Paper Menu >>
Journal Menu >>
 Natural Resources, 2010, 1, 104-109 doi:10.4236/nr.2010.12011 Published Online December 2010 (http://www.SciRP.org/journal/nr) Copyright © 2010 SciRes. NR Adsorption of Pb(II) onto Modified Rice Bran Hengpeng Ye*, Zhijuan Yu School of Chemistry and Material Science, South Central University for Nationalties, Wuhan, China. Email: *yehengpeng@126.com Received November 3rd, 2010; revised November 25th, 2010; accepted November 26th, 2010. ABSTRACT In this study, the modified rice bran was tested to remove Pb(II) from water. Batch experiments were carried out to evaluate the adsorption characteristics of the modified rice bran for Pb(II) removal from aqueous solutions. The ad- sorption isotherms, thermodynamic parameters, kinetics, pH effect, and desorbability were examined. The results show that the maximum adsorption capacity of the modified rice bran was approximately 70.8 mg Pb(II)/g absorbent at tem- perature of 25℃ and at the initial Pb(II) concentration of 400 mg/L and pH 7.0. And the adsorption isotherm data could be well fitted by both Langmuir equation and Freundlich equation. Thermodynamic studies confirmed that the process was spontaneous and endothermic. The adsorbed amounts of Pb(II) tend to increase with the increase of pH. The adsorption kinetic data can be satisfactorily described by either of the power functions and simple Elovich equa- tions. The desorbability of Pb(II) is about 15-20%, and it is relatively difficult for the adsorbed Pb(II) to be desorbed. The relatively low cost and high capabilities of the rice bran make it potentially attractive adsorbent for the removal of Pb(II) from wastewater. Keywords: Rice Bran, Pb(II) Removal, Adsorption Capacity, Adsorption Isotherm 1. Introduction Duo to the toxicological importance in the ecosystem, agriculture and human health, pollution by heavy metals has received wide spread attention in the recent years. Lead, an element which has been used by man for years, can be regarded as a longstanding environmental con- taminant. All lead compounds are cumulative poisons. Several industries like mining, printing, painting, dyeing, battery manufacture and other industries discharge ef- fluent containing lead, to surface water. Lead has toxic effects on neurobehavioral development and on the brain cell function [1]. The accumulation of lead in river beds [2] has been detected and gives cause for concern. This underlines the need for developing methods for effective removal of lead from water at least below the regulatory level. However, there are several methods for the treatment of wastewaters containing Pb(II), including ion exchange, adsorption, precipitation and membrane separation [3]. During last decades, the process of adsorption using ac- tivated carbon [4-5] has been found to be an efficient technology for the removal of Pb(II) from wastewater. Though the removal of Pb(II) through adsorption is quite effective, its use is restricted sometimes due to the higher cost of activated carbon and difficulties associated with regeneration. Attempts have therefore been made to util- ize natural as well as waste materials as alternative ad- sorbents. However, preparation of carbons from low-cost materials [6-7], use of clay minerals [8-9], waste materi- als [10-12], and wheat bran [13-14] are some of the al- ternatives of costly activated carbons. Although many of these adsorbents can effectively remove Pb(II), most of them present some disadvantages such as poor adsorption capacity, low efficiency/cost ratio and ineffectiveness for low metal concentrations. China produces millions of tons of rice annually. Rice bran is a by-product of the rice milling industry and the amount of rice bran available is far in excess of any local uses, thus frequently causing disposal problems. Therefore, rice bran is very inexpensive, with a cost of 1/50-1/40 of that of active carbon, and thus its use would significantly lower the cost of wastewater treatment. In addition, the use of rice bran would represent effective utilization of waste matter. The modified rice bran has been success- fully used to remove Cd(II) from water [15]. The objective of this work was to study the feasibility of using modified rice bran as adsorbents for the removal of Pb(II) from wastewater. Rice bran was chosen due to its granular structure, insolubility in water, chemical sta- bility and local availability. In this work, batch experi- ments were carried out to evaluate the adsorption char-  Adsorption of Pb(II) onto Modified Rice Bran Copyright © 2010 SciRes. NR 105 acteristics of the modified rice bran for Pb(II) removal from aqueous solutions. The adsorption isotherms, ther- modynamic parameters, kinetics, pH effect, and desorb- ability were examined. 2. Materials and Methods 2.1. Materials The used rice bran was purchased at a local market. It was dried in an oven at 105 for a period of℃ 24 h, and then ground and sieved to obtain uniform material (< 75 μm). The chemical compositions and physical properties of rice bran are given in Table 1. The BET surface area was measured by the N2 adsorption-desorption technique using a NOVA-1000 (Ver.3.70) analyzer. The bulk den- sity of the adsorbent was determined with a densitometer. Mercury porositometry determined porosity of the ad- sorbent. Chemical analysis of the rice bran showed the presence of various oxides of Ca, Si, Mn, Mg, and Fe. About 20 g of dried rice husks were treated with 200 mL 3 M sodium hydroxide at 60 for℃ 2 h in a paraffin bath. The sodium hydroxide used was an equivalent amount required to remove all silica present in the husks. The husks were then filtered and washed with distilled water until the filtrate was neutral. The treated husks were dried at 105 in an oven and℃ left overnight. The dried husk was carbonized with 50 mL 30% sodium hy- droxide under nitrogen atmosphere at temperatures rang- ing from 400 to 650, for ℃45 min. Then the carbonized husks were washed with distilled water until the filtrate was about pH 6–7. The samples were then dried at 105 ℃ in an oven overnight and ball-milled into powder that passed through 75 μm mesh. 2.2. Experimental Procedure In the present investigation the batch mode of operation was selected in order to measure the progress of adsorp- tion. It was carried out by shaking 100 mg of modified rice bran with 100 mL aqueous solution of adsorbate (Pb(NO3)2) of different concentrations (30, 60, 90, 120, 150, 200, 300, 400 mg/L) at different temperature (15℃, 25℃, 35℃) and different pHs (3.1, 4.5, 6.2, 7.0, 7.6, 8.1) in different glass bottles in a shaking thermostat at a con- stant speed of 120 rpm. The pH of the adsorbate solution Table 1. Chemical compositions and physical properties of rice bran. Chemical composition Physical properties Moisture 8.25% Surface area (m2/g) 438.05 Volatile matter 42.80% Bulk density (g/cm3) 0.3086 Fixed carbon 30.56% Porosity (fraction) 0.38 Ash (oxides of Ca, Mn, Si, Fe, Mg, etc.) 18.39% was adjusted by manually adding 0.1 M HNO3 or 0.1 M NaOH solutions using a pH meter. The progress of ad- sorption was noted at different time intervals till the at- tainment of saturation. At the completion of predeter- mined time intervals, the adsorbate and adsorbent were separated by centrifugation at 12000 rpm for 25 minutes in a Sorvall RC-5C centrifuge. And the supernatant liq- uid was analyzed for Pb(II). Desorption experiments were conducted by shaking 100 mg of adsorbent con- taining adsorbed Pb(II) with 100 mL of distilled-deion- ized water at 25℃ and pH 7.0. Blank samples were run under similar conditions of concentration, pH, and temperature without adsorbent in all cases to correct for any adsorption on the internal surface of the bottles. The duplicate experiments demon- strated the high repeatability of this adsorption method and the experimental error could be controlled within 5-10%. 2.3. Analysis of Pb(II) Pb(II) was estimated through atomic absorption spectro- photometer using a Varian Spectra AA 10 Plus atomic absorption spectrophotometer, at wavelength of 217 nm. The band pass was 1 nm. The adsorbed Pb(II) was cal- culated from the difference of the Pb(II) in solution and the known initial concentration. Each analysis point was an average of three independent parallel sample solutions. Triplicate tests showed that the standard deviation of the results was ±3%. 3. Results and Discussion 3.1. Pb(II) Adsorption Isotherm Experiments were performed at different initial Pb(II) concentrations (30, 60, 90, 120, 150, 200, 300, 400 mg/L) at temperature of 15, 25, 35℃, respectively, and pH of 7.0. The results of the Pb(II) adsorption isotherm ex- periments are shown in Figure 1. It can be found that the Pb(II) adsorption capacity of the modified rice bran in- creased with the Pb(II) equilibrium concentration in- creasing from 0 to 100 mg/L. This capacity was ap- proximately 59.5, 68.0, 75.8 mg Pb(II)/g absorbents re- spectively at temperature of 15, 25, 35℃ and at the Pb(II) equilibrium concentration of 100 mg/L and pH 7.0. With a further increase of the Pb(II) equilibrium concentration, the increase of the adsorption capacity was less signify- cant. Two typical isotherms, as described below in Equa- tions (1)-(2), were used for fitting the experimental data: Freundlich equation: 1n ee qKC (1) Langmuir equation: 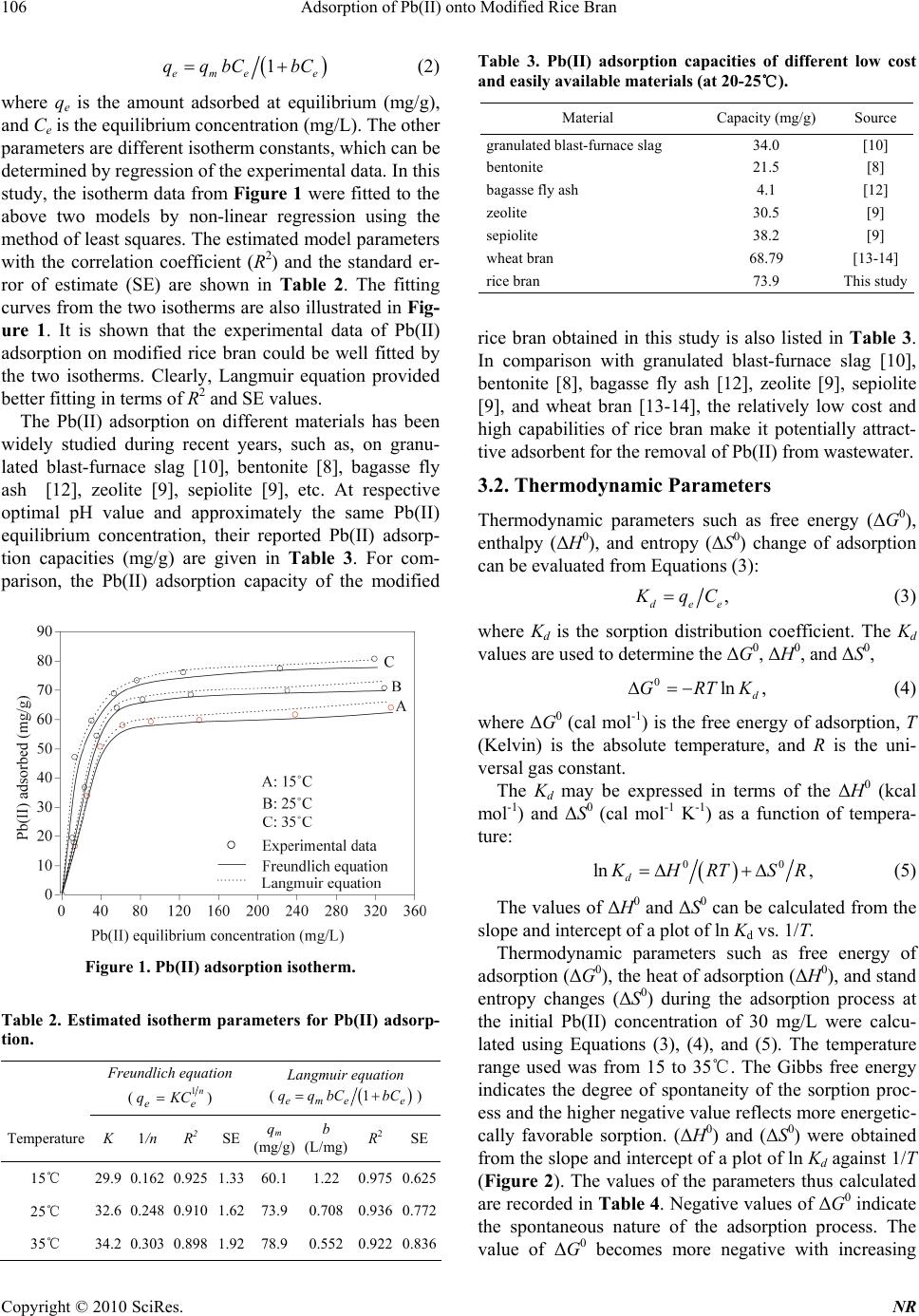 Adsorption of Pb(II) onto Modified Rice Bran Copyright © 2010 SciRes. NR 106 1 emee qqbC bC (2) where qe is the amount adsorbed at equilibrium (mg/g), and Ce is the equilibrium concentration (mg/L). The other parameters are different isotherm constants, which can be determined by regression of the experimental data. In this study, the isotherm data from Figure 1 were fitted to the above two models by non-linear regression using the method of least squares. The estimated model parameters with the correlation coefficient (R2) and the standard er- ror of estimate (SE) are shown in Table 2. The fitting curves from the two isotherms are also illustrated in Fig- ure 1. It is shown that the experimental data of Pb(II) adsorption on modified rice bran could be well fitted by the two isotherms. Clearly, Langmuir equation provided better fitting in terms of R2 and SE values. The Pb(II) adsorption on different materials has been widely studied during recent years, such as, on granu- lated blast-furnace slag [10], bentonite [8], bagasse fly ash [12], zeolite [9], sepiolite [9], etc. At respective optimal pH value and approximately the same Pb(II) equilibrium concentration, their reported Pb(II) adsorp- tion capacities (mg/g) are given in Table 3. For com- parison, the Pb(II) adsorption capacity of the modified Figure 1. Pb(II) adsorption isotherm. Table 2. Estimated isotherm parameters for Pb(II) adsorp- tion. Freundlich equation (1n ee qKC) Langmuir equation ( 1 eme e qqbC bC) Temperature K 1/n R2 SEqm (mg/g) b (L/mg) R2SE 15℃ 29.9 0.162 0.925 1.33 60.1 1.22 0.9750.625 25℃ 32.6 0.248 0.910 1.62 73.9 0.708 0.9360.772 35℃ 34.2 0.303 0.898 1.92 78.9 0.552 0.9220.836 Table 3. Pb(II) adsorption capacities of different low cost and easily available materials (at 20-25℃). Material Capacity (mg/g) Source granulated blast-furnace slag 34.0 [10] bentonite 21.5 [8] bagasse fly ash 4.1 [12] zeolite 30.5 [9] sepiolite 38.2 [9] wheat bran 68.79 [13-14] rice bran 73.9 This study rice bran obtained in this study is also listed in Table 3. In comparison with granulated blast-furnace slag [10], bentonite [8], bagasse fly ash [12], zeolite [9], sepiolite [9], and wheat bran [13-14], the relatively low cost and high capabilities of rice bran make it potentially attract- tive adsorbent for the removal of Pb(II) from wastewater. 3.2. Thermodynamic Parameters Thermodynamic parameters such as free energy (ΔG0), enthalpy (ΔH0), and entropy (ΔS0) change of adsorption can be evaluated from Equations (3): , dee K qC (3) where Kd is the sorption distribution coefficient. The Kd values are used to determine the ΔG0, ΔH0, and ΔS0, 0ln , d GRTK (4) where ΔG0 (cal mol-1) is the free energy of adsorption, T (Kelvin) is the absolute temperature, and R is the uni- versal gas constant. The Kd may be expressed in terms of the ΔH0 (kcal mol-1) and ΔS0 (cal mol-1 K-1) as a function of tempera- ture: 00 ln , d K HRTSR (5) The values of ΔH0 and ΔS0 can be calculated from the slope and intercept of a plot of ln Kd vs. 1/T. Thermodynamic parameters such as free energy of adsorption (ΔG0), the heat of adsorption (ΔH0), and stand entropy changes (ΔS0) during the adsorption process at the initial Pb(II) concentration of 30 mg/L were calcu- lated using Equations (3), (4), and (5). The temperature range used was from 15 to 35℃. The Gibbs free energy indicates the degree of spontaneity of the sorption proc- ess and the higher negative value reflects more energetic- cally favorable sorption. (ΔH0) and (ΔS0) were obtained from the slope and intercept of a plot of ln Kd against 1/T (Figure 2). The values of the parameters thus calculated are recorded in Table 4. Negative values of ΔG0 indicate the spontaneous nature of the adsorption process. The value of ΔG0 becomes more negative with increasing 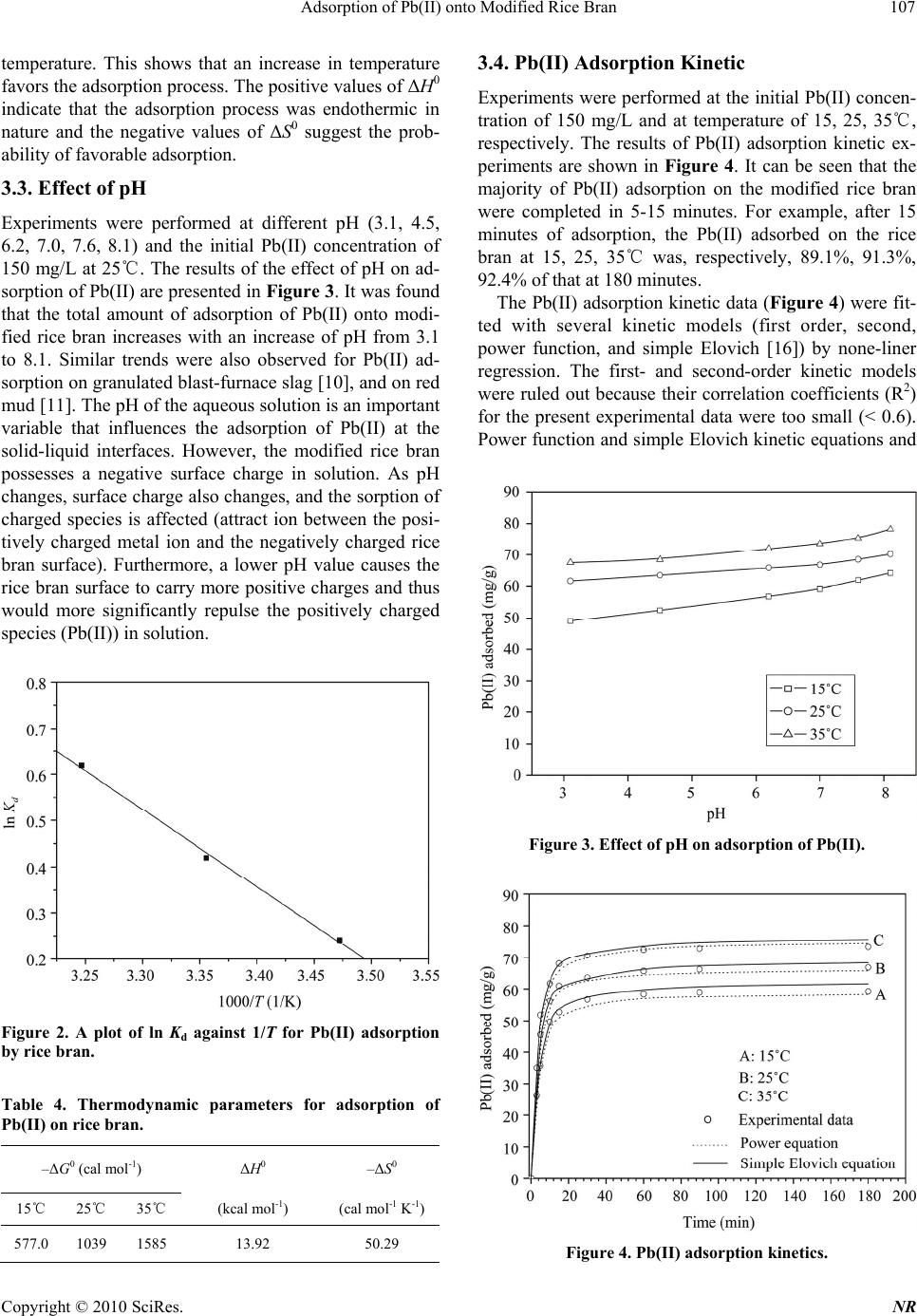 Adsorption of Pb(II) onto Modified Rice Bran Copyright © 2010 SciRes. NR 107 temperature. This shows that an increase in temperature favors the adsorption process. The positive values of ΔH0 indicate that the adsorption process was endothermic in nature and the negative values of ΔS0 suggest the prob- ability of favorable adsorption. 3.3. Effect of pH Experiments were performed at different pH (3.1, 4.5, 6.2, 7.0, 7.6, 8.1) and the initial Pb(II) concentration of 150 mg/L at 25℃. The results of the effect of pH on ad- sorption of Pb(II) are presented in Figure 3. It was found that the total amount of adsorption of Pb(II) onto modi- fied rice bran increases with an increase of pH from 3.1 to 8.1. Similar trends were also observed for Pb(II) ad- sorption on granulated blast-furnace slag [10], and on red mud [11]. The pH of the aqueous solution is an important variable that influences the adsorption of Pb(II) at the solid-liquid interfaces. However, the modified rice bran possesses a negative surface charge in solution. As pH changes, surface charge also changes, and the sorption of charged species is affected (attract ion between the posi- tively charged metal ion and the negatively charged rice bran surface). Furthermore, a lower pH value causes the rice bran surface to carry more positive charges and thus would more significantly repulse the positively charged species (Pb(II)) in solution. Figure 2. A plot of ln K d against 1/T for Pb(II) adsorption by rice bran. Table 4. Thermodynamic parameters for adsorption of Pb(II) on rice bran. –ΔG0 (cal mol-1) ΔH0 –ΔS0 15℃ 25℃ 35℃ (kcal mol-1) (cal mol-1 K-1) 577.0 1039 1585 13.92 50.29 3.4. Pb(II) Adsorption Kinetic Experiments were performed at the initial Pb(II) concen- tration of 150 mg/L and at temperature of 15, 25, 35℃, respectively. The results of Pb(II) adsorption kinetic ex- periments are shown in Figure 4. It can be seen that the majority of Pb(II) adsorption on the modified rice bran were completed in 5-15 minutes. For example, after 15 minutes of adsorption, the Pb(II) adsorbed on the rice bran at 15, 25, 35℃ was, respectively, 89.1%, 91.3%, 92.4% of that at 180 minutes. The Pb(II) adsorption kinetic data (Figure 4) were fit- ted with several kinetic models (first order, second, power function, and simple Elovich [16]) by none-liner regression. The first- and second-order kinetic models were ruled out because their correlation coefficients (R2) for the present experimental data were too small (< 0.6). Power function and simple Elovich kinetic equations and Figure 3. Effect of pH on adsorption of Pb(II). Figure 4. Pb(II) adsorption kinetics. 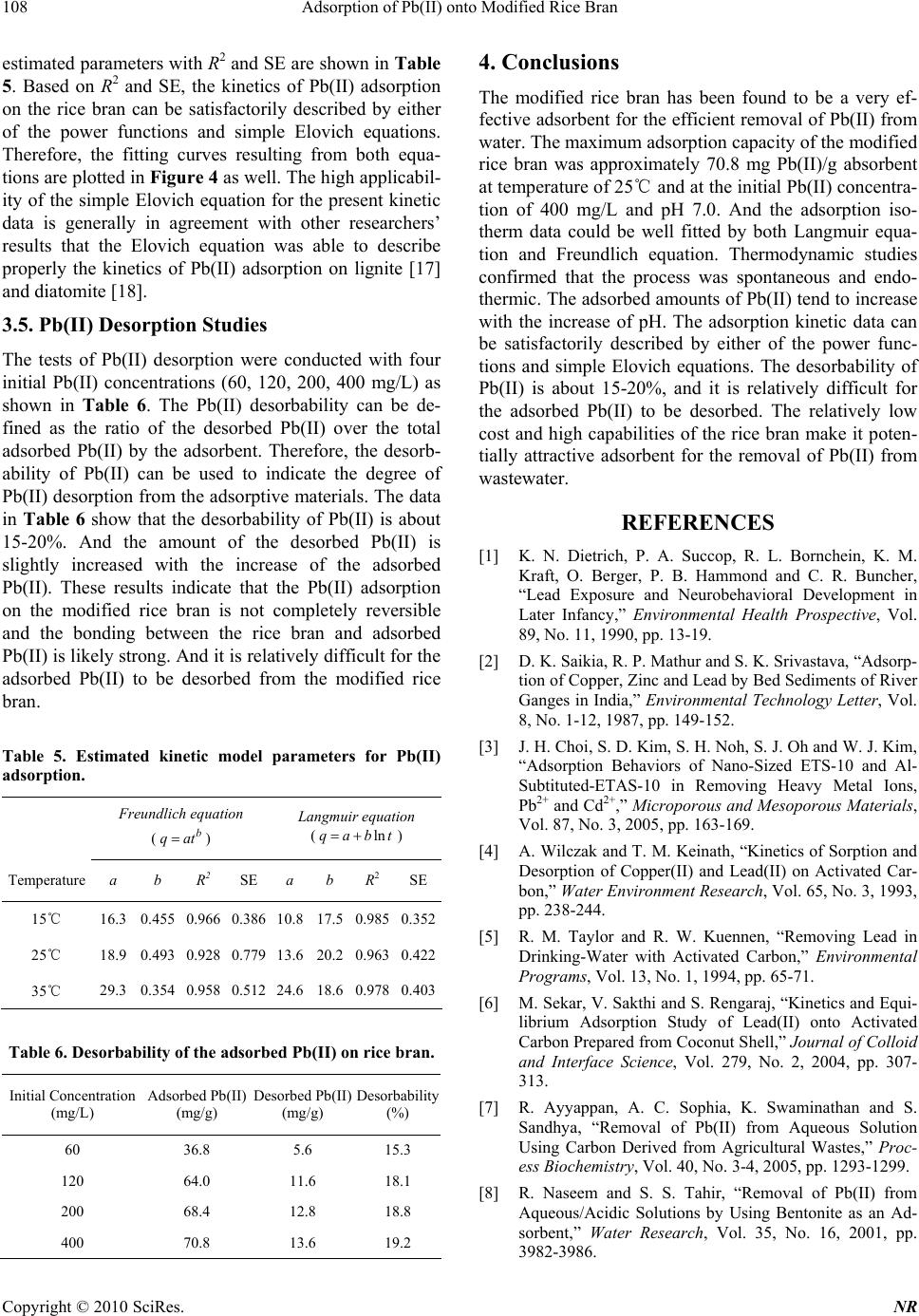 Adsorption of Pb(II) onto Modified Rice Bran Copyright © 2010 SciRes. NR 108 estimated parameters with R2 and SE are shown in Table 5. Based on R2 and SE, the kinetics of Pb(II) adsorption on the rice bran can be satisfactorily described by either of the power functions and simple Elovich equations. Therefore, the fitting curves resulting from both equa- tions are plotted in Figure 4 as well. The high applicabil- ity of the simple Elovich equation for the present kinetic data is generally in agreement with other researchers’ results that the Elovich equation was able to describe properly the kinetics of Pb(II) adsorption on lignite [17] and diatomite [18]. 3.5. Pb(II) Desorption Studies The tests of Pb(II) desorption were conducted with four initial Pb(II) concentrations (60, 120, 200, 400 mg/L) as shown in Table 6. The Pb(II) desorbability can be de- fined as the ratio of the desorbed Pb(II) over the total adsorbed Pb(II) by the adsorbent. Therefore, the desorb- ability of Pb(II) can be used to indicate the degree of Pb(II) desorption from the adsorptive materials. The data in Table 6 show that the desorbability of Pb(II) is about 15-20%. And the amount of the desorbed Pb(II) is slightly increased with the increase of the adsorbed Pb(II). These results indicate that the Pb(II) adsorption on the modified rice bran is not completely reversible and the bonding between the rice bran and adsorbed Pb(II) is likely strong. And it is relatively difficult for the adsorbed Pb(II) to be desorbed from the modified rice bran. Table 5. Estimated kinetic model parameters for Pb(II) adsorption. Freundlich equation (b qat) Langmuir equation (lnqabt ) Temperature a b R2 SE a b R2SE 15℃ 16.3 0.455 0.966 0.386 10.8 17.5 0.9850.352 25℃ 18.9 0.493 0.928 0.779 13.6 20.2 0.9630.422 35℃ 29.3 0.354 0.958 0.512 24.6 18.6 0.9780.403 Table 6. Desorbability of the adsorbed Pb(II) on rice bran. Initial Concentration (mg/L) Adsorbed Pb(II) (mg/g) Desorbed Pb(II) (mg/g) Desorbability (%) 60 36.8 5.6 15.3 120 64.0 11.6 18.1 200 68.4 12.8 18.8 400 70.8 13.6 19.2 4. Conclusions The modified rice bran has been found to be a very ef- fective adsorbent for the efficient removal of Pb(II) from water. The maximum adsorption capacity of the modified rice bran was approximately 70.8 mg Pb(II)/g absorbent at temperature of 25℃ and at the initial Pb(II) concentra- tion of 400 mg/L and pH 7.0. And the adsorption iso- therm data could be well fitted by both Langmuir equa- tion and Freundlich equation. Thermodynamic studies confirmed that the process was spontaneous and endo- thermic. The adsorbed amounts of Pb(II) tend to increase with the increase of pH. The adsorption kinetic data can be satisfactorily described by either of the power func- tions and simple Elovich equations. The desorbability of Pb(II) is about 15-20%, and it is relatively difficult for the adsorbed Pb(II) to be desorbed. The relatively low cost and high capabilities of the rice bran make it poten- tially attractive adsorbent for the removal of Pb(II) from wastewater. REFERENCES [1] K. N. Dietrich, P. A. Succop, R. L. Bornchein, K. M. Kraft, O. Berger, P. B. Hammond and C. R. Buncher, “Lead Exposure and Neurobehavioral Development in Later Infancy,” Environmental Health Prospective, Vol. 89, No. 11, 1990, pp. 13-19. [2] D. K. Saikia, R. P. Mathur and S. K. Srivastava, “Adsorp- tion of Copper, Zinc and Lead by Bed Sediments of River Ganges in India,” Environmental Technology Letter, Vol. 8, No. 1-12, 1987, pp. 149-152. [3] J. H. Choi, S. D. Kim, S. H. Noh, S. J. Oh and W. J. Kim, “Adsorption Behaviors of Nano-Sized ETS-10 and Al- Subtituted-ETAS-10 in Removing Heavy Metal Ions, Pb2+ and Cd2+,” Microporous and Mesoporous Materials, Vol. 87, No. 3, 2005, pp. 163-169. [4] A. Wilczak and T. M. Keinath, “Kinetics of Sorption and Desorption of Copper(II) and Lead(II) on Activated Car- bon,” Water Environment Research, Vol. 65, No. 3, 1993, pp. 238-244. [5] R. M. Taylor and R. W. Kuennen, “Removing Lead in Drinking-Water with Activated Carbon,” Environmental Programs, Vol. 13, No. 1, 1994, pp. 65-71. [6] M. Sekar, V. Sakthi and S. Rengaraj, “Kinetics and Equi- librium Adsorption Study of Lead(II) onto Activated Carbon Prepared from Coconut Shell,” Journal of Colloid and Interface Science, Vol. 279, No. 2, 2004, pp. 307- 313. [7] R. Ayyappan, A. C. Sophia, K. Swaminathan and S. Sandhya, “Removal of Pb(II) from Aqueous Solution Using Carbon Derived from Agricultural Wastes,” Proc- ess Biochemistry, Vol. 40, No. 3-4, 2005, pp. 1293-1299. [8] R. Naseem and S. S. Tahir, “Removal of Pb(II) from Aqueous/Acidic Solutions by Using Bentonite as an Ad- sorbent,” Water Research, Vol. 35, No. 16, 2001, pp. 3982-3986.  Adsorption of Pb(II) onto Modified Rice Bran Copyright © 2010 SciRes. NR 109 [9] T. Mustafa, M. Ugur, Y. Baris and S. Mehmet, “Lead Removal in Fixed-Bed Columns by Zeolite and Sepio- lite,” Chemosphere, Vol. 60, No. 10, 2005, pp. 1487- 1492. [10] S. V. Dimitrova and D. R. Mehandgiev, “Lead Removal from Aqueous Solutions by Granulated Blast-Furnace Slag,” Water Research, Vol. 32, No. 11, 1998, pp. 3289- 3292. [11] V. K. Gupta, M. Gupta and S. Sharma, “Process Devel- opment for the Removal of Lead and Chromium from Aqueous Solutions Using Red Mud - An Aluminium In- dustry Waste,” Water Research, Vol. 35, No. 5, 2001, pp. 1125-1134. [12] V. K.Gupta and I. Ali, “Removal of Lead and Chromium from Wastewater Using Bagasse Fly Ash - A Sugar In- dustry Waste,” Journal of Colloid and Interface Science, Vol. 271, No. 2, 2004, pp. 321-328. [13] Y. Bulut and Z. Baysal, “Removal of Pb(II) from Waste- water Using Wheat Bran,” Journal of Environmental Management, Vol. 78, No. 2, 2006, pp. 107-113. [14] A. Özer, “Removal ofPb(II) Ions from Aqueous Solutions by Sulphuric Acid-Treated Wheat Bran,” Journal of Haz- ardous Materials, Vol. 141, No. 3, 2007, pp. 753-761. [15] H. P. Ye, Q. Zhu and D. Y. Du, “Adsorptive Removal of Cd(II) from Aqueous Solution Using Natural and Modi- fied Rice Husk,” Bioresource Technology, Vol. 101, No. 14, 2010, pp. 5175-5179. [16] D. L. Sparks, “Kinetics of Soil Chemical Process,” Aca- demic Press Inc, New York, 1999. [17] J. M. Gu and D. R. Ding, “A Study on the Characteristics of Adsorption for Zn2+, Cu2+, Pb2+ Ions onto Peat and Lignite,” Environmental Chemistry (in Chinese), Vol. 15, No. 4, 1996, pp. 343-346. [18] Y. B. Shen, Y. M. Zhu, Z. A. Wang and D. Z. Wei, “Ad- sorption of Pb2+ in Hydrofacies by diatomite,” Journal of Northeastern University (Natural Science, in Chinese), Vol. 24, No. 10, 2003, pp. 982-985. |

