetylcholinesterase (AChE) activity is involved. Methods A total of 403 Healthy men and women were included in the study and underwent treadmill exercise testing. Prior to exercise testing the subject’s serum AChE activity levels were assessed by measurin g r ates o f acetylt hi oc ho li ne hydrolysis. Results In male subjects AChE activity was positively cor- related to resting heart rate (r = 0.210, p = 0.001). Complementing this observation, AChE activity was negatively correlated to the exercise-induced heart rate increase (r = –0.181, p = 0.005) and to heart rate recovery at 1, 2 and 5 minutes following cessation of exercise (r = –0.150, p = 0.022; r = –0.157, p = 0.016; r = –0.176, p = 0.008 respec- tively). This indicated that lower than average AChE activities, which presumably reflect increased peripheral ACh levels, might be correlated to favorable heart rate parameters. Similar observations were made in female subjects, ex- cept for lack of correlation to their resting heart rate. Additionally, we observed that we were able to stratify subjects into two groups of significan tly d ifferent AChE activity (p = 0.001 ) ba sed on a cut poin t of h eart ra te reco very belo w 20 beats one minute after cessation of exercise. Conclusion In asymptomatic individuals lower than average AChE activity is associated with favorable indices of exercise-inducible heart rate increase as well as heart rate recovery. Future studies will be needed to evaluate the added prognostic significance gained by implementing this marker into routine practice. Keywords: Exercise, Nervous System, Autonomic, Heart Rate 1. Introduction A vast body of clinical and experimental evidence indi- cates that cardiovascular functioning is tightly linked to autonomous nervous system (ANS) activities. Prior studies have shown that autonomic imbalance, being either a decrease in vagal tone or an increase in sympa- thetic activity is associated with increased mortality due to cardiovascular pathologies including myocardial in- farction [1,2] and cardiac arrhythmias [3]. Indirect meas- ures of cardiac parasympathetic dysfunction obtained during exercise testing such as elevated resting heart rate, delayed heart rate recovery (HRR) from exercise and attenuated heart rate increase (HRI) during exercise have been shown to be independent predictors for adverse cardiovascular outcome [4-6]. The principal neurotransmitter of the parasympathetic branch of the ANS is acetylcholine (ACh), the levels of which are regulated by the enzyme acetylcholinesterase (AChE) and the closely related butyrylcholinesterase (BChE), collectively determining the total cholinesterase activity [7]. Based on previous studies demonstrating the protective effect of ACh on ischemic myocardium [8,9] and that administration of pyridostigmine, an inhibitor of AChE, improved HRR and resting heart rate both in healthy subjects [10] and in heart failure patients [11], we sought to determine whether the peripheral serum levels of AChE and BChE (i.e. the total cholinesterase activity tested by aspecific assay [12]) can serve as bio- markers for assessing the aforementioned parameters of  Serum AChE Activities Predict Exercise Heart Rate Parameters of Asymptomatic Individuals Copyright © 2010 SciRes. NM 44 autonomic activity. Specifically, we hypothesized that in apparently healthy individuals, increased serum levels of AChE and/or BChE, which presumably reflect lower peripheral ACh levels would be correlated with an abnormal heart rate profile including resting heart rate, heart rate in- crease during exercise and heart rate recovery after exer- cise. 2. Methods 2.1. Study Population The study included 403 men and women who were re- cruited to the Tel Aviv Medical Center Inflammation Survey (TAMCIS) which comprises a population of ap- parently healthy individuals attending the Tel Aviv Sourasky Medical Center for routine health examinations [13,14]. Exclusion criteria included underlying inflammatory dis- ease (arthritis, inflammatory bowel disease, etc.), as well as any infections or other inflammatory conditions such as myocardial infarction, surgery or angiography during the 6 months preceding study enrollment. Cancer patients were excluded as well. To minimize the effect of some important confounders, we excluded an additional 44 subjects based on their medication usage, including alpha and beta blockers and Amiodarone (owing to their chrono- tropic effects). All individuals included in the present study provided written consent according to the instruc- tions of the Institutional Ethics Committee which ap- proved this study (approval number 02-049). 2.2. Clinical Data Prior to exercise testing, a review of each patient’s chart and a structured interview were conducted to gather data on symptoms, medications, coronary risk factors, previ- ous cardiac events, and other diagnoses. Hypertension was defined as a systolic blood pressure of ≥ 140 mm Hg at rest, a diastolic blood pressure of ≥ 90 mm Hg at rest in two separate measurements, or treatment with anti- hypertensive medication. Dyslipidemia was defined as low density lipoprotein (LDL) cholesterol concentration or non-high density lipoprotein (HDL) cholesterol con- centrations, for individuals with triglyceride concentra- tions of > 200 mg/dl, above the recommended number according to the risk profile defined by the updated ATP III recommendations [15] or the use of lipid-lowering medication. 2.3. Exercise Test Protocol All subjects performed treadmill exercise testing using the standardized Bruce protocol [16]. The Quinton Q STRESS TM 55 (Quinton Instrument Company, Bothell, Washington) hardware and software were used for recording and analyz- ing stress test data. Heart rate was measured at rest, before exercise, and every two minutes during exercise, at peak ex- ercise, and at one, two and five minutes during recovery. The heart-rate increase was defined as the difference between the peak exercise rate and the resting rate, and heart-rate recovery was defined as the reduction in rate from the peak exercise level to the rate one, two and five minutes following cessa- tion of exercise. Exercise capacity was measured in watts, which were converted to maximum oxygen consumption calculated as metabolic equivalents (MET) [17]. 2.4. Enzyme Activity Measurements Blood was drawn in the morning (7:15-10:30 am) after a fasting period of at least 12 hours. Following centrifuga- tion, serum samples were checked for lack of red blood cells contaminants and were then stored at –80˚C until cholinesterase activities were determined. AChE activity levels were assessed in a microtiter plate assay by meas- uring rates of acetylthiocholine (ATCh, Sigma, 1 mM) hydrolysis following 20 min pre-incubation in the dark, with 500 micromolar tetraisopropyl pyrophosphoramide (iso-OMPA, Sigma), a specific BChE inhibitor [18]. The non-enzymatic breakdown of substrate was subtracted from the total rate of hydrolysis. Enzyme activities were calculated using the e405 for 5-thio-2-nitrobenzoate, 13.600 M/cm [19]. The total cholinesterase activity (combined activity of AChE and BChE) was determined by measur- ing the rates of ATCh hydrolysis without a cholinesterase inhibitor [12]. All laboratory methods were performed by a blinded technician, each method by the same person for all measures. Several samples were routinely thawed and re-tested in subsequent days to evaluate between-days variability of measurements, which were found to be lower than 10%, excluding batch effects. 2.5. Statistical Analysis Collected data was summarized and displayed as mean SD, Continuous values with non-Gaussian distributions were compared by the Wilcoxon rank-sum or Mann- Whitney U test. The 2 test was used to assess associa- tions among categorical variables. Correlations between the cholinesterase activities and exercise test parameters were determined using the two-tailed Spearman rank correlation. Significance was set at p < 0.05. Analyzing data with the One-Sample Kolmogorov-Smirnov test yielded a p-value of 0.001, indicating that AChE activity did not distribute normally. SPSS/WIN (version 15.0, SPSS INC, Chicago, IL, USA) software was used to carry out all statistical analyses. 3. Results Baseline clinical characteristics of study subjects are listed in Table 1. The study cohort consisted of 403 sub- 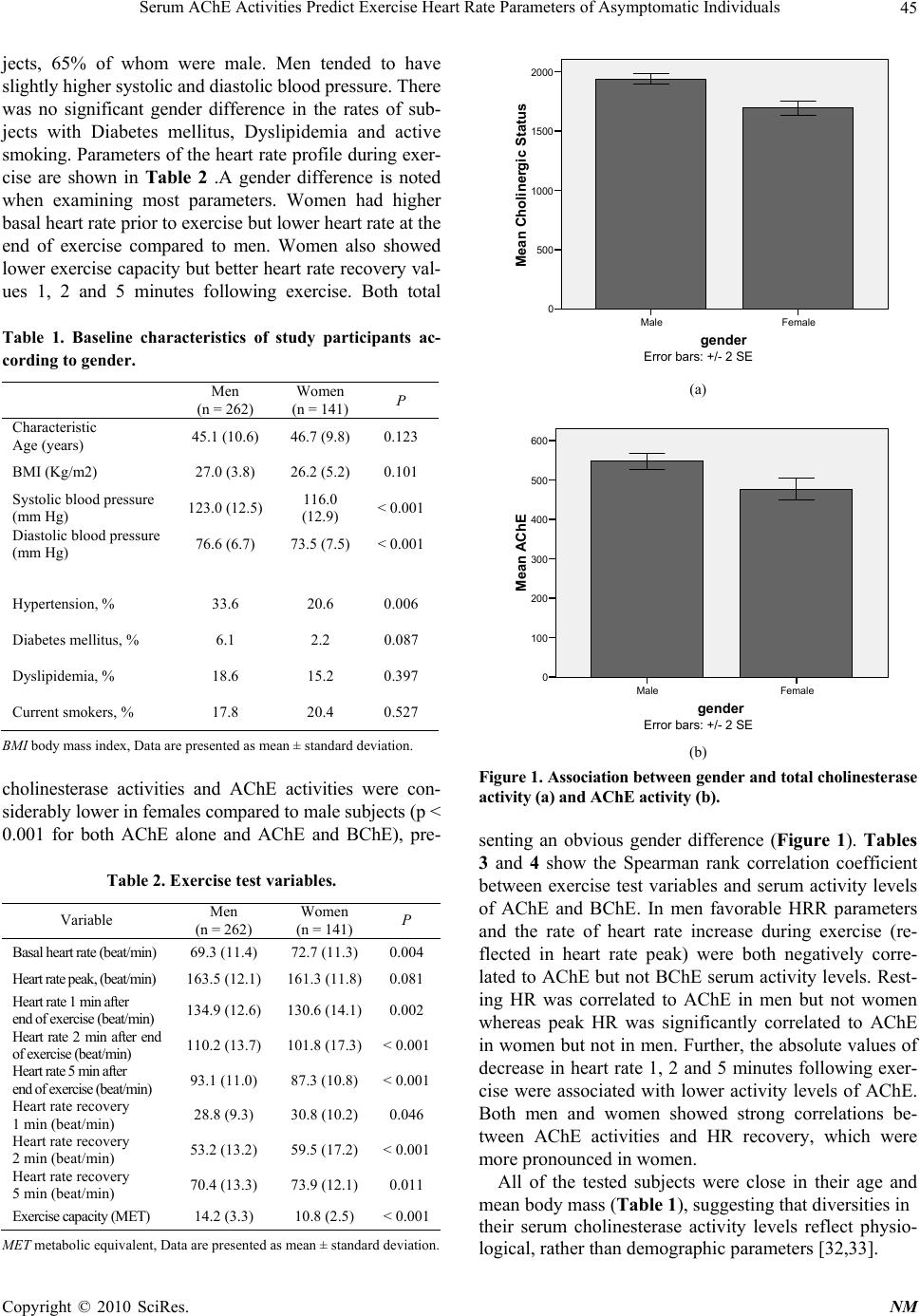 Serum AChE Activities Predict Exercise Heart Rate Parameters of Asymptomatic Individuals Copyright © 2010 SciRes. NM 45 jects, 65% of whom were male. Men tended to have slightly higher systolic and diastolic blood pressure. There was no significant gender difference in the rates of sub- jects with Diabetes mellitus, Dyslipidemia and active smoking. Parameters of the heart rate profile during exer- cise are shown in Table 2 .A gender difference is noted when examining most parameters. Women had higher basal heart rate prior to exercise but lower heart rate at the end of exercise compared to men. Women also showed lower exercise capacity but better heart rate recovery val- ues 1, 2 and 5 minutes following exercise. Both total Table 1. Baseline characteristics of study participants ac- cording to gender. Men (n = 262) Women (n = 141) P Characteristic Age (years) 45.1 (10.6) 46.7 (9.8) 0.123 BMI (Kg/m2) 27.0 (3.8) 26.2 (5.2) 0.101 Systolic blood pressure (mm Hg) 123.0 (12.5) 116.0 (12.9) < 0.001 Diastolic blood pressure (mm Hg) 76.6 (6.7) 73.5 (7.5) < 0.001 Hypertension, % 33.6 20.6 0.006 Diabetes mellitus, % 6.1 2.2 0.087 Dyslipidemia, % 18.6 15.2 0.397 Current smokers, % 17.8 20.4 0.527 BMI body mass index, Data are presented as mean ± standard deviation. cholinesterase activities and AChE activities were con- siderably lower in females compared to male subjects (p < 0.001 for both AChE alone and AChE and BChE), pre- Table 2. Exercise test variables. Variable Men (n = 262) Women (n = 141) P Basal heart rate (beat/min) 69.3 (11.4) 72.7 (11.3) 0.004 Heart rate peak, (beat/min) 163.5 (12.1) 161.3 (11.8)0.081 Heart rate 1 min after end of exercise (beat/min) 134.9 (12.6) 130.6 (14.1)0.002 Heart rate 2 min after end of exercise (beat/min) 110.2 (13.7) 101.8 (17.3)< 0.001 Heart rate 5 min after end of exercise (beat/min) 93.1 (11.0) 87.3 (10.8) < 0.001 Heart rate recovery 1 min (beat/min) 28.8 (9.3) 30.8 (10.2) 0.046 Heart rate recovery 2 min (beat/min) 53.2 (13.2) 59.5 (17.2) < 0.001 Heart rate recovery 5 min (beat/min) 70.4 (13.3) 73.9 (12.1) 0.011 Exercise capacity (MET) 14.2 (3.3) 10.8 (2.5) < 0.001 MET metabolic equivalent, Data are presented as mean ± standard deviation. gender FemaleMale Mean Cholinergic Status 2000 1500 1000 500 0 Error bars: +/- 2 SE (a) gender FemaleMale Mean AChE 600 500 400 300 200 100 0 Error bars: +/- 2 SE (b) Figure 1. Association between gender and total cholinesterase activity (a) and AChE activity (b). senting an obvious gender difference (Figure 1). Tables 3 and 4 show the Spearman rank correlation coefficient between exercise test variables and serum activity levels of AChE and BChE. In men favorable HRR parameters and the rate of heart rate increase during exercise (re- flected in heart rate peak) were both negatively corre- lated to AChE but not BChE serum activity levels. Rest- ing HR was correlated to AChE in men but not women whereas peak HR was significantly correlated to AChE in women but not in men. Further, the absolute values of decrease in heart rate 1, 2 and 5 minutes following exer- cise were associated with lower activity levels of AChE. Both men and women showed strong correlations be- tween AChE activities and HR recovery, which were more pronounced in women. All of the tested subjects were close in their age and mean body mass (Table 1), suggesting that diversities in their serum cholinesterase activity levels reflect physio- logical, rather than demographic parameters [32,33]. 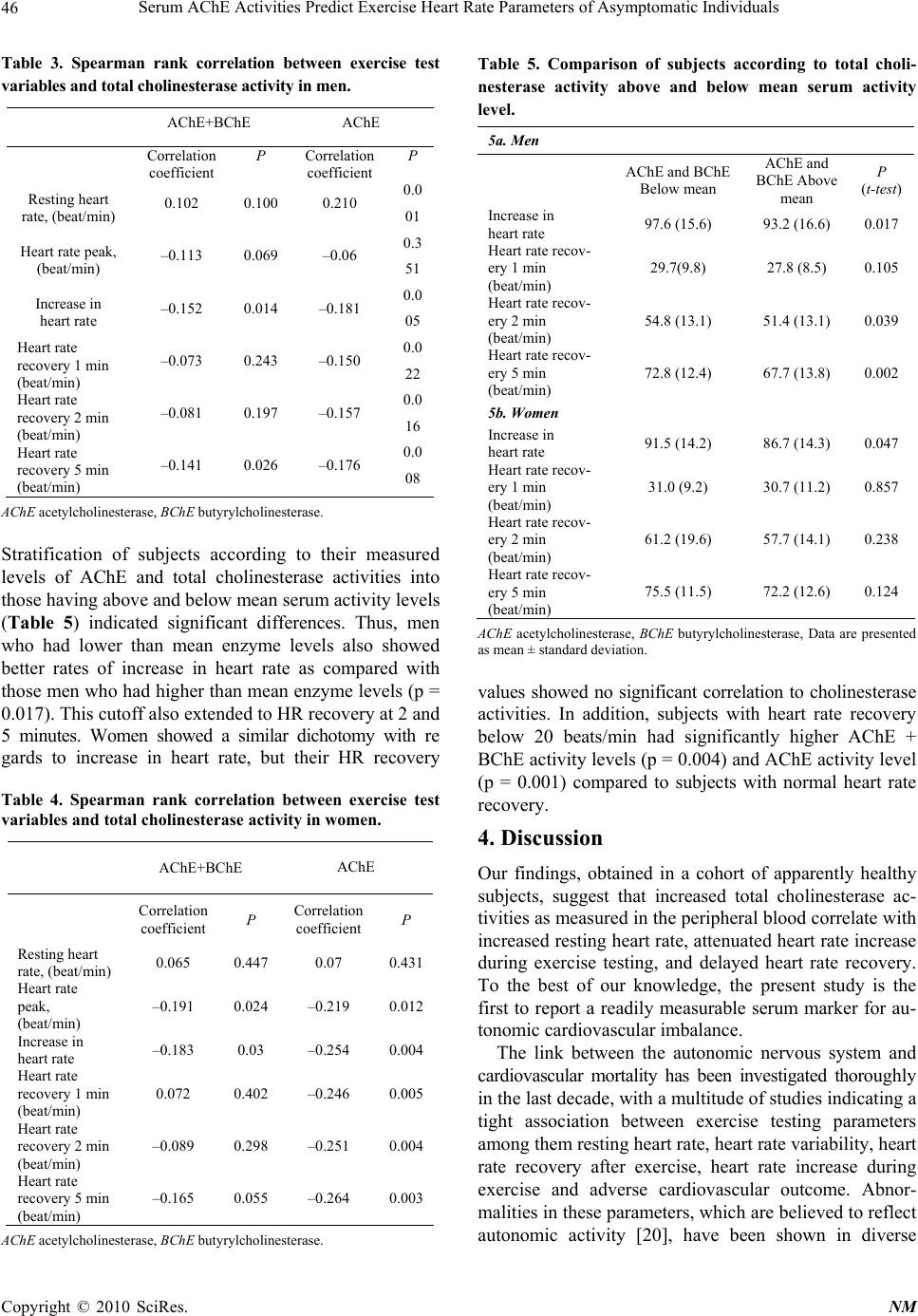 Serum AChE Activities Predict Exercise Heart Rate Parameters of Asymptomatic Individuals Copyright © 2010 SciRes. NM 46 Table 3. Spearman rank correlation between exercise test variables and total cholinesterase activity in men. AChE+BChE AChE Correlation coefficient P Correlation coefficient P Resting heart rate, (beat/min) 0.102 0.100 0.210 0.0 01 Heart rate peak, (beat/min) –0.113 0.069 –0.06 0.3 51 Increase in heart rate –0.152 0.014 –0.181 0.0 05 Heart rate recovery 1 min (beat/min) –0.073 0.243 –0.150 0.0 22 Heart rate recovery 2 min (beat/min) –0.081 0.197 –0.157 0.0 16 Heart rate recovery 5 min (beat/min) –0.141 0.026 –0.176 0.0 08 AChE acetylcholinesterase, BChE butyrylcholinesterase. Stratification of subjects according to their measured levels of AChE and total cholinesterase activities into those having above and below mean serum activity levels (Table 5) indicated significant differences. Thus, men who had lower than mean enzyme levels also showed better rates of increase in heart rate as compared with those men who had higher than mean enzyme levels (p = 0.017). This cutoff also extended to HR recovery at 2 and 5 minutes. Women showed a similar dichotomy with re gards to increase in heart rate, but their HR recovery Table 4. Spearman rank correlation between exercise test variables and total cholinesterase activity in women. AChE+BChE AChE Correlation coefficient P Correlation coefficient P Resting heart rate, (beat/min) 0.065 0.447 0.07 0.431 Heart rate peak, (beat/min) –0.191 0.024 –0.219 0.012 Increase in heart rate –0.183 0.03 –0.254 0.004 Heart rate recovery 1 min (beat/min) 0.072 0.402 –0.246 0.005 Heart rate recovery 2 min (beat/min) –0.089 0.298 –0.251 0.004 Heart rate recovery 5 min (beat/min) –0.165 0.055 –0.264 0.003 AChE acetylcholinesterase, BChE butyrylcholinesterase. Table 5. Comparison of subjects according to total choli- nesterase activity above and below mean serum activity level. 5a. Men AChE and BChE Below mean AChE and BChE Above mean P (t-test) Increase in heart rate 97.6 (15.6) 93.2 (16.6) 0.017 Heart rate recov- ery 1 min (beat/min) 29.7(9.8) 27.8 (8.5) 0.105 Heart rate recov- ery 2 min (beat/min) 54.8 (13.1) 51.4 (13.1) 0.039 Heart rate recov- ery 5 min (beat/min) 72.8 (12.4) 67.7 (13.8) 0.002 5b. Women Increase in heart rate 91.5 (14.2) 86.7 (14.3) 0.047 Heart rate recov- ery 1 min (beat/min) 31.0 (9.2) 30.7 (11.2) 0.857 Heart rate recov- ery 2 min (beat/min) 61.2 (19.6) 57.7 (14.1) 0.238 Heart rate recov- ery 5 min (beat/min) 75.5 (11.5) 72.2 (12.6) 0.124 AChE acetylcholinesterase, BChE butyrylcholinesterase, Data are presented as mean ± standard deviation. values showed no significant correlation to cholinesterase activities. In addition, subjects with heart rate recovery below 20 beats/min had significantly higher AChE + BChE activity levels (p = 0.004) and AChE activity level (p = 0.001) compared to subjects with normal heart rate recovery. 4. Discussion Our findings, obtained in a cohort of apparently healthy subjects, suggest that increased total cholinesterase ac- tivities as measured in the peripheral blood correlate with increased resting heart rate, attenuated heart rate increase during exercise testing, and delayed heart rate recovery. To the best of our knowledge, the present study is the first to report a readily measurable serum marker for au- tonomic cardiovascular imbalance. The link between the autonomic nervous system and cardiovascular mortality has been investigated thoroughly in the last decade, with a multitude of studies indicating a tight association between exercise testing parameters among them resting heart rate, heart rate variability, heart rate recovery after exercise, heart rate increase during exercise and adverse cardiovascular outcome. Abnor- malities in these parameters, which are believed to reflect autonomic activity [20], have been shown in diverse  Serum AChE Activities Predict Exercise Heart Rate Parameters of Asymptomatic Individuals Copyright © 2010 SciRes. NM 47 study populations to be associated with sudden cardiac death in asymptomatic men (resting heart rate and heart rate recovery) [4,21], all cause mortality in cardiac pa- tients (heart rate recovery) [5,22], and all cause mortality and cardiovascular mortality correlated with attenuated heart rate increase [6,23]. These exercise test parameters are postulated to reflect ANS activity consisting mainly of parasympathetic con- trol and to a lesser degree of sympathetic components [20].Specifically, heart rate recovery and heart rate in- crease during exercise are presumed to be mainly con- trolled by the parasympathetic branch of the ANS [6] whereas resting heart rate is determined by both sympa- thetic and parasympathetic activities [24]. Considering that parasympathetic activity is mediated via ACh release from efferent vagal nerve discharge, we searched for possible associations between the peripheral activity of serum cholinesterases, AChE and BChE and exercise test parameters. Our results clearly demonstrate for both genders that decreased activity of AChE, most likely reflecting increased peripheral ACh levels, was corre- lated to faster heart rate recovery 1,2 and 5 minutes fol- lowing cessation of exercise. Additionally, we found that decreased AChE activity was correlated with a rapid heart rate increase in both genders and more profoundly so in women. Two findings which showed genders speci- fity were that higher than average heart rate peak was correlated to decreased AChE activity in women only and that higher than average resting heart rate was asso- ciated with increased AChE activity in men only. An- other interesting observation was that using a cut point of 20 beats per minute we were able to stratify subjects into two groups of significantly different AChE activity. These results are in accordance with previous studies. First, Kakinuma et al. [8] have shown that in a model of acute myocardial ischemia, increasing local ACh secre- tion in rat cardiomyocytes by electrical stimulation of the vagal efferent nerve decreased infarct size via the PI3K/ Akt/HIF-1alpha pathway. Complementing this study, Ando et al. [9] demonstrated, also in a rat model of myocardial ischemia, that vagal nerve stimulation which elevates release of ACh exerted antiarrhythmogenic effects ac- companied by prevention of the loss of phosphorylated Cx43 during acute myocardial infarction. In line with these data, atria from elderly and diabetic patients were shown to have locally reduced ACh secretion, thus re- flecting impaired local parasympathetic activity [25]. Second, previous studies by Katz and coworkers have characterized the systemic effect of AChE inhibition with Pyridostigmine, a short-acting, brain-impenetrable re- versible AChE inhibitor which only affects peripheral cholinergic activity. The first study, in chronic heart fail- ure (CHF) patients, showed marked improvement in heart rate recovery [11] and a more recent work showed that Pyridostigmine improved heart rate recovery and resting heart rate in sedentary adults [10]. Strengths of our study include the exclusion of sub- jects using beta blockers, since the issue of the potential effects of beta blockers on heart rate recovery is still un- resolved [29]. Previous studies used statistical adjustment as a means of rectifying this effect, but we chose to re- move this possible confounding factor altogether. Addi- tionally, our study population consisted of relatively young subjects without any overt cardiovascular disease thus enabling us to characterize their total cholinesterase activities with less possible confounding factors as would be expected in patients with known cardiovascular dis- ease. Finally, heart rate recovery is a modifiable factor as was previously shown in exercise training of cardiovas- cular patients [30,31] and with pharmacological therapy with AChE inhibition as noted above. The limitations of our study involve our ignoring of psychological and/or inflammatory confounding factors; thus, higher than average serum AChE activity may be due to a transient anxiety state [32] or reflect the out- come of infection [12], in which case it would not nec- essarily predict cardiovascular risk. Additionally, inher- ited and/or experience-derived changes in the total cho- linesterase activities may be caused by polymorphisms in these and/or other genes, e.g. paraoxonase [33] or by altered micro-RNA levels under stress [34,35]. The ap- parent associations between serum cholinesterase activi- ties and cardiovascular parameters thus merit further in-depth studies. 5. Conclusions Our study links heart rate parameters during exercise testing to a simple, measurable serum marker. We be- lieve that our findings provide important additional in- sights into the pathophysiology of ANS dysfunction in cardiovascular disease. In view of previous research our findings suggest a possible future avenue for novel ther- apy modalities of autonomic imbalance. Future studies will be needed to evaluate the added prognostic signifi- cance gained by implementing this marker into routine practice. REFERENCES [1] P. J. Schwartz, M. T. La Rovere and E. Vanoli, “Auto- nomic nervous system and sudden cardiac death. Experi- mental basis and clinical observations for post-myocardial infarction risk stratification,” Circulation, Vol. 85, No. S1, 1992, pp. 77-91. [2] P. J. Schwartz, “The Autonomic Nervous System and  Serum AChE Activities Predict Exercise Heart Rate Parameters of Asymptomatic Individuals Copyright © 2010 SciRes. NM 48 Sudden Death,” European Heart Journal, Vol. 19, 1998, pp. F72-F80. [3] M. T. La Rovere, G. D. Pinna, S. H. Hohnloser, F. I. Mar- cus, A. Mortara, R. Nohara, J. T. Bigger, A. J. Camm and P. J. Schwartz, “Baroreflex Sensitivity and Heart Rate Variability in the Identification of Patients at Risk for Life-Threatening Arrhythmias: Implications for Clinical Trials,” Circulation, Vol. 103, No. 16, 2001, pp. 2072- 2077. [4] X. Jouven, J. P. Empana, P. J. Schwartz, M. Desnos, D. Courbon and P. Ducimetiere, “Heart-Rate Profile during Exercise as a Predictor of Sudden Death,” New England Journal of Medicine, Vol. 352, No. 19, 2005, pp. 1951- 1958. doi:10.1056/NEJMoa043012 [5] C. R. Cole, E. H. Blackstone, F. J. Pashkow, C. E. Snader, M. S. Lauer, “Heart-Rate Recovery Immediately after Exercise as a Predictor of Mortality,” New England Jour- nal of Medicine, Vol. 341, No. 18, 1999, pp. 1351-1357. doi:10.1056/NEJM199910283411804 [6] N. J. Leeper, F. E. Dewey, E. A. Ashley, M. Sandri, S. Y. Tan, D. Hadley, J. Myers and V. Froelicher, “Prognostic Value of Heart Rate Increase at Onset of Exercise Test- ing,” Circulation, Vol. 115, No. 4, 2007, pp. 468-474. doi:10.1161/CIRCULATIONAHA.106.666388 [7] Y. Loewenstein-Lichtenstein, M. Schwarz, D. Glick, B. Norgaard-Pedersen, H. Zakut and H. Soreq, “Genetic pre- disposition to adverse consequences of anti-cholinesterases in ‘atypical’ BCHE carriers,” Nature Medicine, Vol. 1, No. 10, 1995, pp. 1082-1085. doi:10.1038/nm1095-1082 [8] Y. Kakinuma, M. Ando, M. Kuwabara, R. G. Katare, K. Okudela, M. Kobayashi and T. Sato, “Acetylcholine from Vagal Stimulation Protects Cardiomyocytes against Ische- mia and Hypoxia Involving Additive Non-Hypoxic In- duction of Hif-1alpha,” FEBS Letters, Vol. 579, No. 10, 2005, pp. 2111-2118. doi:10.1016/j.febslet.2005.02.065 [9] M. Ando, R. G. Katare, Y. Kakinuma, D. Zhang, F. Ya- masaki, K. Muramoto and T. Sato, “Efferent Vagal Nerve Stimulation Protects Heart against Ischemia-Induced Ar- rhythmias by Preserving Connexin43 Protein,” Circula- tion, Vol. 112, No. 2, 2005, pp. 164-170. doi:10.1161/CIRCULATIONAHA.104.525493 [10] T. A. Dewland, A. S. Androne, F. A. Lee, R. J. Lampert and S. D. Katz, “Effect of Acetylcholinesterase Inhibition with Pyridostigmine on Cardiac Parasympathetic Func- tion in Sedentary Adults and Trained Athletes,” American Journal of Physiology and Heart Circulation Physiology, Vol. 293, No. 1, 2007, pp. 86-92. doi:10.1152/ajpheart.01339.2006 [11] A. S. Androne, K. Hryniewicz, R. Goldsmith and A. Ar- wady, S. D. Katz, “Acetylcholinesterase Inhibition with Pyridostigmine Improves Heart Rate Recovery after Maximal Exercise in Patients with Chronic Heart Fail- ure,” Heart, Vol. 89, No. 8, 2003, pp. 854-858. doi:10.1136/heart.89.8.854 [12] K. Ofek, K. S. Krabbe, T. Evron, M. Debecco, A. R. Niel- sen, H. Brunnsgaad, R. Yirmiya, H. Soreq and B. K. Pedersen, “Cholinergic Status Modulations in Human Volunteers under Acute Inflammation,” Journal of Mo- lecular Medicine, Vol. 85, No. 11, 2007, pp. 1239-1251. doi:10.1007/s00109-007-0226-x [13] A. Steinvil, A. Shirom, S. Melamed, S. Toker, D. Justo, N. Saar, I. Shapira, S. Berliner and O. Rogowski, “Relation of Educational Level to Inflammation-Sensitive Bio- marker Level,” American Journal of Cardiology, Vol. 102, No. 8, 2008, pp. 1034-1039. doi:10.1016/j.amjcard.2008.05.05 5 [14] O. Rogowski, S. Toker, I. Shapira, S. Melamed, A. Shi- rom, D. Zeltser and S. Berliner, “Values of High-Sensitiv- ity C-Reactive Protein in Each Month of the Year in Ap- parently Healthy Individuals,” American Journal of Car- diology, Vol. 95, No. 1, 2005, pp. 152-155. doi:10.1016/j.amjcard.2004.08.08 6 [15] “Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III),” JAMA, Vol. 285, No. 19, 2001, pp. 2486-2497. [16] P. M. Rautaharju, R. J. Prineas, W. J. Eifler, C. D. Fur- berg, J. D. Neaton, R. S. Crow, J. Stamler and J. A. Cutler, “Prognostic Value of Exercise Electrocardiogram in Men at High Risk of Future Coronary Heart Disease: Multiple Risk Factor Intervention Trial Experience,” Journal of the American College of Cardiology, Vol. 8, No. 1, 1986, pp. 1-10. doi:10.1016/S0735-1097(8 6)80084 -5 [17] ACSM’s Metabolic Calculations Handbook, Philadelphia, Lippincott Williams & Wilkins, PA, 2006. [18] A. Berson, M. Knobloch, M. Hanan, S. Diamant, M. Sharoni, D. Schuppli, B. C. Geyer, R. Ravid, T. S. Mor, R. M. Nitsch and H. Soreq, “Changes in Readthrough Ace- tylcholinesterase Expression Modulate Amyloid-Beta Pathology,” Brain, Vol. 131, No. Pt1, 2008, pp. 109-119. [19] A. Gilboa-Geffen, P. P. Lacoste, L. Soreq, G. Cize- ron-Clairac, R. Le Panse, F. Truffault, I. Shaked, H. Soreq and S. Berrih-Aknin, “The Thymic Theme of Ace- tylcholinesterase Splice Variants in Myasthenia Gravis,” Blood, Vol. 109, No. 10, 2007, pp. 4383-4391. doi:10.1182/blood-2006- 07-0333 73 [20] M. K. Lahiri, P. J. Kannankeril, J. J. Goldberger, “As- sessment of Autonomic Function in Cardiovascular Dis- ease: Physiological Basis and Prognostic Implications,” Journal of the American College of Cardiology, Vol. 51, No. 18, 2008, pp. 1725-1733. doi:10.1016/j.jacc.2008.01.038 [21] Adabag AS, Grandits GA, Prineas RJ, Crow RS, Bloom- field HE, Neaton JD. Relation of heart rate parameters during exercise test to sudden death and all-cause mortal- ity in asymptomatic men. American Journal of Cardiol- ogy 2008; 101(10):1437-1443. doi:10.1016/j.amjcard.2008.01.02 1 [22] R. Arena, M. Guazzi, J. Myers and M. A. Peberdy, “Prog- nostic Value of Heart Rate Recovery in Patients with Heart Failure,” American Heart Journal, Vol. 151, No. 4, 2006, pp. 851-857. doi:10.1016/j.ahj.2005.09.012 [23] K. P. Savonen, V. Kiviniemi, J. A. Laukkanen, T. A. Lakka, T. H. Rauramaa, J. T. Salonen and R. Rauramaa,  Serum AChE Activities Predict Exercise Heart Rate Parameters of Asymptomatic Individuals Copyright © 2010 SciRes. NM 49 “Chronotropic Incompetence and Mortality in Mid- dle-Aged Men with Known or Suspected Coronary Heart Disease,” European Heart Journal, Vol. 29, No. 15, 2008, pp. 1896-1902. doi:10.1093/eurheartj/ehn269 [24] D. Robertson, G. A. Johnson, R. M. Robertson, A. S. Nies, D. G. Shand and J. A. Oates, “Comparative As- sessment of Stimuli That Release Neuronal and Adrenome- dullary Catecholamines in Man,” Circulation, Vol. 59, No. 4, 1979, pp. 637-643. [25] V. Oberhauser, E. Schwertfeger, T. Rutz, F. Beyersdorf and L. C. Rump, “Acetylcholine Release in Human Heart Atrium: Influence of Muscarinic Autoreceptors, Diabetes, and Age,” Circulation, Vol. 103, No. 12, 2001, pp. 1638-1643. [26] D. P. Vivekananthan, E. H. Blackstone, C. E. Pothier and M. S. Lauer, “Heart Rate Recovery after Exercise is a Predictor of Mortality, Independent of the Angiographic Severity of Coronary Disease,” Journal of the American College of Cardiology, Vol. 42, No. 5, 2003, pp. 831-838. doi:10.1016/S0735-1097(03)00833-7 [27] B. Aijaz, R. W. Squires, R. J. Thomas, B. D. Johnson and T. G. Allison, “Predictive Value of Heart Rate Recovery and Peak Oxygen Consumption for Long-Term Mortality in Patients with Coronary Heart Disease,” American Journal of Cardiology, Vol. 103, No. 12, 2009, pp. 1641-1646. doi:10.1016/j.amjcard.2009.02.013 [28] L. R. Davrath, S. Akselrod, I. Pinhas, E. Toledo, A. Beck, D. Elian and M. Scheinowitz, “Evaluation of Autonomic Function Underlying Slow Postexercise Heart Rate Re- covery,” Medical Science Sports Exercise, Vol. 38, No. 12, 2006, pp. 2095-2101. doi:10.1249 /01. mss.0 0002 3536 0.243 08. c7 [29] A. P. Morise, “Heart Rate Recovery: Predictor of Risk Today and Target of Therapy Tomorrow?” Circulation, Vol. 110, No. 18, 2004, pp. 2778-2780. doi:10.1161/01.CIR.0000147615.62634.48 [30] P. Kligfield, A. McCormick, A. Chai, A. Jacobson, P. Feuerstadt and S. C. Hao, “Effect of Age and Gender on Heart Rate Recovery after Submaximal Exercise during Cardiac Rehabilitation in Patients with Angina Pectoris, Recent Acute Myocardial Infarction, or Coronary Bypass Surgery,” American Journal of Cardiology, Vol. 92, No. 5, 2003, pp. 600-603. doi:10.1016/S0002-9149(03)00733-1 [31] J. Myers, D. Hadley, U. Oswald, K. Bruner, W. Kottman, L. Hsu and P. Dubach, “Effects of Exercise Training on Heart Rate Recovery in Patients with Chronic Heart Fail- ure,” American Heart Journal, Vol. 153, No. 6, 2007, pp. 1056-1063. doi:10.1016/j.ahj.2007.02.038 [32] E. H. Sklan, A. Lowenthal, M. Korner, Y. Ritov, D. M. Landers, T. Rankinen, C. Bouchard, A. S. Leon, T. Rice, D. C. Rao, J. H. Wilmore, J. S. Skinner and H. Soreq, “Acetylcholinesterase/Paraoxonase Genotype and Ex- pression Predict Anxiety Scores in Health, Risk Factors, Exercise Training, and Genetics Study,” Proceedings of the National Academy of Sciences USA, Vol. 101, No. 15, 2004, pp. 5512-5517. doi:10.1073/pnas.0307659101 [33] B. Bryk, L. BenMoyal-Segal, E. Podoly, O. Livnah, A. Eisenkraft, S. Luria, A. Cohen, Y. Yehezkelli, A. Hour- vitz and H. Soreq, “Inherited and Acquired Interactions between Ache and Pon1 Polymorphisms Modulate Plasma Acetylcholinesterase and Paraoxonase Activities,” Journal of Neurochemistry, Vol. 92, No. 5, 2005, pp. 1216-1227. doi:10.1111/j.1471-4159.2004.02959.x [34] C. Guimaraes-Sternberg, A. Meerson, I. Shaked and H. Soreq, “MicroRNA Modulation of Megakaryoblast Fate Involves Cholinergic Signaling,” Leukocyte Research, Vol. 30, No. 5, 2006, pp. 583-595. doi:10.1016/j.leukres.2005.09.005 [35] I. Shaked, A. Meerson, Y. Wolf, R. Avni, D. Greenberg, A. Gilboa-Geffen and H. Soreq, “MicroRNA-132 Poten- tiates Cholinergic Anti-Inflammatory Signaling by Tar- geting Acetylcholinesterase,” Immunity, Vol. 31, No. 6, 2010, pp. 965-73. doi:10.1016/j.immuni.2009.09.019 |

