 Engineering, 2013, 5, 577-586 http://dx.doi.org/10.4236/eng.2013.57070 Published Online July 2013 (http://www.scirp.org/journal/eng) A Kinetic Study of Anaerobic Biodegradat ion of Food and Fruit Residues during Biogas Generation Using Initial Rate Method William Wanasolo, Samwel Victor Manyele, John Makunza Department of Chemical and Mining Engineering, College of Engineering and Technology, University of Dar es Salaam, Dar es Salaam, Tanzania Email: wanasolo@gmail.com, smanyele@udsm.ac.tz, makunzaj@gmail.com Received May 23, 2013; revised June 23, 2013; accepted July 1, 2013 Copyright © 2013 William Wanasolo et al. This is an open access article distributed under the Creative Commons Attribution Li- cense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT A kinetic study of biogas production from Urban Solid Waste (USW) generated in Dar es Salaam city (Tanzania) is presented. An experimental bioreactor simulating mesophilic conditions of most USW landfills was developed. The goal of the study was to generate the kinetic order of reaction with respect to biodegradable organic waste and use it to model biogas production from food residues mixed with fruit waste. Anaerobic biodegradation was employed under temperature range of 28˚C - 38˚C. The main controls were leachate recirculation and pH adjustments to minimize acid inhibitory effects and accelerate waste biodegradation. The experimental setup was comprised of three sets of bioreactors. A biodegradation rate law in differential form was proposed and the numerical values of kinetic order and rate constant were determined using initial rate method as 0.994 and 0.3093 mol0.006·day−1, respectively. Results obtained were con- sistent with that found in literature and model predictions were in reasonable agreement with experimental data. Keywords: Urban and Municipal Solid Waste; Biogas Production; Anaerobic Biodegradation; Mesophilic Conditions; Order of Reaction; Kinetic Model; Initial Rate Method; Renewable Energy; Bioreactor; Landfill; Biodegradable Organic Waste 1. Introduction One of the main global challenges of the 21st Century is the rapidly growing energy demand where a high per- centage is met by supplies from fossil fuels. It has been reported that during this century energy demand will increase by a factor of two or three [1]. The use of fossil fuels contributes significantly to the rising concentrations of greenhouse gases (GHGs) in the stratosphere resulting in global warming. The quest for alternative energy sources has become inevitably important with renewable energy sources as the most credible alternative. Renew- able energy is energy derived from natural processes such as sunlight and wind that are replenished at a higher rate than they are consumed. Solar, wind, geothermal, hydro, biomass and biogas are common examples of re- newable energy [2]. It has been proposed that in the next few decades, bio-energy will be the most significant re- newable energy source compared to fossil fuels [3]. This shows the increasing attention towards use of renewable energy for solving global energy needs and environ- mental problems. In Tanzania and most African countries, biogas is the common type of renewable energy in use. It is produced mainly from animal waste excreta. No at- tempt has been made to produce biogas from Urban Solid Waste (USW) in this region. In addition, the science be- hind biogas production rates from USW is yet to be studied. Biogas production from USW elsewhere in the world has been studied. However, the kinetic orders have always been assumed and applied retrospectively in re- searches related to the bioconversion of Solid Waste Or- ganic Matter (SWOM). This paper focuses on kinetics by determining the kinetic order of the biodegradation proc- ess during biogas generation, using initial rate method. The study employed wet digesters to ferment food resi- dues mixed with fruit waste obtained from staff Canteens and student Cafeterias at the University of Dar es Salaam (UDSM) in Tanzania. 2. Literature Review 2.1. Biogas Potential There is great potential for biogas generation from Solid C opyright © 2013 SciRes. ENG  W. WANASOLO ET AL. 578 Waste (SW). Elango, Pulikesi, Baskaralingam, Rama- murthi and Sivanesan 2007 [4] generated biogas from Municipal Solid Waste (MSW) enhanced by addition of domestic sewage. The amount of biogas production re- ported was 0.36 m3/kg of volatile solids (VS) added per day at optimal organic loading rate of 2.9 kg VS/m3/day and the biogas produced during anaerobic digestion had methane composition in the range of 68% - 72%. Rao, Baral, Dey and Mutnuri 2010 [5] estimated the bio-en- ergy potential of MSW, crop remains and farm waste, wastewater sludge, animal refuse, industrial waste in India to be 40,734 Mm3/year. Furthermore, it has been reported that USW contains an easily biodegradable or- ganic fraction of up to 40% [6]. Methane generated from fermentation of sewage sludge, Organic Fraction of Mu- nicipal Solid Waste (OFMSW) was investigated and biogas obtained contained about 60% methane gas. The biogas productivity varied between 0.4 and 0.6 dm3/g. All these show that there is a high potential of biogas generation from anaerobic biodegradation of SW, espe- cially, SWOM. The prospects of biogas generation from anaerobic di- gestion of other waste of biomass elsewhere have also been studied. In a comparative study of digestion of sewage sludge (SS) and OFMSW, the cumulative biogas production for SS was found to be lower than that for OFMSW [7]. MSW has been one of the most potential feedstock for many anaerobic digestion processes [8,9]. These studies also show a high prospect of generating biogas from SWOM. 2.2. Advantages of USW The availability and abundance of SWOM in urban cen- ters is of great advantage for biogas generation. The bio- conversion of USW process is non-polluting and envi- ronmentally friendly, involves less capital investment in relation to other renewable energy resources such as hy- dro-power, solar and wind energy. Also, biogas is avail- able as a domestic resource in rural areas, which makes it not subject to world price fluctuations and unpredictable supplies of conventional fuels [5]. 2.3. Factors Affecting Biodegradation Process Properties of the raw USW particularly the amount of biodegradable matter affect its biodegradability. Wang 2004 [10] classified the biodegradable fraction as rapidly, moderately and slowly biodegradable organic matter basing on physical parameters of solubility and particu- late level and biological factor of presence and type of microbes. There are many other factors that influence biodegradation process among which are retention time, recycle leachate, pH, organic loading rates and substrate type. It has been found that the mean daily biogas pro- duction and yield per unit weight of waste increased for high retention time. The mean daily biogas yield per unit waste was 51.6 L biogas/kg day for high retention time compared to 48.7 L biogas/kg day for low retention time, attributed to longer digestion period [11]. Nevertheless, the volume of biogas generated per unit weight of par- tially solid fruit waste combined with assimilated sludge at shorter retention time had higher biogas production compared to that produced with high retention time but without recycled digested sludge [11]. This shows that recycle digested sludge influence biogas production more than retention time. In addition, several authors found that the time required for complete digestion was large because the SW dissolution and its hydrolysis to lower- molecular-weight compounds were the rate limiting steps in the anaerobic digestion process [5,12,13]. Furthermore, the stability of the anaerobic process and the rate of bio- gas production depended on organic loading rates [5]. It was also shown that anaerobic digestion became more stable when a variety of substrates were applied [7]. In the general sense, during anaerobic digestion, microor- ganisms utilize carbon 25 - 30 times faster than nitrogen [14]. Co-digestion improved nutrient balance by adding large quantities of carbon being readily biodegradable resulting in enhanced biogas yields [7]. One of the important parameters affecting OFMSW biodegradation is moisture content, which can be regu- lated by way of leachate recirculation [15]. The idea of enhancing refuse decomposition by addition of water and/or re-circulating leachate was first proposed several decades ago [16]. Leachate re-circulation promotes bio- degradation process because liquid movement spreads out the microbial inoculum, mitigates local nutrient shor- tages and offsets potential toxins. However, in the ab- sence of active acetogenic and methanogenic populations, re-circulated leachate may cause an accumulation of volatile fatty acids (VFA). Sosnowski, Klepacz-Smolka, Kaczorek and Ledakowicz 2008 [7] found that accumu- lation of VFA caused pH decrease and strongly inhibited subsequent biogas production. A combination of leachate recirculation and pH adjustment can minimize the in- hibitory effects of acid accumulation and accelerate the rate of SW biodegradation. Leachate recycle is therefore an important component in biogas production from USW leading to its pH recovery. This technique was employed in the present research 2.4. Compositing Kinetics Composting (that is, biodegradation in the presence of oxygen) has gained an important role in USW manage- ment. Composting kinetics has been investigated recently by many researchers to describe the decomposition of organic wastes. For example, the kinetics of co-com- posting of Rose processing waste and OFMSW under Copyright © 2013 SciRes. ENG  W. WANASOLO ET AL. 579 aerobic conditions was evaluated and the results showed first order kinetics as the best fitting kinetic model [13]. No model has been proposed to fix the kinetic orders for anaerobic processes of USW. Several other researchers used first order kinetic models to describe different bio- degradation processes. Kirchmann and Bernal, 1997 [17] applied first-zero-order model for aerobic biodegradation of different material types such as cattle dung, pig dung, and sewage sludge-cotton waste mixture. Paredes, Bernal, Cegarra and Roig, 2002 [18] found that organic matter losses followed a first-order kinetic equation for aerobic biodegradation of olive mill wastewater sludge. Baptista, Antunes, Gonçalves, Morvan and Silveira, 2010 [19] investigated the kinetics of solid waste compositing based on VS change and the experimental data were fit- ted with a first-order kinetic model, and a rate constant of composting under optimum conditions was obtained. 2.5. Modeling Kinetics Modeling has often been used as the main tool in the study of composting as well as anaerobic biodegradation processes, frequently with the aim of optimizing the de- sign and operation of full-scale plant. Such studies yielded models such as the Anaerobic Digestion Model No. 1 (ADM 1) [20] and several other mathematical models. However, few studies have applied such models to full scale plants [19]. Besides, Kinetic models for an- aerobic digestion of organic substrates were derived for substrate utilization and methane production [21]. The model equations considered hydrolyzed products as lim- iting nutrients for microbial growth and biogas produc- tion according to Monod kinetics. Additionally, a kinetic model for investigation of the anaerobic digestion of wastewater generated from orange rind pressing during orange juice making was proposed basing on the experi- mental results determined at mesophilic conditions [22]. Monod type kinetic models were also widely used to describe process kinetics of anaerobic digesters success- fully [15,23]. Although there was some success in ap- plying Monod type kinetics to the anaerobic process, some researchers found it difficult to apply them for their systems [15,23]. Furthermore, a two-stage model com- bining zero and first order kinetics based on enzyme re- action and Monod type micro-organism growth rate equation was proposed and developed for handling hos- pital waste biodegradation in landfills [10]. This model successfully predicted both the cumulative biogas pro- duction and its rate. It assumed zero order kinetics at the start of the process followed by a first order kinetics with respect to biodegradable organic carbon. The model did not differentiate between the time when the zero order ends and the start of first order and therefore it was used ambiguously. Also, Garcı́a-Ochoa, Santos, Naval, Guar- diola and Lopez, 1999 [12] developed two separate ki- netic models to explain the anaerobic digestion of live- stock refuse. This model replicated the experimental data obtained for cow manure anaerobic digestion with more accuracy. In this study, the simulation model developed by Wang, 2004 [10] was adopted and modified in derivation. It was assumed that the biodegradation of organic matter depended on both the amount of biodegradable organic matter present and moisture content as the primary limit- ing factors. The model selection was influenced by the strength of experimental support and mathematical deri- vations. 3. Methods and Equipment 3.1. Design Features of Experimental Setup The experimental set-up comprised of three sets of bio- reactor cells shown in Figure 1. The first set had three cells in series labeled BA1, BA2 and BA3. The second had two: BB1 and BB2 and the third had one, BC1. Each of these cells was of volume 3 liters. Leachate was col- lected in tanks Lt1, Lt2 and Lt3 and recycled using pumps P1, P2 and P3, respectively. The pipe system was made of IPS material to limit corrosion and contamination. Bio- gas was tapped to the gas measuring device LI1 con- nected to a calibrated manometer LI2. Using control valve CV1, the gas volume was measured at regular time intervals and collected in gas collection bag GB. In gen- eral a batch type of bioreactors in series was operated at temperature range of 28˚C - 38˚C. 3.2. Data Collection Three batches of 12.0, 12.9 and 13.2 kg were prepared at three different times. Batch one of 12.0 kg was distrib- uted in six bioreactor cells of Figure 1. Three experi- ments of weight (Mw) = 6 kg (set one), 4 kg (set two) and 2 kg (set three) each were carried out simultaneously over a period of about one week. This was repeated for batches two and three. All the batches comprised of food residues, fruit waste and non biodegradables shown in Figure 2. The SW was sorted and categorized as biodegradable food residues (BFR), biodegradable fruit waste (BFW) and non-biodegradable waste (NBW). This categoriza- tion is shown in Table 1 while Table 2 shows the moles of biodegradable matter of batches one, two and three. In the first batch, the different categories of waste were mixed and distributed in the six bioreactor cells each taking about 2 kg. Water of pH 7.04 and microbial in- oculum were added at temperature of 32˚C. The bioreac- tors were then completely sealed to avoid oxygen inter- ference and allow mesophilic biodegradation process to take place. The biogas volume measurements were done Copyright © 2013 SciRes. ENG 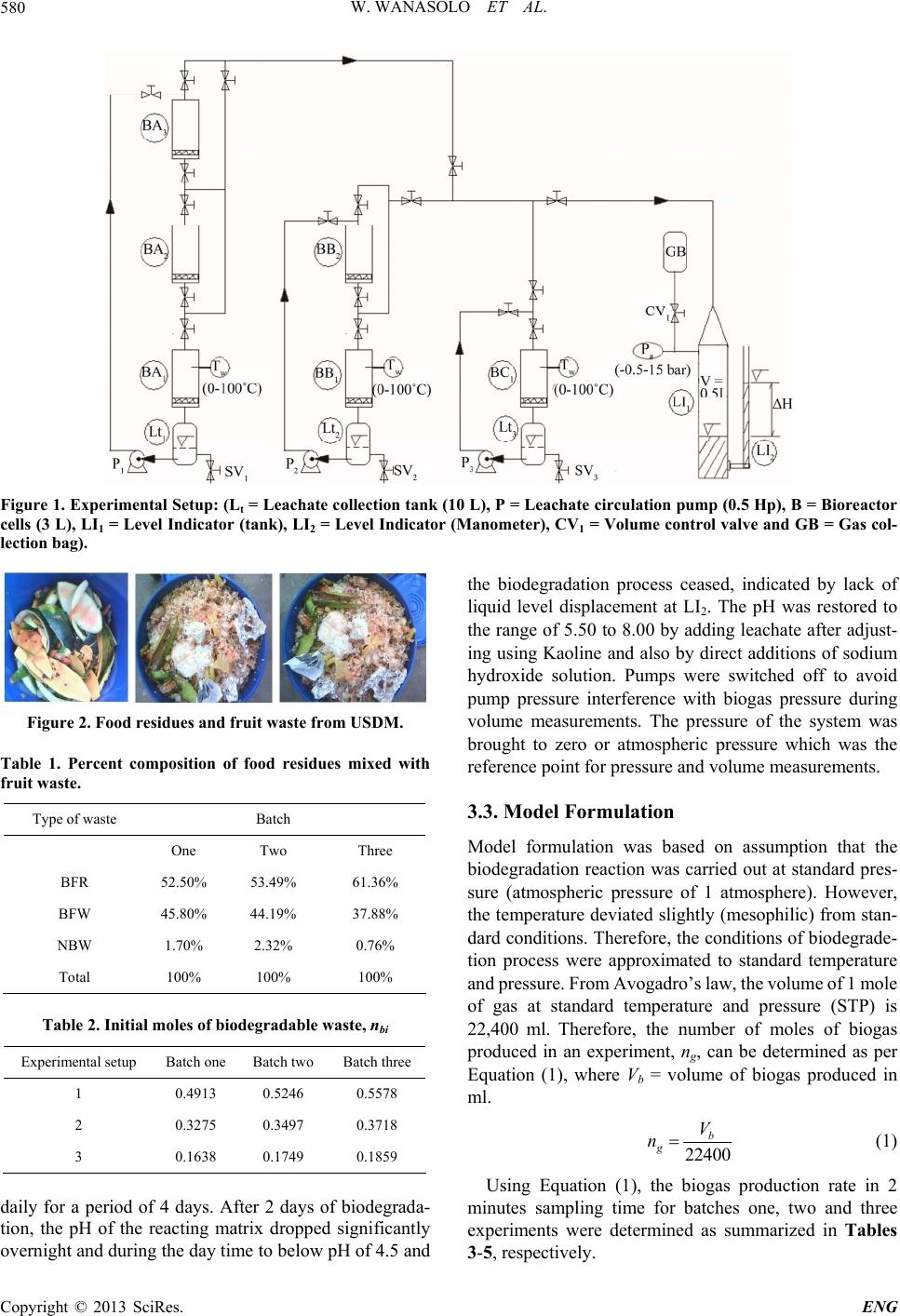 W. WANASOLO ET AL. Copyright © 2013 SciRes. ENG 580 Figure 1. Experimental Setup: (Lt = Leachate collection tank (10 L), P = Leachate circulation pump (0. 5 Hp), B = Bioreactor cells (3 L), LI1 = Level Indicator (tank), LI2 = Level Indicator (Manometer), CV1 = Volume control valve and GB = Gas col- lection bag). the biodegradation process ceased, indicated by lack of liquid level displacement at LI2. The pH was restored to the range of 5.50 to 8.00 by adding leachate after adjust- ing using Kaoline and also by direct additions of sodium hydroxide solution. Pumps were switched off to avoid pump pressure interference with biogas pressure during volume measurements. The pressure of the system was brought to zero or atmospheric pressure which was the reference point for pressure and volume measurements. Figure 2. Food residues and fruit waste from USDM. Table 1. Percent composition of food residues mixed with fruit waste. Type of waste Batch One Two Three BFR 52.50% 53.49% 61.36% BFW 45.80% 44.19% 37.88% NBW 1.70% 2.32% 0.76% Total 100% 100% 100% 3.3. Model Formulation Table 2. Initial moles of biodegradable waste, nbi Experimental setup Batch oneBatch two Batch three 1 0.4913 0.5246 0.5578 2 0.3275 0.3497 0.3718 3 0.1638 0.1749 0.1859 Model formulation was based on assumption that the biodegradation reaction was carried out at standard pres- sure (atmospheric pressure of 1 atmosphere). However, the temperature deviated slightly (mesophilic) from stan- dard conditions. Therefore, the conditions of biodegrade- tion process were approximated to standard temperature and pressure. From Avogadro’s law, the volume of 1 mole of gas at standard temperature and pressure (STP) is 22,400 ml. Therefore, the number of moles of biogas produced in an experiment, ng, can be determined as per Equation (1), where Vb = volume of biogas produced in ml. 22400 b g V n (1) Using Equation (1), the biogas production rate in 2 minutes sampling time for batches one, two and three experiments were determined as summarized in Tables daily for a period of 4 days. After 2 days of biodegrada- tion, the pH of the reacting matrix dropped significantly overnight and during the day time to below pH of 4.5 and 3-5, respectively.  W. WANASOLO ET AL. 581 3.4. Order of Reaction and Rate Constant ion of goving the ationattd in USW. The m molof orga is r by Aof The general biochemical equation for biodegradat organic matter can be written as per Equation (2). ABrCsD tEuF (2) where A, B, C, D, E and F are biodegrada matter, moisture, methane, carbon dioxide, a ble organic mmonia and biomass, respectively; α, β, r, s, t and u are stoichiomet- ric coefficients. The rate of biogas production is given by Equation (3): d d q C rkAB t (3) where p and q were the proposed ord determined and which are not necessarily equal to the ers of reaction to be stoichiometric coefficients of reactants in Equation (2). The biogas produced was assumed to be a mixture of methane, carbon dioxide and ammonia in Equation (2). Equation (3) is the proposed biodegradation rate law Table 3. Batch one gas rate (mol/min) at 2 min sampling me. ti Residence Experiment 3 Experiment 2 Experiment 1 Time (days) M = 2 kg M = 4 kg M = 6 kg w w w 1 5.208 × 10−5 1.105 × 10−4 1.548 × 10−4 2 4.464 × 10 −58.929 × 10 −51.289 × 10 −4 3 4.911 × 10 −5−4−4 −5−5−4 1.049 × 10 1.406 × 10 4 4.688 × 10 8.482 × 10 1.384 × 10 Table 4. Batche ( 2 me. two gas ratmol/min) atmin sampling ti Residence Experiment 3 Experiment 2 Experiment 1 Time (days) Mw = 2.15 kg Mw = 4.30 kg Mw = 6.45 kg 1 3. −5 311 × 10423 × 10026 × 10 −5−5−4 −5−5−5 −56. −5 −51. −4 −5 2 3.571 × 10 6.679 × 10 1.111 × 10 3 3.823 × 10 7.422 × 10 1.199 × 10 4 2.065 × 10 4.088 × 10 5.802 × 10 Table 5. Batceng me. h three gas rat (mol/min) at 2 min sampli ti Residence Experiment 3 Experiment 2 Experiment 1 Time (days) M = 2.25 kg M = 4.50 kg M = 6.75 kg w ww 1 1.53 × 10 −5 −53.05 × 10 −5 −54.58 × 10 −4 −4 2 3.91 × 10 7.81 × 10 1.17 × 10 3 4.13 × 10 ernbiodegrad of organic mer containe equation is in nic matter differential for epresented where the and that es moisture by B. The exact numerical value of p was de- termined from the experimental data using initial rate method. The rate of organic matter consumption can be expressed as the rate of biogas production and it is equal to the rate of decomposition of substrate organic matter, A, and that of moisture as summarized in Equation (4). ddd ddd CAB rttt (4) The value of p was established by making the amount of organic matter, A, a limiting factor whi present in excess. All other factors namely temperature, pH e experiments of batches one, two and three (Tables 3-5) a (5) gives a set of three equations an tions were determined after evaluating the , from Equation (5): le moisture was , nutrient level, micro-organisms, etc., were kept at optimal quantities, and assumed to be constant. Using any of Equation (3) or (4) the biodegradation rate law is reduced to Equation (5), i.e., p rkA (5) Based on data from the nin nd applying Equation with two unknowns p d k, solutions of which yield three values of p. Using the arithmetic average value of p, three values of k were also determined. Hence the arithmetic average values of p and k for the three experiments of batch one at resi- dence time of 1 day were determined. This process was repeated for residence times of 2, 3, and 4 days for batch one and for batches two and three. Results are as shown in Table 6. 3.5. Model Equations Model equa values of pav, and kav. Thus d d av A rkA t (6) Substituting for p = pav = 0.994 into Equation (6) and integrating between limits t = (0, t) a to Equation (7). s the solution to Equation (6). At is the kmol of biodegradable organic matter rem time t, A is the initial kmol of biodegradab m nd A = (Ao, At) leads 166.67 0.006 0.006 to av AA kt (7) Equation (7) i aining after le organic o atter and kav is the average rate constant in kmol0.006· min−1. Differentiating Equation (7) with respect to time, gives the rate of biodegradation in kmol/min at any time, t, in minutes as per Equation (8): −5−5−4 4 3.79 × 10−5 7.59 × 10−5 1.14 × 10−4 8.26 × 10 1.24 × 10 165.67 0.006 d0.006 d t av oav AkA kt t (8) Equation (8) is the model equation for rate of reaction Copyright © 2013 SciRes. ENG  W. WANASOLO ET AL. 582 Table 6. Arithmetic average rate orders and ra R te constants. t (days) kinetic order, p Rate constant, k Batch one Batch two Batch three Batch one Batch two Batch three 1 0.969 1.047 0. 0.00038 Avge Overall average 0.006·m −1 06· 985 1 0.0002 0.0000 2 0.957 1.063 0.963 0.00026 0.00021 0.00020 3 0.925 1.061 0.947 0.00029 0.00023 0.00021 4 1.016 0.930 1.065 0.00026 0.00012 0.00021 era0.967 1.025 0.990 0.00028 0.00019 0.00018 0.994 0.0002 or 0.3 2 mol 93 mol0.00 in day−1 Re d inorem ining biodegtter The kmol of biodegradable or- biodegraded after time t (minutes), t = residenc time inays. terms f the aradable organic ma At, at time t (minutes). anic matter that wasg denoted by ∆Aot, can be determined as per Equation (9): oto t AA (9) According to Wang 2004 [10], this corresponds to the kmol of biogas produced in time t (min). From Avo- gadro’s law, the volume of 1 mole of gas at standard temperature and pressure is 22.4 liters·mol−1. This law applies to atomic and gaseous species. That is, 1 mole of atomic species occupies 22.4 liters·mol−1 of volume. Since 1 mole of biodegraded carbon atoms corresponds to 1 mole of biogas produced, then 1 mole of biodegraded car- bon atoms corresponds to 22.4 liters of biogas. Thus, (Ao − At) kmol of biodegraded carbon corresponds to 22400 (Ao − A t) 103 liters of biogas. If this volume of biogas produced in time t is denoted by Dt, then: 22400 tot DAA (10) Substituting for At from Equation (7) into Equation (10) leads to: (11) is the model equation for the volume of gas produced in sampling time t (minutes). gas production in sampling time t is obtaine en 166.67 0.006 00 22400 0.006 tav DAAkt (11) Equation The rate of d by differ- tiating Equation (11) which gives Equation (12). 165.67 0.006 d22400 0.006 d t av oav DkA kt t (12) Equation (12) is the kinetic model equation for biogas production rate in dm3·min−1 at time, t, in min maximum volume of biogas occurs when the rat to th utes. The e is equal zero. By equating Equation (12) to zero and solving, e result is as per Equation (13): 0.006 max 0.006 o av A tk (13) Equation (13) gives the time at which maximum bio- gas volume occurs. Furthermore, the approximate time, t1/2, for half of the initial substrate to half-life can be obtained as per Equation (14). biodegrade, called 0.006 12 0.012 o av A tk (14) 4. Data Presentation and Discussion 4.1. Model Equations and Para The arithmetic average kinetic order and rate constant 1, respec- ers and rate in Table 7. work Bernal (1997) (2002) (2004) meters obtained were 0.994 and 0.3093 mol0.006·day− tively. These were compared with kinetic ord constants from other researches as shown From this table, the zero and first orders were used by other researchers and not the second and third orders. Secondly, the kinetic order of 0.994 obtained in this re- search is close to the first order used by most other re- searchers. It can also be seen that different substrate ma- terials, namely, cow dung, pig dung, poultry excreta, olive mill waste and hospital waste were used by differ- ent researchers while this research used food residues mixed with fruit wastes. All these and the results indicate that the kinetic order of USW biodegradation processs can be zero, first, close to first or both zero and first or- ders depending on the substrate material under investiga- tion and the conditions of biodegradation process. This can be attributed to the type, complexity, and nature of SW containing the biodegrading organic matter. Since this research was done under a pH range of 6 to 8, the kinetic order obtained being close to first order kinetics is appropriate and suitably used to model the kinetic equa- tions of the biodegradation process under study. In con- clusion, the rate constant was slightly higher than that of other substrate materials indicating that food residues mixed with fruit waste gave a slightly higher biodegrada- tion rate than that obtained using pig dung, cow dung, poultry, etc., used by other researchers to generate bio- gas. Table 7. A comparison of rate orders and rate constants. Name This Kirchmann and Parades et al. Wang et al. Average k or inetic der 0.994 Zero and first order First order Zero and first Average ·day−1ung) reta) ay−1 (olive mill ay t . rate constant kav 0.3093 mol0.006 0.078 day−1 (cow dung), 0.131 (pig d and 0.093 (poultry exc 0.0181 d waste) 0.0018 d−1 (hospital waste), firs order Copyright © 2013 SciRes. ENG  W. WANASOLO ET AL. 583 4.2. Effect of Substrate Weight and Residen Tim Figures ce s rate and e on Cu 3 ad 4 sh m lu nriatibioga cumulative volume,with substrate weight nd he biogutes sampling ulative Vo ow the va respectively, me on of and residence time. From the figures the biogas rate a cumulative volume increase with substrate weight. T as rate and cumulative volume at 2 min time are almost doubled when the substrate weight dou- bles from 2 to 4 kg for batch one, 2.15 to 4.30 kg for batch two and 2.25 to 4.5 kg for batch three. These val- ues go up almost by three times when substrate weight triples. For example, at 1 day residence time and for 2 minutes sampling time of batch one, the cumulative volume is 2.5 ml for the 2 kg substrate weight. This volume becomes about 5 ml when the substrate weight is increased to 4 kg and it is about 7 ml when the substrate weight triples to 6 kg. This implies that increasing substrate weight increases volume of gas generated. This observation agrees well with research findings by Rao, Baral, Dey and Mutnuri 2010 [5] who found that biogas generation directly de- pends on organic loading rates. Increasing organic load- ing rates increases the amount of biogas generated. On the whole, the cumulative gas volume increases with weight of substrate material at all residence times. There- fore, in order to generate a high volume of biogas the weight of substrate material has to be increased. Sampling time (min) Batch one 2 4 6 8 10 2 4 6 8 1 4 2 3 02 4 6 82 4 6 8 Batch two 1 4 3 2 0 Gas rate (ml/min) Rt = 4 days Batch three Rt = 3 days Rt = 2 days 5 1 4 2 3 0 Rt = 1 day Figure 3. Variation of biogas rate with substrate weight and residence time. Sampling time (min) 2 4 6 8 2 0 5 10 15 20 2 4 6 8 22 4 6 8 22 4 6 8 10 Batch oneBatch two Cumulative volume (ml) 0 5 10 Rt = 4 days 15 20 Batch three Rt = 3 days Rt = 2 days 0 5 10 25 15 20 Rt = 1 day Figure 4. Variation of cumulative volume with substrate weight and residence time. Secondly, from the figures it can also be seen that ini- tially the cumulative volume increases with residence time and generally declines after 2 days residence time for all batches. This implies that the longer the residence time, the lower the increment in cumulative volume of gas generated for a given sampling time. Therefore, for high volume of biogas generation at longer residence time, fresh substrate material should be added to the bio- reactor cell. Furthermore, the figures also show that at high sampling time of 6 minutes and above, the bioga erimental and model values. From these figures, the deviation between model and experi- First, the residence time has s rate and cumulative volume are generally constant, espe- cially for low substrate weight. This indicates that the rate of gas generation decreases with sampling time and residence time. The fairly constant biogas rates and cu- mulative volume at high sampling time can be attributed to the increase in gas pressure which ultimately has a negative impact on biogas production. High pressures in the bioreactor cell inhibit biodegradation reaction leading to the almost constant biogas rate and cumulative volume at 6 minutes and above of sampling time. This implies that for continuous biodegradation and gas generation, the already generated biogas should be evacuated from the bioreactor space above the SW material in order to reduce bioreactor cell pressure and foster subsequent biogas production. 4.3. Model Validation In order to validate the model equations the amount of biogas generated experimentally was measured and com- pared with that calculated using Equation (11). Figures 5-7 show a comparison of cumulative volume of biogas obtained using the model equation and that determined experimentally for batches one, two and three, respec- tively. The three figures show the effect of initial amount of substrate weight on the cumulative volume of biogas. They also show the effect of residence time on the devia- tions between exp mental data is discussed. negligible impact on variations between model and ex- perimental data for all values of initial substrate weight. This implies that the deviation between model and ex- perimental data is negligible for different residence times at a given initial substrate weight. This is because the almost constant substrate weight does not have an impact on the quantity or volume of biogas generated at different residence times, other fac- tors of pH and temperature remaining constant. There- fore, the mathematical model can predict the experimen- tal data of the same substrate weight at different resi- dence times within experimental error. This means a high reproducibility of model data for the same initial sub- strate weight. Copyright © 2013 SciRes. ENG  W. WANASOLO ET AL. 584 Rt = 1 day 0 5 10 15 20 25 30 4 kg exp'tal 6 kg exp'tal 2 kg exp'tal Rt = 3 days Sampling time (min) Cumulative volume (ml) 0 5 10 15 20 25 2 4 6 8 Rt = 4 days 246810 Rt = 2 days 2 kg model 4 kg model 6 kg model Figure 5. Comparison of model and experimental data for batch one. Rt = 1 day 0 5 10 15 20 25 30 2.15 kg expt 4.30 kg expt 6.45 kg expt Rt = 2 days 2.15 kg mdl 4.30 kg mdl 6.45 kg mdl Rt = 4 days 24681 Rt = 3 days Sampling time (min) Cumulative volume (ml) 2 4 6 8 0 5 10 15 20 25 Figure 6. Comparison of model and experimental data for atch two. b Rt = 2 days 2.25 kg mdl 4.50 kg mdl 6.75 kg mdl Rt = 1 day 0 5 10 15 20 25 30 2.25 kg expt 4.50 kg expt 6.75 kg expt Rt = 4 days 24681 Rt = 3 days Sampling time (min) Cumulative volume (ml) 2 4 6 8 0 5 10 15 20 25 Figure 7. Comparison of model and experimental data for batch three. Secondly, the initial weight of substrate has a direct impact on the variation between model and experimental data. This implies that increasing initial amount of sub- strate increases the deviations between model and ex- perimental data. This can be attributed to the increase in cumulative volume of biogas generated as a result of increase in substrate weight. This increase in cumulative volume causes a corresponding pressure increase in bio- reactor cell which suppresses subsequent generation of biogas, resulting in little or no increment in quantity of bserved. Thus, for the mathematical model to predict well the experimental data, the biogas generated should no model data. Generally, the m total cumulative volume of experimental data. Eventually a high variation between model and experimental data is o t be allowed to accumulate in the bioreactor cell such that pressure increases which reduce subsequent gas generation do not occur. This results in high predictabil- ity of experimental data. Furthermore, the deviation between model and ex- perimental data of cumulative volume of biogas was ob- served to be minimal for the low SW weight throughout the sampling time. This is because the small substrate weight generates small volume of biogas and therefore low pressures which do not have great impact on subse- quent biogas generation. Consequently at low pressures the experimental data do not vary so much from model data, enhancing the reliability of model data. Beyond 6 minutes of sampling time and at high SW weight the deviations were extraordinarily high. This can be attrib- uted to high cumulative volume of biogas caused by long sampling time and high substrate weight, resulting in reduced accuracy of the odel and experimental data agreed most with ANOVA, p = 0.0000063 (Table 8) and least with ANOVA, p = 0.21 (Table 9). The reproducibility of model data is high for different residence times at constant substrate weight. The predictability and reliability are high with low substrate weight while the accuracy of the model data is reduced with high substrate weight and longer sampling time. 5. Conclusion Using initial rate method the arithmetic average values of kinetic order and rate constant obtained were consistent with values assumed and used in other researches in lit- erature. The rate constant for food residues mixed with fruit waste was slightly higher than that of other substrate Table 8. Statistical significance of batch one, experiment 1 at 3 days residence time . Source of Variation df F P-value F crit Rows 4 688.1551 6.31E-06 705.7732 Columns 1 51.20627 0.002018 996.6675 Error 4 Total 9 Table 9. Statistical significance of batch one, experiment 3 at 1 day residence time. Source of Variation dfF p-value F crit Rows 4 2.384484 0.21031 2.3873 Columns 1 2.8574 8 Total 9 810.1684 2.2258 Error 4 Copyright © 2013 SciRes. ENG  W. WANASOLO ET AL. 585 maplyg aggrade than obtainng pig dung, cow dung, poultr usedher researchers to generate ogas. This i portnsidrenewable energy sources for im roved efficiencies and yield. The cumulative gas idence times. In order to gen a hie of biogas the weight of substrate material hascreased. Alsor high volud high tion e atsidee, fb- straterial shold beo thctor for us bdegandnerae alreaneratebiogas ould be removed fro the bioreactor in order to ena le more biogas production. Biotechnology, Vo 85, No. 4, 2010, pp. 849-860. 246-7 terials imin slightly hiher biodeation rat that by ot ed usiy, etc., s im-bi ant coering - vop l- ume and gas generation rate increase with weight of sub- strate material at all reserate gh volum to be in, fo biogasme an genera mate rat u longer re added t nce tim e biorea resh su cell and continuoioradation gas getion, th dy ged shm b Increasing the substrate weight increased the absolute deviation, absolute mean and standard deviations, attrib- uted to the increase in biogas pressure caused by cumula- tive gas volume in the manometer. The longer the resi- dence time the lower the absolute deviation, absolute mean and standard deviations, attributing to the low gas pressure due to low cumulative volume. The model pre- dicted well the experimental data for low substrate weight at all residence times and for high substrate weight at longer residence time. For the model which has predicted well the experimental data at higher substrate weight and shorter residence time, the biogas generated should have been removed from the bioreactor cell immediately when it is formed. It would be helpful to pursue further re- search about the study and experimental design consid- ering detaching the cumulative volume measuring unit from the bioreactor cell thereby eliminating biogas ac- cumulation within the bioreactor cell. 6. Acknowledgements The success of this work has been made possible with financial assistance from Kyambogo University, Uganda and University of Dar es Salaam, Tanzania. REFERENCES [1] OECD/IEA, “World Energy Outlook, International En- ergy Agency,” OECD/IEA, Paris, 2006. [2] OECD/IEA, “Working Together to Ensure Reliable, Af- fordable and Clean Energy, International Energy Agency,” 2012, [3] P. Weiland, “Biogas Production: Current State and Per- spectives,” Applied Microbiology andl. doi:10.1007/s00253-009-2 007, pp. 301- 304. doi:10.1016/j.jhazmat.2006.07.003 [4] D. Elango, M. Pulikesi, P. Baskaralingam, V. Rama- murthi and S. Sivanesan, “Production of Biogas from Municipal Solid Waste with Domestic Sewage,” Journal of Hazardous Materials, Vol. 141, No. 1, 2 [5] P. V. Rao, S. . Mutnuri, “Biogas o. 7, 2010, pp. S. Baral, R. Dey and S Generation Potential by Anaerobic Digestion for Sus- tainable Energy Development in India,” Renewable and Sustainable Energy Reviews, Vol. 14, N 2086-2094. doi:10.1016/j.rser.2010.03.031 [6] P. Sosnowski, A. Wieczorek and S. Ledakowicz, “An- aerobic Co-Digestion of Sewage Sludge and Organic 49-7 Fraction of Municipal Solid Wastes,” Advances in Envi- ronmental Research, Vol. 7, No. 3, 2003, pp. 609-616. doi:10.1016/S1093-0191(02)000 a, K. Kaczorek and S. [7] P. Sosnowski, A. Klepacz-Smolk Ledakowicz, “Kinetic Investigations of Methane Co- Fermentation of Sewage Sludge and Organic Fraction of Municipal Solid Wastes,” Bioresource Technology, Vol. 99, No. 13, 2008, pp. 5731-5737. doi:10.1016/j.biortech.2007.10.019 [8] K. Braber, “Anaerobic Digestion of Municipal Solid Waste: A Modern Waste Disposal Option on the Verge of Breakthrough,” Biomass and Bioenergy, Vol. 9, No. 1-5, 1995, pp. 365-376. doi:10.1016/0961-9534(95)00103-4 [9] A. Ambulkar and A. Shekdar, “Prospects of Biomethana- tion Technology in the Indian Context: A Pragmatic Ap- proach,” Resources, Conservation and Recycling, Vol. 40, No. 2, 2004, pp. 111-128. doi:10.1016/S0921-3449(03)00037-5 [10] Q. Wang, “Aspects of Pretreated Hospital Waste Biodeg- s Production and Its d Management, Vol. radation in Landfills,” Department of Mechanical Engi- neering, University of Duisburg-Essen, Essen, 2004. [11] G. Lastella, C. Testa, G. Cornacchia, M. Notornicola, F. Voltasio and V. K. Sharma, “Anaerobic Digestion of Semi-Solid Organic Waste: Bioga Purification,” Energy Conversion an 43, No. 1, 2002, pp. 63-75. doi:10.1016/S0196-8904(01)00011-5 [12] F. Garcı́a-Ochoa, V. Santos, L. Naval, E. Guardiola and B. Lopez, “Kinetic Model for Anaerobic Digestion of Live- stock Manure,” Enzyme and Microbial Technology, Vol. 25, No. 1-2, 1999, pp. 55-60. doi:10.1016/S0141-0229(99)00014-9 [13] I. Tosun, M. Gönüllü, E. “Co-Composting Kinetics of Rose Pro Arslankaya and A. Günay, cessing Waste with Organic Fraction of Municipal Solid Waste,” Bioresource Technology, Vol. 99, No. 14, 2008, pp. 6143-6149. doi:10.1016/j.biortech.2007.12.039 [14] T. Sreekrishnan, S. Kohli and V. Rana, “Enhancement of Biogas Production from Solid Substrates Using Different Techniques—A Review,” Bioresource Technology, Vol. 95, No. 1, 2004, pp. 1-10. doi:10.1016/j.biortech.2004.02.010 002/bit.10450 [15] V. Vavilin, S. Rytov, L. Lokshina, S. Pavlostathis and M. Barlaz, “Distributed Model of Solid Waste Anaerobic Digestion: Effects of Leachate Recirculation and pH Ad- justment,” Biotechnology and Bioengineering, Vol. 81, No. 1, 2003, pp. 66-73. doi:10.1 Stabilization with Bio- [16] F. G. Pohland, “Sanitary Landfill Leachate Recycle and Residual Treatment, Epa,” Civil Engineering, Vol. 106, No. 6, 1975, pp. 1057-1069 [17] H. Kirchmann and M. Bernal, “Organic Waste Treatment and C Stabilization Efficiency,” Soil Biology and Copyright © 2013 SciRes. ENG  W. WANASOLO ET AL. Copyright © 2013 SciRes. ENG 586 pp. 1747-1753. chemistry, Vol. 29, No. 11-12, 1997, doi:10.1016/S0038-0717(97)00065-5 [18] C. Paredes, M. Bernal, J. Cegarra and A. Roig, “Bio- Degradation of Olive Mill Wastewater Sludge by Its Co-Composting with Agric Technology, Vol. 85, No. 1, 2002, pp ultural Wastes,” Bioresource . 1-8. doi:10.1016/S0960-8524(02)00078-0 [19] M. Baptista, F. Antunes, M. S. Gonçalves, B. Morvan and A. Silveira, “Composting Kinetics in Full-Scale Me- chanical-Biological Treatment Plants,” Waste Manage- ment, Vol. 30, 2010, pp. 1908-1921. [20] R. K. Dereli, M. E. Ersahin, H. Ozgun, I. Ozturk and A. F. Aydin, “Applicability of Anaerobic Digestion Model No. 1 (Adm1) for a Specific Industrial Wastewater: Opium Alkaloid Effluents,” Chemical Engineering Journal, Vol. 165, No. 1, 2010, pp. 89-94. doi:10.1016/j.cej.2010.08.069 [21] A. Barthakur, M. Bora and H. D. Singh, “Kinetic Model for Substrate Utilization and Methane Production in the Anaerobic Digestion of Organic Feeds,” Biotechnology Progress, Vol. 7, 1991, pp. 369-376. doi:10.1021/bp00010a012 [22] J. Siles, M. Martin, A. Chica and R. Borja, “Kinetic Mod- elling of the Anaerobic Digestion of Wastewater Derived from the Pressing of Orange Juice Manufacturing,” Chemi Rind Produced in Orange cal Engineering Journal, Vol. 140, No. 1-3, 2008, pp. 145-156. doi:10.1016/j.cej.2007.09.026 [23] K. P. Singh, S. Gupta, A. K. Singh and S. Sinha, “Opti- mizing Adsorption of Crystal Violet D Magnetic Nanocomposite U ye from Water by sing Response Surface Mod- eling Approach,” Journal of Hazardous Materials, Vol. 186, 2011, pp. 1462-1473.
|