Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio ns, 2010, 1, 358-368 doi:10.4236/msa.2010.16052 Published Online December 2010 (http://www.scirp.org/journal/msa) Copyright © 2010 SciRes. MSA Plasticization Effect on the Photodegradation of Poly (4-Chlorostyrene) and Poly (4-Bromostyrene) Films Khalid E. Al Ani1, Afrah Essa Ramadhan2 1Department of Petroleum Engineering, The British University of Science and Technology, Irbil, Iraq; 2Department of Industrial Chemistry, Institute of Technology, Baghdad, Iraq. E-mail: khalidalani44@yahoo.com Received September 15th, 2010; revised October 16th, 2010; accepted November 19th, 2010. ABSTRACT The photodegradation of thin films of poly (4-chlorostyrene) and poly (4-bromostyrene) with 265 nm radiation in the presence of oxygen and as a function of irradiation time has been studied mainly using fluorescence, FT-IR, and UV-VIS spectroscopic techniques. The influence of phthalate and terephthalate plasticizers on photo-oxidative degra- dation was also investigated. Phthalate and terephthalate-plasticizers were found to increase the photodegradation processes in polymeric chains. On the other hand, the intensity of absorption was also found to increase with irradia- tion time and in the intensity of a new absorption band at longer wavelength. The appearance of new fluorescence bands in the irrad iated polymer films can well indicate a possibility o f photodegradation of po lymer films. In addition, the observed increase in the intensities of the carbonyl and hydroxyl regions of the FT-IR spectra, providing evidence for the photodegradation as well as the photo-oxidation of polymeric chains. The increase in the analyzed ranges was attributed to the forma tion of alcohols, aliphatic keton es and to the increase in the number of (C=C) that resulted from hydrogen abstraction during chains-scission. Keywords: Excimer Fluorescence, Poly (4-Bromostyrene), Poly (4-Chlorostyrene), Photodegradation Kinetics, Phthalate Plasticizers 1. Introduction The study of degradation and stabilization of polymers is significant for both practical and theoritical viewpoints [1]. The thermal behaviour of halogenated polymers re- ceived a considerable attention, owing mainly to the in- dustrial importance of these polymers [2-6]. Indeed thermal degradation behavior of poly (4-bromostyrene) (PBS), and Poly (4-chlorostyrene) (PCS), has been found to be closely analogous to that of polystyrene (PS) [7]. A blend of PBS with poly (methyl methacrylate) showed some interaction during degradation resulting in some stabilization of the PBS. Thus, bromonation and chlrori- nation in polymer chromophore, losse the backbone helide as hydrogen helide at high temperature or during irradiation. The resulting unsaturation in the polymer backbone provides points of weakness for chain scission, which occurs at lower temperature than for polystyrene [8]. Ring-brominated polystyrene is less stable than ring chlorinated polystyrene, as it lacks the backbone haloge- nations which are unavoidable in the ring chlorination in polystyrene. The halogenated polystyrenes have fairly similar thermal stability to polystyrene and degrade in essentially the same way giving mainly the monomer, as well as styrene and hydrogen halide [2]. The knowledge of photodegradation process in poly- mers is quite essential from a practical point of view, es- pecially when it comes to its outdoor applications. Weath- ering of polymer materials which involves physical and chemical changes during the exposure of polymer to light and heat [9-11] is often investigated in accelerated condi- tions, though they can significantly differ from that in a natural environment [12]. It should be indicated that irra- diated polymeric materials undergo a series of oxidative reactions that lead to photochemical degradation [13-15] with consequences like brittleness, loss of brightness, and color changes. Besides the cross-linking processes, a number of other changes may take place in polymeric chains during photodegradation [16,17].  Plasticization Effect on the Photodegradation of Poly (4-Chlorostyrene) and Poly (4-Bromostyrene) Films 359 Many investigations concerning the photodegradation of polystyrene [18-20] and substituted polystyrene [21] have been carried out. It has been reported that photode- gradation in PS films occurs when irradiated with UV-radiation; under atmospheric oxygen. Hence the ini- tiation of photo-oxidation is expected to result from the absorption by polymeric chromophores. From the latter process, a number of different photoproducts were re- ported. More specifically, hydro peroxides, carbonyl and hydroxyl compounds were the main products that re- sulted from the photo-oxidation of PS films [22-25]. On the other hand polymeric additives were found to accelerate the radiation-induced degradation of PS and poly (4-substituted styrene) films (SPS) [20]. It was also found that the photo-stability of PS was reduced by the addition of bromine-containing flame retardants, and appeared to depend upon the chemical structure of the polymeric additive [19]. It was reported that the photo- degradation of the PS containing carbonyl group was increased with the increase in the time of irradiation. The changes in the average molecular weight in photo-oxi- dized PS were produced as a consequence of chain dis- sociation by the Norrish Type II reaction [26]. The aim of this work is to examine the photostability of pure and blended PBS and PCS films, which contain a small amount of doped dimethyl terephthalate, diethyl terephthalate, dioctyl terephthalate, dibutyl phthalate and dioctyl phthalate plasticizers upon UV-irradiation. The present study also seeks to check if plasticization affects the photostability of these polymers in solid films, and to investigate the effect of the bulkiness of plasticizer molecules on the photodegradation processes of PBS and PCS in films. The photodegradation processes of the irradiated polymer have been characterized by UV-visible, fluo- rescence, and FT-IR spectroscopic techniques. The effi- ciency of photodegradation of PCS and PBS is compared to that of blended polystyrene with polymeric additives. 2. Experimental Part 2.1. Materials The samples of PCS and PBS were supplied by Across- Organics with high purity, PCS, (Mw = 65,000, Mn = 31,000), and PBS, (Mw = 7,500, Mn = 3,300) [27]. Spectroscopic-quality, dichloroethane was found to give no detectable absorption in the range 250-400 nm, and was used in preparation of solid films. It was purchased from Fluka GMBH and was used as received. The used plasticizes were dimethyl terephthalate (DMT), diethyl terephthalate (DET), dioctyl terephthalate (DOT), dibutyl phthalate (DBP), and dioctyl phthalate (DOP), were of high-purity of 99.8% which was purchased from Across- Organics. They were also found to give no detectable absorption or emission in the range 265-400 nm. 2.2. Preparation of Plasticized Polymeric Solid Films PCS and PBS thin films with thickness of approximately 0.02 mm were prepared by solution casting of 20 wt.% polymer in dichloroethane solvent (DCE) on a spectro- scopic window (quartz plate of 1.0 mm × 20 mm diame- ter). Moreover, about 0.02 mm thick PCS and PBS- plas- ticizer films, containing different wt.% plasticizers were prepared by solution casting of a 20 wt.% polymer + added plasticizer, in DCE solvent. All solid films were dried in a vacuum oven at 300 K for 6 hrs, as to ensure the complete removal of solvent traces [27]. 2.3. Absorption and Fluorescence Spectra Measurements Fluorescence spectra were recorded on JASCO FP 6500 Spectrofluorometer for each of the prepared samples. The parameters were constant for all measurements, and the excitation wavelength was 265 nm. The emission wavelength range was 270-500 nm, and all fluorescence spectra of the solid films were obtained using a thermo- stated solid sample holder at 298 K. The UV absorption spectra for PCS and PBS solid films were recorded before and immediately after UV irradiation with a Cary 100 Bio UV-visible Spectropho- tometer at 298 K. 2.4. Irradiation of Polymeric Films PCS and PBS solid films were exposed to UV-radiation for different intervals of time, from (0.0-4.0 hrs), using a JASCO spectrometer with a built in Hydrogen-Xenon lamp (6808-J007A model number ESC-333). It is sup- ported with monochromator of holographic grating with 1800 grooves/mm. Each film was irradiated for different interval of time with a monochromatic light of 265 nm in a thermostated solid sample holder in presence of air. The intensity of incident radiation was (4.9 mW/cm2), and the distance between light source and samples was (5 cm). 2.5. FT-IR Spectroscopy The FT-IR-spectroscopy system that was employed in this work was NICOLET-MAGNA-IR-560 spectrometer, while the transmission mode was employed in these measure- ments. The FT-IR spectra were recorded for the irradiated and the non-irradiated films, whereas the transmittance was plotted as function of the wavenumbers. 2.6. Calculation of Io [PH-PH]*/I[PH-PH]* Ratios In an earlier work, an equation was invented to calculate the fluorescence quenching effect and the photo quench- ing rate constant as function of increase in exposure time Copyright © 2010 SciRes. MSA 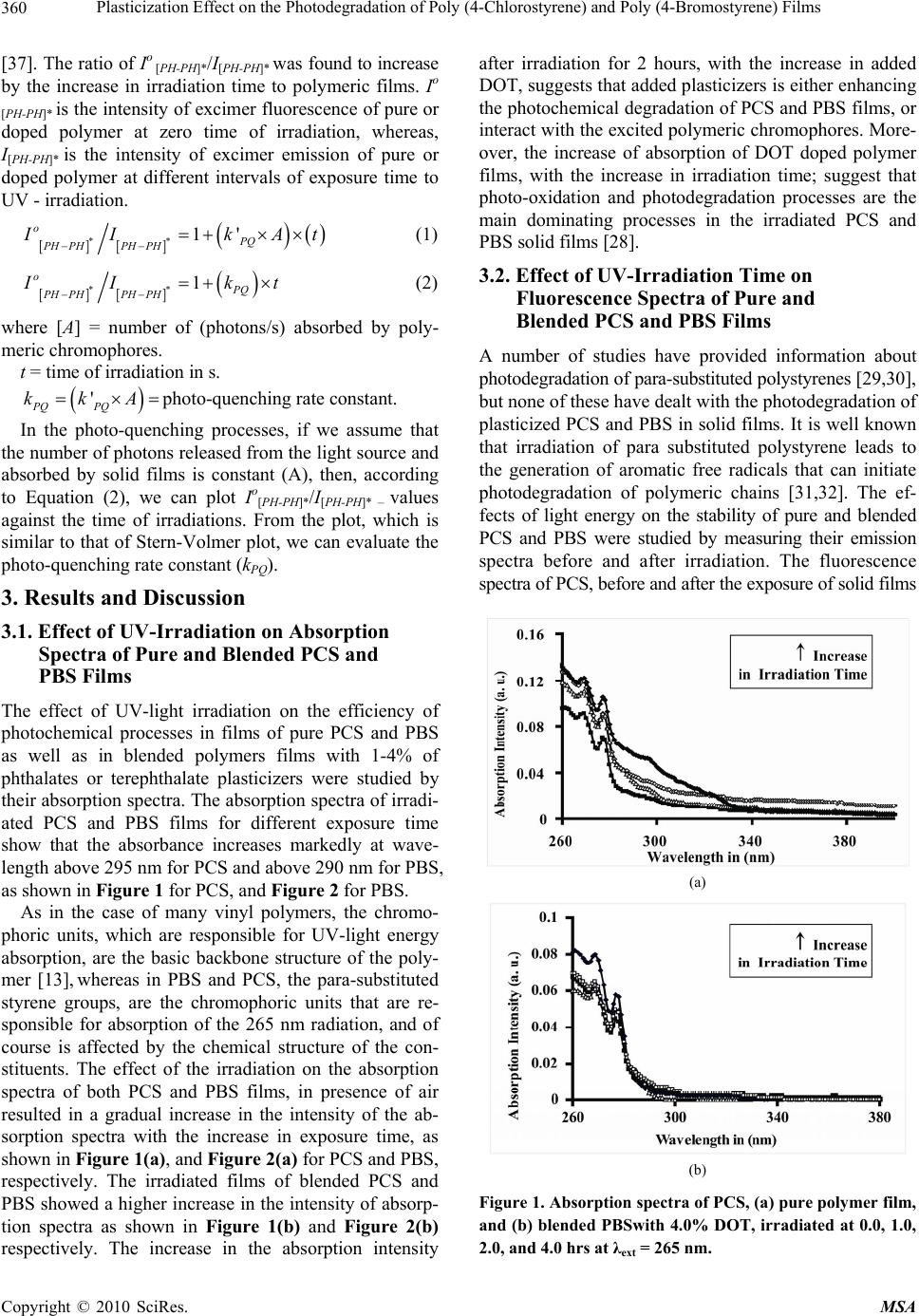 Plasticization Effect on the Photodegradation of Poly (4-Chlorostyrene) and Poly (4-Bromostyrene) Films 360 [37]. The ratio of Io [PH-PH]*/I[PH-PH]* was found to increase by the increase in irradiation time to polymeric films. Io [PH-PH]* is the intensity of excimer fluorescence of pure or doped polymer at zero time of irradiation, whereas, I[PH-PH]* is the intensity of excimer emission of pure or doped polymer at different intervals of exposure time to UV - irradiation. [][] () () ** 1' oPQ PH PHPH PH I IkA −− =+× ×t (1) [][] () ** 1 oPQ PH PHPH PH I Ik −− =+ ×t ) = (2) where [A] = number of (photons/s) absorbed by poly- meric chromophores. t = time of irradiation in s. ( ' PQ PQ kkA=×photo-quenching rate constant. In the photo-quenching processes, if we assume that the number of photons released from the light source and absorbed by solid films is constant (A), then, according to Equation (2), we can plot Io[PH-PH]*/I[PH-PH]* – values against the time of irradiations. From the plot, which is similar to that of Stern-Volmer plot, we can evaluate the photo-quenching rate constant (kPQ). 3. Results and Discussion 3.1. Effect of UV-Irradiation on Absorption Spectra of Pure and Blended PCS and PBS Films The effect of UV-light irradiation on the efficiency of photochemical processes in films of pure PCS and PBS as well as in blended polymers films with 1-4% of phthalates or terephthalate plasticizers were studied by their absorption spectra. The absorption spectra of irradi- ated PCS and PBS films for different exposure time show that the absorbance increases markedly at wave- length above 295 nm for PCS and above 290 nm for PBS, as shown in Figure 1 for PCS, and Figure 2 for PBS. As in the case of many vinyl polymers, the chromo- phoric units, which are responsible for UV-light energy absorption, are the basic backbone structure of the poly- mer [13], whereas in PBS and PCS, the para-substituted styrene groups, are the chromophoric units that are re- sponsible for absorption of the 265 nm radiation, and of course is affected by the chemical structure of the con- stituents. The effect of the irradiation on the absorption spectra of both PCS and PBS films, in presence of air resulted in a gradual increase in the intensity of the ab- sorption spectra with the increase in exposure time, as shown in Figure 1(a), and Figure 2(a) for PCS and PBS, respectively. The irradiated films of blended PCS and PBS showed a higher increase in the intensity of absorp- tion spectra as shown in Figure 1(b) and Figure 2(b) respectively. The increase in the absorption intensity after irradiation for 2 hours, with the increase in added DOT, suggests that added plasticizers is either enhancing the photochemical degradation of PCS and PBS films, or interact with the excited polymeric chromophores. More- over, the increase of absorption of DOT doped polymer films, with the increase in irradiation time; suggest that photo-oxidation and photodegradation processes are the main dominating processes in the irradiated PCS and PBS solid films [28]. 3.2. Effect of UV-Irradiation Time on Fluorescence Spectra of Pure and Blended PCS and PBS Films A number of studies have provided information about photodegradation of para-substituted polystyrenes [29,30], but none of these have dealt with the photodegradation of plasticized PCS and PBS in solid films. It is well known that irradiation of para substituted polystyrene leads to the generation of aromatic free radicals that can initiate photodegradation of polymeric chains [31,32]. The ef- fects of light energy on the stability of pure and blended PCS and PBS were studied by measuring their emission spectra before and after irradiation. The fluorescence spectra of PCS, before and after the exposure of solid films (a) (b) Figure 1. Absorption spectra of PCS, (a) pure polymer film, and (b) blended PBSwith 4.0% DOT, irradiated at 0.0, 1.0, 2.0, and 4.0 hrs at λext = 265 nm. Copyright © 2010 SciRes. MSA 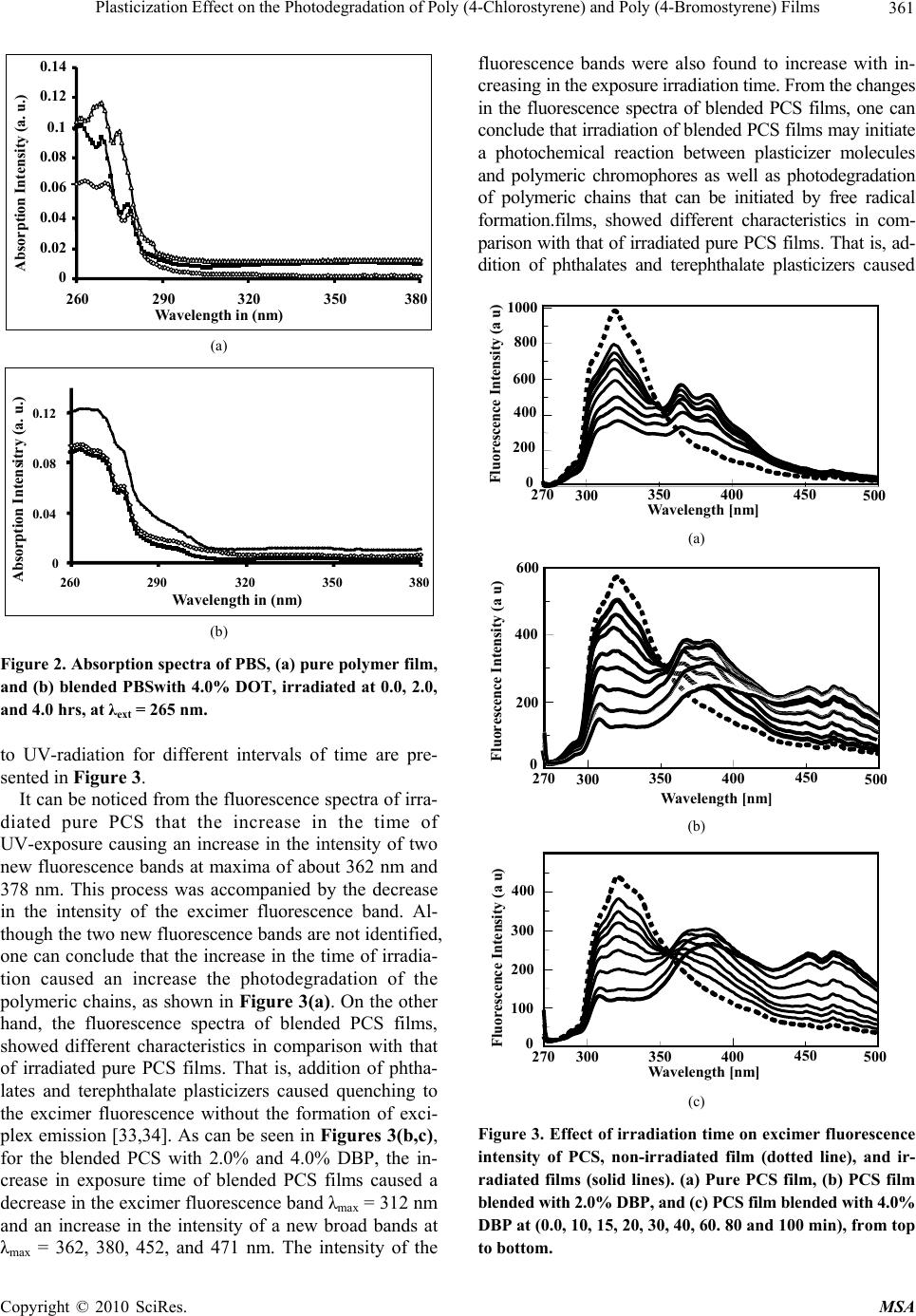 Plasticization Effect on the Photodegradation of Poly (4-Chlorostyrene) and Poly (4-Bromostyrene) Films 361 0 0.02 0.04 0.06 0.08 0.1 0.12 0.14 260 290 320 350 380 Absorption Intensity (a. u.) Wavelength in (nm) (a) 0 0.04 0.08 0.12 260 290 320 350 380 Wavelen g th in (nm) Absorption Intensitry (a. u.) (b) Figure 2. Absorption spectra of PBS, (a) pure polymer film, and (b) blended PBSwith 4.0% DOT, irradiated at 0.0, 2.0, and 4.0 hrs, at λext = 265 nm. to UV-radiation for different intervals of time are pre- sented in Figure 3. It can be noticed from the fluorescence spectra of irra- diated pure PCS that the increase in the time of UV-exposure causing an increase in the intensity of two new fluorescence bands at maxima of about 362 nm and 378 nm. This process was accompanied by the decrease in the intensity of the excimer fluorescence band. Al- though the two new fluorescence bands are not identified, one can conclude that the increase in the time of irradia- tion caused an increase the photodegradation of the polymeric chains, as shown in Figure 3(a). On the other hand, the fluorescence spectra of blended PCS films, showed different characteristics in comparison with that of irradiated pure PCS films. That is, addition of phtha- lates and terephthalate plasticizers caused quenching to the excimer fluorescence without the formation of exci- plex emission [33,34]. As can be seen in Figures 3(b,c), for the blended PCS with 2.0% and 4.0% DBP, the in- crease in exposure time of blended PCS films caused a decrease in the excimer fluorescence band λmax = 312 nm and an increase in the intensity of a new broad bands at λmax = 362, 380, 452, and 471 nm. The intensity of the fluorescence bands were also found to increase with in- creasing in the exposure irradiation time. From the changes in the fluorescence spectra of blended PCS films, one can conclude that irradiation of blended PCS films may initiate a photochemical reaction between plasticizer molecules and polymeric chromophores as well as photodegradation of polymeric chains that can be initiated by free radical formation.films, showed different characteristics in com- parison with that of irradiated pure PCS films. That is, ad- dition of phthalates and terephthalate plasticizers caused 0 200 400 600 800 Fluorescence Intensity (a u) 1000 270 300 400 Wavelength [nm] 450 350 500 (a) 0 600 200 400 Fluorescence Intensity (a u) 270 300 400 450 350 50 0 Wavelength [nm] (b) 0 100 200 300 400 270 300 400 Wavelength [nm] 450 350 500 Fluorescence Intensity (a u) (c) Figure 3. Effect of irradiation time on excimer fluorescence intensity of PCS, non-irradiated film (dotted line), and ir- radiated films (solid lines). (a) Pure PCS film, (b) PCS film blended with 2.0% DBP, and (c) PCS film blended with 4.0% DBP at (0.0, 10, 15, 20, 30, 40, 60. 80 and 100 min), from top to bottom. Copyright © 2010 SciRes. MSA 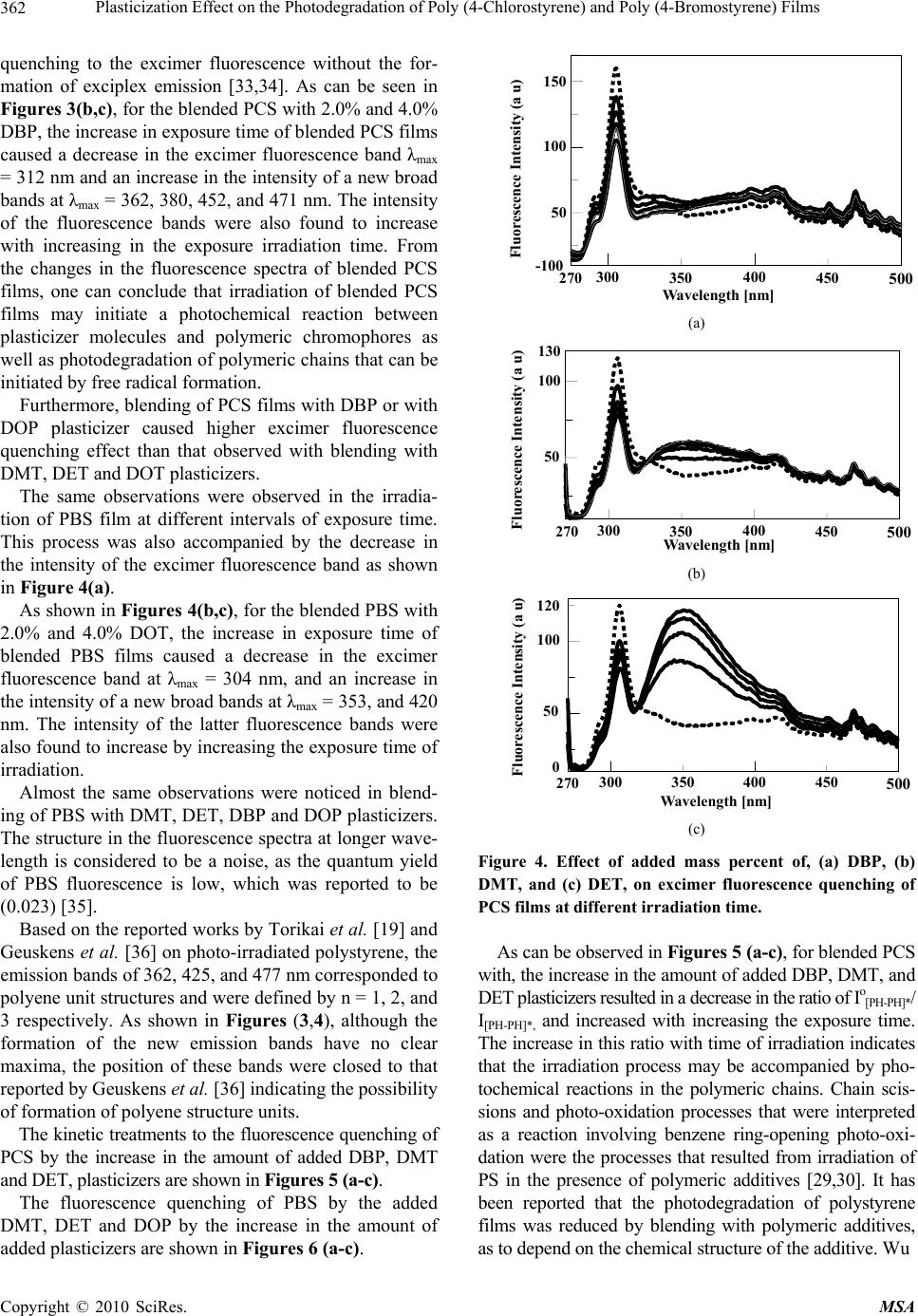 Plasticization Effect on the Photodegradation of Poly (4-Chlorostyrene) and Poly (4-Bromostyrene) Films 362 quenching to the excimer fluorescence without the for- mation of exciplex emission [33,34]. As can be seen in Figures 3(b,c), for the blended PCS with 2.0% and 4.0% DBP, the increase in exposure time of blended PCS films caused a decrease in the excimer fluorescence band λmax = 312 nm and an increase in the intensity of a new broad bands at λmax = 362, 380, 452, and 471 nm. The intensity of the fluorescence bands were also found to increase with increasing in the exposure irradiation time. From the changes in the fluorescence spectra of blended PCS films, one can conclude that irradiation of blended PCS films may initiate a photochemical reaction between plasticizer molecules and polymeric chromophores as well as photodegradation of polymeric chains that can be initiated by free radical formation. Furthermore, blending of PCS films with DBP or with DOP plasticizer caused higher excimer fluorescence quenching effect than that observed with blending with DMT, DET and DOT plasticizers. The same observations were observed in the irradia- tion of PBS film at different intervals of exposure time. This process was also accompanied by the decrease in the intensity of the excimer fluorescence band as shown in Figure 4(a). As shown in Figures 4(b,c), for the blended PBS with 2.0% and 4.0% DOT, the increase in exposure time of blended PBS films caused a decrease in the excimer fluorescence band at λmax = 304 nm, and an increase in the intensity of a new broad bands at λmax = 353, and 420 nm. The intensity of the latter fluorescence bands were also found to increase by increasing the exposure time of irradiation. Almost the same observations were noticed in blend- ing of PBS with DMT, DET, DBP and DOP plasticizers. The structure in the fluorescence spectra at longer wave- length is considered to be a noise, as the quantum yield of PBS fluorescence is low, which was reported to be (0.023) [35]. Based on the reported works by Torikai et al. [19] and Geuskens et al. [36] on photo-irradiated polystyrene, the emission bands of 362, 425, and 477 nm corresponded to polyene unit structures and were defined by n = 1, 2, and 3 respectively. As shown in Figures (3,4), although the formation of the new emission bands have no clear maxima, the position of these bands were closed to that reported by Geuskens et al. [36] indicating the possibility of formation of polyene structure units. The kinetic treatments to the fluorescence quenching of PCS by the increase in the amount of added DBP, DMT and DET, plasticizers are shown in Figures 5 (a-c). The fluorescence quenching of PBS by the added DMT, DET and DOP by the increase in the amount of added plasticizers are shown in Figures 6 (a-c). 50 100 150 -100 270 500 300400 Wavelength [nm] 450350 Fluorescence Intensity (a u) (a) 130 50 100 270 500 300400 Wavelength [nm] 450350 Fluorescence Intensity (a u) (b) 0 120 50 100 270 500 300 400 Wavelength [nm] 450350 Fluorescence Intensity (a u) (c) Figure 4. Effect of added mass percent of, (a) DBP, (b) DMT, and (c) DET, on excimer fluorescence quenching of PCS films at different irradiation time. As can be observed in Figures 5 (a-c), for blended PCS with, the increase in the amount of added DBP, DMT, and DET plasticizers resulted in a decrease in the ratio of Io [PH-PH]*/ I[PH-PH]*, and increased with increasing the exposure time. The increase in this ratio with time of irradiation indicates that the irradiation process may be accompanied by pho- tochemical reactions in the polymeric chains. Chain scis- sions and photo-oxidation processes that were interpreted as a reaction involving benzene ring-opening photo-oxi- dation were the processes that resulted from irradiation of PS in the presence of polymeric additives [29,30]. It has been reported that the photodegradation of polystyrene films was reduced by blending with polymeric additives, as to depend on the chemical structure of the additive. Wu Copyright © 2010 SciRes. MSA  Plasticization Effect on the Photodegradation of Poly (4-Chlorostyrene) and Poly (4-Bromostyrene) Films 363 0 1 2 3 4 5 6 0 2040 60 80 100 Irradiation Time in (min) PCS + 0.0%DBP PCS + 1.0%DBP PCS + 3.0%DBP PCS + 2.0%DBP PCS + 4.0%DBP I oEX / I EX (a) 0 1 2 3 4 5 6 020 406080 100 Irradiation Time in (min) PCS+ 0.0% DMT PCS+ 1.0% DMT PCS+ 3.0% DMT PCS+ 4.0% DMT IoEX / IEX (b) 0 1 2 3 4 5 020406080 100 Irradiation Time in (min) I oEX / I EX PCS + 0.0% DET PCS + 1.0% DET PCS + 3.0% DET PCS + 2.0% DET PCS + 4.0% DET (c) Figure 5. Effect of added mass percent of, (a) DBP, (b) DMT, and (c) DET, on excimer fluorescence quenching of PCS films at different irradiation time. et al. [38] also studied the photo-oxidation of polystyrene by fluorescence spectra, and proposed that photo-quen- ching of polystyrene fluorescence was attributed to the quenching effect of peroxides formed during polymer ir- radiation. PCS and PBS showed lower photo- stability in comparison with other substituted polystyrene like, poly (4-methoxystyrene), Poly (4-methylstyrene) and poly (4- tert-butylstyrene) in solutions [39]. Similarly, as can be observed in Figures 6(a-c), for PBS, the increase in the amount of added DMT, DET, and DOP plasticizers resulted in a decrease in the ratio of Io [PH-PH]*/I[PH-PH]*, and increased with increasing the ex- posure time. The efficiency of photo-quenching for plasticized poly- mer showed a lower value than that for pure polymer, and was also found to increase with the increase in the bulkiness of plasticizer molecules. 0.5 1 1.5 2 2.5 02040 60 80 100 Irradiation Time in (min) PBS + 0.0% DMT PBS + 1.0% DMT PBS + 2.0% DMT PBS + 3.0% DMT PBS + 4.0% DMT I oEX / I EX (a) 0 0.5 1 1.5 2 2.5 0204060 80 100 Irradiation Time in (min) PBS + 0.0% DET PBS + 1.0% DET PBS + 2.0% DET PBS + 3.0% DET PBS + 4.0% DET I oEX / I E X (b) 0.5 1 1.5 2 2.5 02040 60 80 100 Irradiation Time in (min) PBS + 0%DOP PBS + 1%DOP PBS + 2%DOP PBS + 3%DOP PBS + 4%DOP I oEX / I EX (c) Figure 6. Effect of added mass percent of, (a) 4.0% DMT, (b) 4.0% DET, and (c) 4.0 % DOP on excimer fluorescence quenching of PBS films at different irradiation time, at (λ = 265 nm). From Figures 5 and 6, where the ratio Io [PH-PH]*/I[PH-PH]* was plotted against different intervals of the exposure time, from the slope of the obtained lines, kPQ values were calculated for PCS and PBS plasticized with 4.0% of DMT, DET, DOT, DBP, and DOP, and are presented in Table 1. As can be observed from Figures 5 and 6, and Table 1, the photo-quenching rate constant kPQ values, were found to increase with the increase in molar mass of the used plasticizer. Phthalate plasticizers, on the other hand, displayed a higher efficiency of photo-quenching than that which was observed by terephthalate plasticizers. This fact also correlates well with the thermal quenching of the polymers excimer fluorescence. In other words the Copyright © 2010 SciRes. MSA 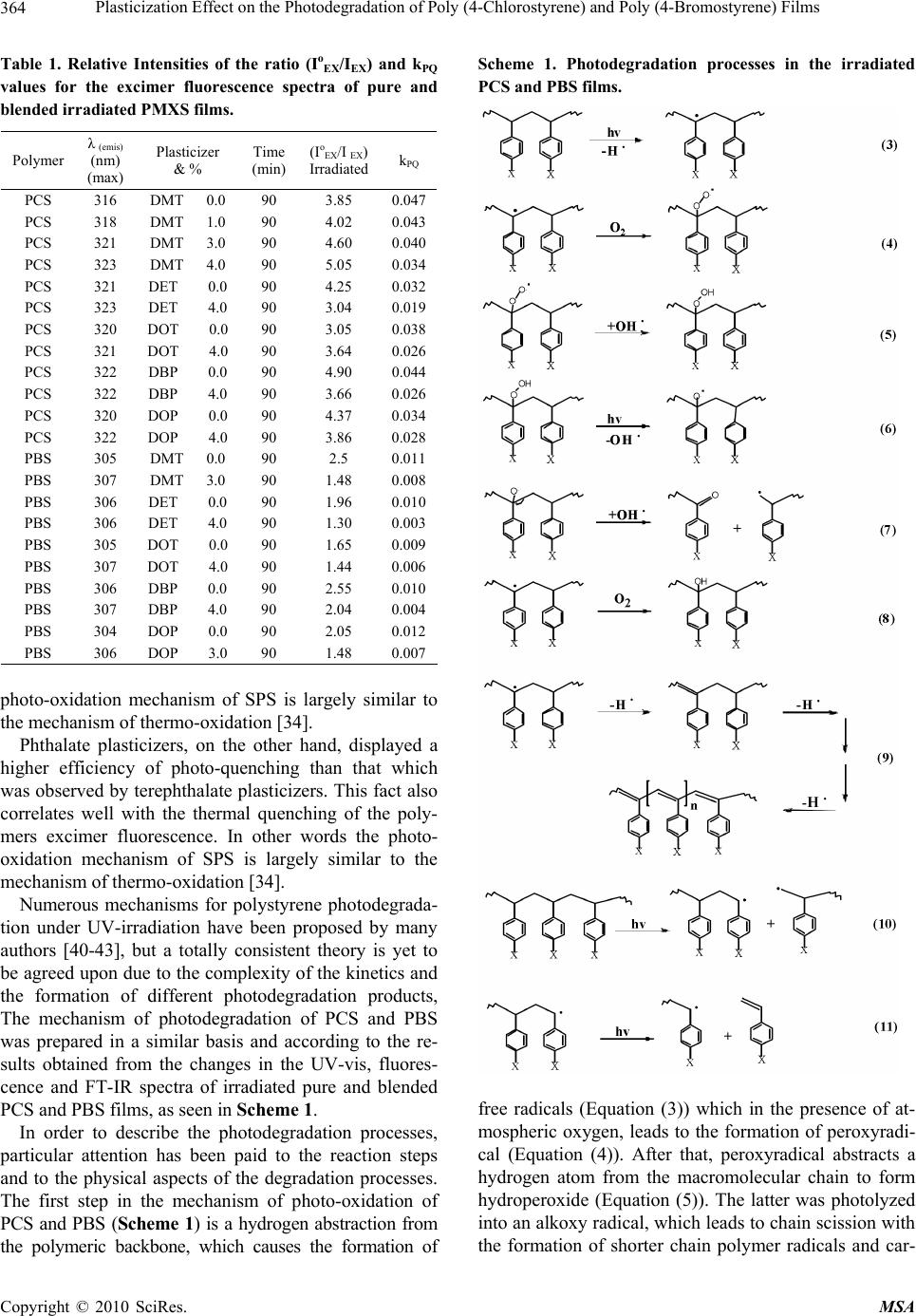 Plasticization Effect on the Photodegradation of Poly (4-Chlorostyrene) and Poly (4-Bromostyrene) Films 364 Table 1. Relative Intensities of the ratio (Io EX/IEX) and kPQ values for the excimer fluorescence spectra of pure and blended irradiated PMXS films. kPQ (IoEX/I EX) Irradiated Time (min) Plasticizer & % λ (emis) (nm) (max) Polymer 0.0473.85 90 DMT 0.0 316 PCS 0.0434.02 90 DMT 1.0 318 PCS 0.0404.60 90 DMT 3.0 321 PCS 0.0345.05 90 DMT 4.0 323 PCS 0.0324.25 90 DET 0.0321 PCS 0.0193.04 90 DET 4.0323 PCS 0.0383.05 90 DOT 0.0320 PCS 0.0263.64 90 DOT 4.0321 PCS 0.0444.90 90 DBP 0.0322 PCS 0.0263.66 90 DBP 4.0322 PCS 0.0344.37 90 DOP 0.0320 PCS 0.0283.86 90 DOP 4.0322 PCS 0.0112.5 90 DMT 0.0 305 PBS 0.0081.48 90 DMT 3.0 307 PBS 0.0101.96 90 DET 0.0306 PBS 0.0031.30 90 DET 4.0306 PBS 0.0091.65 90 DOT 0.0305 PBS 0.0061.44 90 DOT 4.0307 PBS 0.0102.55 90 DBP 0.0306 PBS 0.0042.04 90 DBP 4.0307 PBS 0.0122.05 90 DOP 0.0304 PBS 0.0071.48 90 DOP 3.0306 PBS photo-oxidation mechanism of SPS is largely similar to the mechanism of thermo-oxidation [34]. Phthalate plasticizers, on the other hand, displayed a higher efficiency of photo-quenching than that which was observed by terephthalate plasticizers. This fact also correlates well with the thermal quenching of the poly- mers excimer fluorescence. In other words the photo- oxidation mechanism of SPS is largely similar to the mechanism of thermo-oxidation [34]. Numerous mechanisms for polystyrene photodegrada- tion under UV-irradiation have been proposed by many authors [40-43], but a totally consistent theory is yet to be agreed upon due to the complexity of the kinetics and the formation of different photodegradation products, The mechanism of photodegradation of PCS and PBS was prepared in a similar basis and according to the re- sults obtained from the changes in the UV-vis, fluores- cence and FT-IR spectra of irradiated pure and blended PCS and PBS films, as seen in Scheme 1. In order to describe the photodegradation processes, particular attention has been paid to the reaction steps and to the physical aspects of the degradation processes. The first step in the mechanism of photo-oxidation of PCS and PBS (Scheme 1) is a hydrogen abstraction from the polymeric backbone, which causes the formation of Scheme 1. Photodegradation processes in the irradiated PCS and PBS films. free radicals (Equation (3)) which in the presence of at- mospheric oxygen, leads to the formation of peroxyradi- cal (Equation (4)). After that, peroxyradical abstracts a hydrogen atom from the macromolecular chain to form hydroperoxide (Equation (5)). The latter was photolyzed into an alkoxy radical, which leads to chain scission with the formation of shorter chain polymer radicals and car- Copyright © 2010 SciRes. MSA 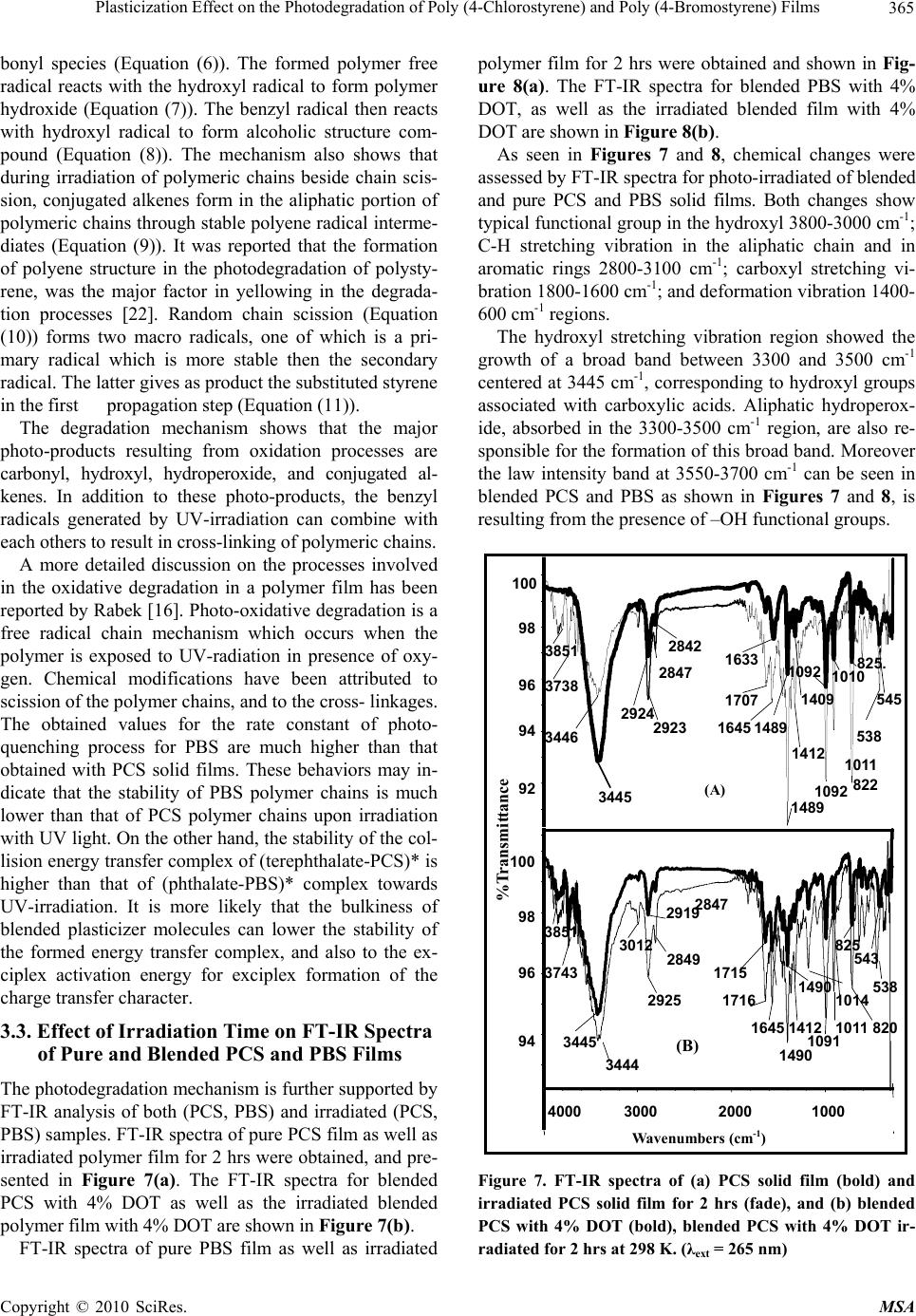 Plasticization Effect on the Photodegradation of Poly (4-Chlorostyrene) and Poly (4-Bromostyrene) Films 365 bonyl species (Equation (6)). The formed polymer free radical reacts with the hydroxyl radical to form polymer hydroxide (Equation (7)). The benzyl radical then reacts with hydroxyl radical to form alcoholic structure com- pound (Equation (8)). The mechanism also shows that during irradiation of polymeric chains beside chain scis- sion, conjugated alkenes form in the aliphatic portion of polymeric chains through stable polyene radical interme- diates (Equation (9)). It was reported that the formation of polyene structure in the photodegradation of polysty- rene, was the major factor in yellowing in the degrada- tion processes [22]. Random chain scission (Equation (10)) forms two macro radicals, one of which is a pri- mary radical which is more stable then the secondary radical. The latter gives as product the substituted styrene in the first propagation step (Equation (11)). The degradation mechanism shows that the major photo-products resulting from oxidation processes are carbonyl, hydroxyl, hydroperoxide, and conjugated al- kenes. In addition to these photo-products, the benzyl radicals generated by UV-irradiation can combine with each others to result in cross-linking of polymeric chains. A more detailed discussion on the processes involved in the oxidative degradation in a polymer film has been reported by Rabek [16]. Photo-oxidative degradation is a free radical chain mechanism which occurs when the polymer is exposed to UV-radiation in presence of oxy- gen. Chemical modifications have been attributed to scission of the polymer chains, and to the cross- linkages. The obtained values for the rate constant of photo- quenching process for PBS are much higher than that obtained with PCS solid films. These behaviors may in- dicate that the stability of PBS polymer chains is much lower than that of PCS polymer chains upon irradiation with UV light. On the other hand, the stability of the col- lision energy transfer complex of (terephthalate-PCS)* is higher than that of (phthalate-PBS)* complex towards UV-irradiation. It is more likely that the bulkiness of blended plasticizer molecules can lower the stability of the formed energy transfer complex, and also to the ex- ciplex activation energy for exciplex formation of the charge transfer character. 3.3. Effect of Irradiation Time on FT-IR Spectra of Pure and Blended PCS and PBS Films The photodegradation mechanism is further supported by FT-IR analysis of both (PCS, PBS) and irradiated (PCS, PBS) samples. FT-IR spectra of pure PCS film as well as irradiated polymer film for 2 hrs were obtained, and pre- sented in Figure 7(a). The FT-IR spectra for blended PCS with 4% DOT as well as the irradiated blended polymer film with 4% DOT are shown in Figure 7(b). FT-IR spectra of pure PBS film as well as irradiated polymer film for 2 hrs were obtained and shown in Fig- ure 8(a). The FT-IR spectra for blended PBS with 4% DOT, as well as the irradiated blended film with 4% DOT are shown in Figure 8(b). As seen in Figures 7 and 8, chemical changes were assessed by FT-IR spectra for photo-irradiated of blended and pure PCS and PBS solid films. Both changes show typical functional group in the hydroxyl 3800-3000 cm-1; C-H stretching vibration in the aliphatic chain and in aromatic rings 2800-3100 cm-1; carboxyl stretching vi- bration 1800-1600 cm-1; and deformation vibration 1400- 600 cm-1 regions. The hydroxyl stretching vibration region showed the growth of a broad band between 3300 and 3500 cm-1 centered at 3445 cm-1, corresponding to hydroxyl groups associated with carboxylic acids. Aliphatic hydroperox- ide, absorbed in the 3300-3500 cm-1 region, are also re- sponsible for the formation of this broad band. Moreover the law intensity band at 3550-3700 cm-1 can be seen in blended PCS and PBS as shown in Figures 7 and 8, is resulting from the presence of –OH functional groups. 545 825. 1010 1092 1409 1489 1633 2842 2924 3445 538 822 1011 1092 1412 1489 1645 1707 2847 2923 3446 3738 92 94 96 98 100 %Transmittance 3851 (A) (B) Wavenumbers (cm -1 ) 94 96 98 100 538 8201011 1091 1412 1490 1645 1716 2925 3444 3743 543 825 1014 1490 1715 2847 2919 3445 3851 3000 4000 2000 1000 2849 3012 Figure 7. FT-IR spectra of (a) PCS solid film (bold) and irradiated PCS solid film for 2 hrs (fade), and (b) blended PCS with 4% DOT (bold), blended PCS with 4% DOT ir- radiated for 2 hrs at 298 K. (λext = 265 nm) Copyright © 2010 SciRes. MSA 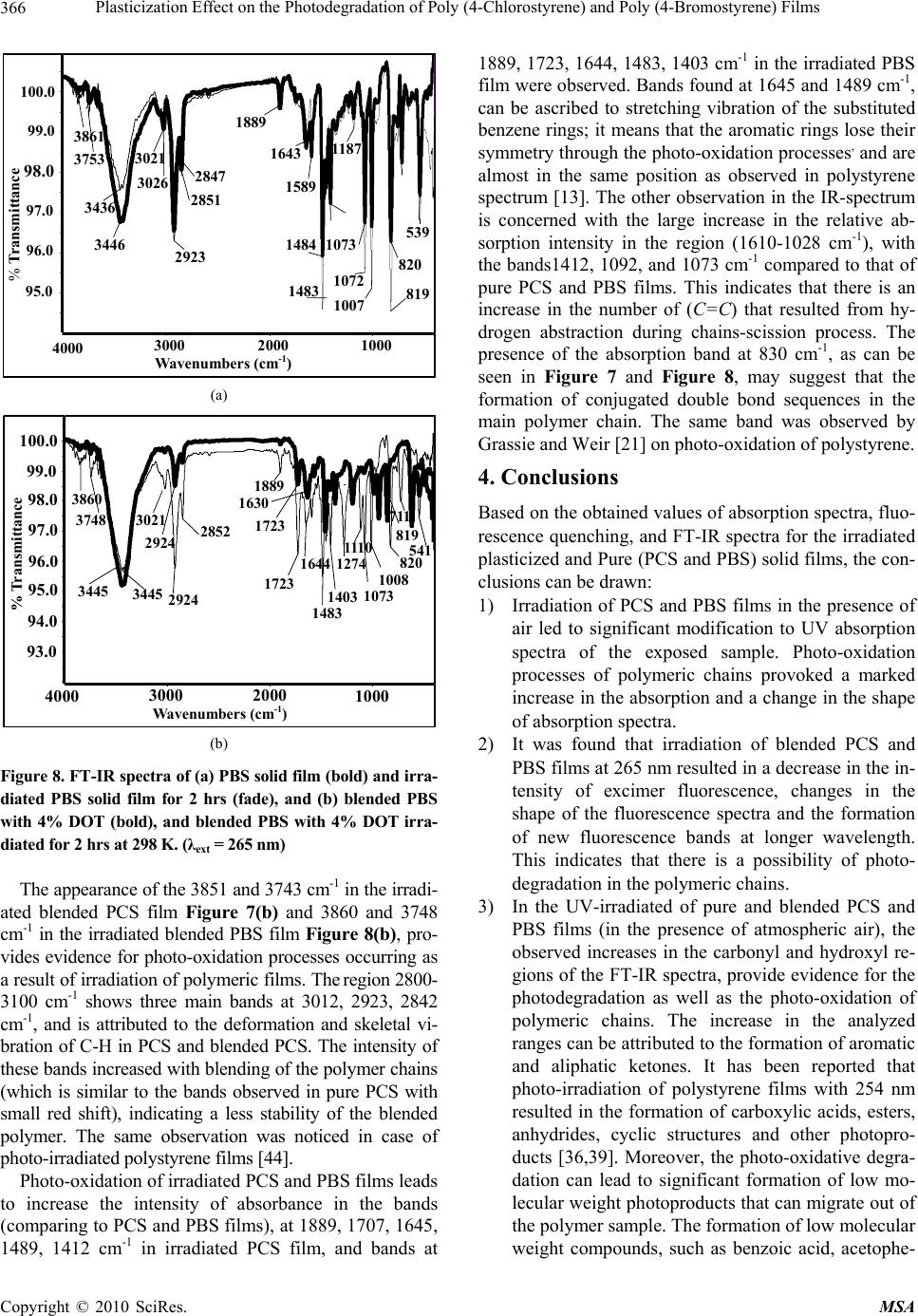 Plasticization Effect on the Photodegradation of Poly (4-Chlorostyrene) and Poly (4-Bromostyrene) Films 366 539 1484 1589 2851 2923 3021 3446 3861 1072 11 87 1483 1643 1889 2847 3026 3436 3753 95.0 96.0 97.0 98.0 99.0 100.0 1000 2000 3000 819 820 1073 1007 % Transmitt ance 4000 Wavenumbers (cm -1 ) (a) 541 711 820 1008 1073 1110 1274 1403 1483 1644 1723 1889 2852 2924 3445 3748 819 1630 1723 2924 3445 93.0 94.0 95.0 96.0 97.0 98.0 99.0 100.0 % Transmittanc e 1000 2000 3000 3021 3860 Wavenumbers (c m -1 ) 4000 (b) Figure 8. FT-IR spectra of (a) PBS solid film (bold) and irra- diated PBS solid film for 2 hrs (fade), and (b) blended PBS with 4% DOT (bold), and blended PBS with 4% DOT irra- diated for 2 hrs at 298 K. (λext = 265 nm) The appearance of the 3851 and 3743 cm-1 in the irradi- ated blended PCS film Figure 7(b) and 3860 and 3748 cm-1 in the irradiated blended PBS film Figure 8(b), pro- vides evidence for photo-oxidation processes occurring as a result of irradiation of polymeric films. The region 2800- 3100 cm-1 shows three main bands at 3012, 2923, 2842 cm-1, and is attributed to the deformation and skeletal vi- bration of C-H in PCS and blended PCS. The intensity of these bands increased with blending of the polymer chains (which is similar to the bands observed in pure PCS with small red shift), indicating a less stability of the blended polymer. The same observation was noticed in case of photo-irradiated polystyrene films [44]. Photo-oxidation of irradiated PCS and PBS films leads to increase the intensity of absorbance in the bands (comparing to PCS and PBS films), at 1889, 1707, 1645, 1489, 1412 cm-1 in irradiated PCS film, and bands at 1889, 1723, 1644, 1483, 1403 cm-1 in the irradiated PBS film were observed. Bands found at 1645 and 1489 cm-1, can be ascribed to stretching vibration of the substituted benzene rings; it means that the aromatic rings lose their symmetry through the photo-oxidation processes, and are almost in the same position as observed in polystyrene spectrum [13]. The other observation in the IR-spectrum is concerned with the large increase in the relative ab- sorption intensity in the region (1610-1028 cm-1), with the bands1412, 1092, and 1073 cm-1 compared to that of pure PCS and PBS films. This indicates that there is an increase in the number of (C=C) that resulted from hy- drogen abstraction during chains-scission process. The presence of the absorption band at 830 cm-1, as can be seen in Figure 7 and Figure 8, may suggest that the formation of conjugated double bond sequences in the main polymer chain. The same band was observed by Grassie and Weir [21] on photo-oxidation of polystyrene. 4. Conclusions Based on the obtained values of absorption spectra, fluo- rescence quenching, and FT-IR spectra for the irradiated plasticized and Pure (PCS and PBS) solid films, the con- clusions can be drawn: 1) Irradiation of PCS and PBS films in the presence of air led to significant modification to UV absorption spectra of the exposed sample. Photo-oxidation processes of polymeric chains provoked a marked increase in the absorption and a change in the shape of absorption spectra. 2) It was found that irradiation of blended PCS and PBS films at 265 nm resulted in a decrease in the in- tensity of excimer fluorescence, changes in the shape of the fluorescence spectra and the formation of new fluorescence bands at longer wavelength. This indicates that there is a possibility of photo- degradation in the polymeric chains. 3) In the UV-irradiated of pure and blended PCS and PBS films (in the presence of atmospheric air), the observed increases in the carbonyl and hydroxyl re- gions of the FT-IR spectra, provide evidence for the photodegradation as well as the photo-oxidation of polymeric chains. The increase in the analyzed ranges can be attributed to the formation of aromatic and aliphatic ketones. It has been reported that photo-irradiation of polystyrene films with 254 nm resulted in the formation of carboxylic acids, esters, anhydrides, cyclic structures and other photopro- ducts [36,39]. Moreover, the photo-oxidative degra- dation can lead to significant formation of low mo- lecular weight photoproducts that can migrate out of the polymer sample. The formation of low molecular weight compounds, such as benzoic acid, acetophe- Copyright © 2010 SciRes. MSA  Plasticization Effect on the Photodegradation of Poly (4-Chlorostyrene) and Poly (4-Bromostyrene) Films 367 none, benzaldehyde, formic acid, acetic acid, styrene monomer, and benzene were identified in UV- irradiated polystyrene. Thus infrared analysis of the oxidative evolution of the irradiated solid sample does not permit recording the formation of all the photoproducts. 5. Acknowledgements We gratefully acknowledge the financial support of Ab- dul Hammed Showman Foundation. Thanks are also due to Manal al buzor, Muna Hawi, May Anabtawi, and Suha Khanfar for their help in this research work, and to Pro- fessor Mikdad Al Arif for useful discussions. REFERENCES [1] S. S. Stivala, J. Kimum and L. Reich. “Degradation and Stability of Polymers,” In: H. G. Jellinek, Ed., Aspects of Degradation and Stabilization of Polymers, Elsevier, Amsterdam, 1983, pp. 1-66. [2] I. C. McNeill and M. Coskun, “Structure and Stability of Halogenated Polymers: Part 3—Ring Brominated Poly- styrene,” Polymer Degradation and Stability, Vol. 23, No. 2, 1989, pp. 175-183. [3] I. C. McNeill and M. Coskun, “Structure and Stability of Halogenated Polymers: Part 1-Ring Chlorinated Polysty- rene.” Polymer Degradation and Stability, Vol. 17, No. 4, 1987, pp. 347-357. [4] D. de Schryver, S. D. Landry and J. S. Reed, “Latest De- velopments on the Flame redundancy of Engineering Thermoplastics-Brominated Polystyrene in Glass Filled Engineering Thermoplastics,” Polymer Degradation and Stability, Vol. 64, No. 3, 1999, pp. 471-477. [5] F. Bertini, G. Audisio and J. Kiji, “Thermal Degradation of Chlorinated Polystyrenes,” Journal of Analytical & Applied Pyrolysis, Vol. 28, No. 2, 1994, pp. 205-217. [6] B. Boinon, W. Abboud, A. Bouras and D. Ainad-Tabet, “Kinetics of the Thermal Degradation of Partially para- Brominated Polystyrenes,” Polymer Degradation and Stability, Vol. 42, No. 1, 1993, pp. 75-80. [7] A. Rincon and I. C. McNeill, “Thermal Degradation of Poly (Methacrylate)-Poly-4-Bromostyrene Blends and Methyl Methacrylate-4-Bromostyrene Copolymers,” Poly- mer Degradation and Stability, Vol. 40, No. 1, 1993, pp. 125-135. [8] I. C. McNeill and M. Coskun, “Structure and Stability of Halogenated Polymers: Part 2—Chain-Chlorinated Poly- styrene,” Polymer Degradation and Stability, Vol. 18, No. 3, 1987, pp. 213-224. [9] N. Grassie, “Developments in Polymer Degradation,” Applied Science Publication, London, 1977. [10] B. Ranby and J. F. Rabek, “Studies on the Photo- Oxidation Mechanism of Polymers, I. Photolysis and Photo-Oxidation of Polystyrene,” .Journal of Polymer Science, Polymer Chemistry Edition, Vol. 12, No. 2, 1974, pp. 273-294. [11] F. Bertini, G. Audisio and J. Kiji. “Thermal Behaviors and Degradation Mechanism of Brominated Polysty- renes,” Journal of Analytical & Applied Pyrolysis, Vol. 33, No. 1, 1995, pp. 213-230. [12] H. Kaczmarek, “Photodegradation of Polystyrene and Poly (Vinyl Acetate) Blends, II. Irradiation of PS/PVAc Blends by Fluorescent Lamp,” European Polymer Jour- nal, Vol. 31, No. 12, 1995, pp. 1175-1184. [13] Z. Zhang, X. Hu and Z. Luo, “Wavelength Sensitivity of Photo-Oxidation of Polypropylene,” Polymer Degra- dation and Stability, Vol. 51, No. 1, 1996, pp. 93-97. [14] H. Kaczmarek and A. Podgorski, “Photochemical and Thermal Behaviors of Poly (Vinyl alcohol) Graphite Ox- ide Composite,” Polymer Degradation and Stability, Vol. 92, No. 6, 2007, pp. 939-946. [15] K. R. White and A. V. Shyichuk, “Effect of Stabilization on Scission and Cross-Linking Rate Changes during Photo-Oxidation of Polypropylene,” Polymer Degrada- tion and Stability, Vol. 92, No. 11, 2007, pp. 2095-2101. [16] J. F. Rabek, “Polymer Photodegradation, Mechanisms and Experimental Methods,” Chapman and Hall, London, 1995. [17] G. M. Fechine, M. S. Rabello and R. M. Souto-Maior, “The Effect of Ultraviolet Stabilizers on Photodegrada- tion of Poly (Ethylene Terephthalate),” Polymer Degra- dation and Stability, Vol. 75, No. 1, 2002, pp. 153-159. [18] J. Kowal and M. Nwakowska, “Photo-Oxidation of Poly- styrene in Dichloromethane Solvent,” Polymer, Vol. 23, No. 2, 1982, pp. 281-282. [19] A. Torikai, T. Kobatake, F. Okisaki and H. Shuyama, “Photodegradation of Polystyrene Containing Fame- retardant Wavelength Sensitivity and Efficiency of Deg- radation,” Polymer Degradation and Stability, Vol. 50, No. 3, 1995, pp. 261-267. [20] N. A. Weir and T. H. Milkie, “Photochemistry of Ring- Substituted Polystyrenes. II. Photolysis of Poly (p-Fluoro, p-Chloro, and p-Bromostyrene),” Journal of Polymer Sci- ence, Polymer Chemistry Edition, Vol. 17, No. 11, 1979, pp. 3735-3749. [21] K. Subramanian, “Photodecomposition of Poly (Styrene Peroxide),” European Polymer Journal, Vol. 38, No. 6, 2002, pp. 1167-1173. [22] G. Geuskens, P. Bastin, Q. Lu-Vinh and M. Rens, “Photo- Oxidation of Polymers: Part IV—Influence of the Proc- essing Conditions on the Photo-Oxidation Stability of Polystyrene,” Polymer Degradation and Stability, Vol. 3, No. 4, 1981, pp. 295-306. [23] K. C. Tse, F. M. Ng and K. N. Yu, “Photodegradation of DAVC by UV-Radiation at Various Wavelengths,” Poly- mer Degradation and Stability, Vol. 91, No. 10, 2006, pp. 2380-2388. [24] P. C. Lucas and R. S. Porter, “Auto Inhibition in Polysty- rene Photo-Oxidation,” Polymer Degradation and Stabil- ity, Vol. 26, No. 3, 1989, pp. 203-208. [25] A. Torikai, T. Takeuchi and K, Fueki, “Photodegradation of Polystyrene and Polystyrene Containing Benzophe- Copyright © 2010 SciRes. MSA  Plasticization Effect on the Photodegradation of Poly (4-Chlorostyrene) and Poly (4-Bromostyrene) Films Copyright © 2010 SciRes. MSA 368 none,” Polymer Photochemistry, Vol. 3 No. 4, 1983, pp. 307-320. [26] W. M. Choi, I. D. Jung, C. Sik and W. Cho, “Synthesis and Properties of Photodegradable Polystyrene-Containing Carbonyl Group,” Journal of Applied Polymer Science Vol. 67, No. 7, 1998, pp. 1237-1242. [27] A. E. Ramadhan, R. K. Ahmed and K. E. Al Ani, “Ther- mal Effect and Quenching of Exciplex Fluorescence of Polystyrene Derivatives by Dimethyl Terephthalate in Solid Films,” Polymer Journal, Vol. 38, No. 4, 2006, pp. 355-363. [28] K. E. Al Ani and M. A. Hawi, “Effect of Plasticization on the Photodegradation of Poly (para-Methoxystyrene) Films,” Journal of Material Science, Vol. 44, No. 10, 2009, pp. 2674-268. [29] S. Stokes and R. B. Fox, “Photolysis of Poly-α-Methy- lstyrene,” Journal of Polymer Science, Vol. 56, No. 2, 1962, pp. 507-517. [30] N. A. Weir, “Photolysis of Poly (p-Methylstyrene),” Jour- nal of Applied polymer Science, Vol. 17, No. 2, 1973, pp. 401-419. [31] V. V. Zuev, P. V. Zgonnik, L. D. Turkova and L. A. Shi- baev, “Thermal Degradation of Poly (p-Nitrostyrene),” Polymer Degradation and Stability, Vol. 63, No. 1, 1999, pp. 15-17. [32] E. K. Fields and S. Meyerson, “Advances in Free-Radical Chemistry,” Elek Science, London, 1975. [33] K. E. Al Ani and A. M. Suleiman, “Substituent Effect on Fluorescence Quenching of Polystyrene Derivatives by Polymeric Plasticizers,” Journal of Photochemistry, A, Chemistry. Vol. 188, No. 2, 2007, pp. 177-184. [34] K. E. Al Ani, M. Al Barghouthi and M. Buzour, “Solvent Effect on Thermal Degradation of Plasticized para- Substituted Polystyrenes,” Polymer Degradation and Sta- bility, Vol. 91, No. 12, 2006, pp. 3252-3258. [35] K. E. Al Ani, Y. Yousef and T. Akasheh, “Effect of Sol- vents on Fluorescence and Fluorescence Lifetimes of p- Substituted Polystyrenes,” Asian Journal of Chemistry, Vol. 18, No. 3, 2006, pp. 1675-1684. [36] C. David, D. Baeyens-Volant, G. Delaunois, Q. Lu-Vinh, W. Piret and G. Geuskens, “Photo-Oxidation of Poly- mers—III Molecular Weight Changes in the Photolysis and Photo-Oxidation of Polystyrene,” European Polymer Journal, Vol. 14, No. 7, 1978, pp. 501-507. [37] K. E. Al Ani and A. E. Ramadhan. “Study of the Influ- ence of UV-Irradiation on the Photodegradation of Plasti- cized Poly (para-Tert-Butylstyrene) Films,” International Journal of Material Research, Revised, 2010. [38] S. K. Wu, L. H. Liu and G. S. Dai, Polymer Communica- tions, Vol. 2, No.2 ,1984, p.153. [39] K. E. Al Ani and A. E. Ramadhan, “Photodegradation of Plasticized Poly (para-Substituted Styrene) in Solution,” Polymer Degradation and Stability, Vol. 93, No. 8, 2008, pp. 1590-1596. [40] N. A. Weir, “Reactions of Hydroxyl Radical with Poly- styrene,” European Polymer Journal, Vol. 14, No. 1, 1978, pp. 9-14. [41] E. P. Otocka, S. Curran and R. S. Porter, “Photo- Oxidation of Polystyrene: Irradiation at 254 and 365 nm,” Journal of Applied Polymer Science, Vol. 28, No. 10, 1983, pp. 3227-3233. [42] T. Tamai, I. Hashida, N. Ichinose, S. Kawanishi, H. Inoue and K. Mizuno, “UV-Irradiation of Thin Film of Polysty- rene Derivatives: Formation of Carboxylic Group and Cross-Linking from 4-Trimethylsilylmethyl Sub- stituent,” Polymer, Vol. 37, No. 24, 1996, pp. 5525-5528. [43] M. D. Millan, J. Locklin, T. Fulghum, A. Baba and R. C. Advincula, “Polymer Thin Film Photodegradation and Photochemical Cross-Linking FT-IR Imaging Evanescent Waveguide Spectroscopy, and QCM Investigation,” Poly- mer, Vol. 46. No. 15, 2005, pp. 5556-5568. [44] H. Kaczmarek, A. Felczak, A. Szalla, “Studies of Photo- chemical Transformation in Polystyrene-maleic Anhy- dride Copolymer,” Polymer Degradation and Stability, Vol. 93, No. 7, 2008, pp. 1259-1266. |

