 Vol.2, No.12, 1341-1348 (2010) doi:10.4236/ns.2010.212163 Copyright © 2010 SciRes. Openly accessible at http:// www.scirp.org/journal/NS/ Natural Science Zinc oxide nanocomposites with antitumor activity Emma Arakelova1*, Ashot Khachatryan1, Karapet Avjyan1, Zoya Farmazyan1, Alvard Mirzoyan1, Lilia Savchenko1, Sedrak Ghazaryan2, Flora Arsenyan2 1Scientific Research Division, State Engineering University of Armenia,Yerevan, Armenia; *Corresponding Author: emma_arakelova@yahoo.com 2Laboratory of Syntheses Derivatives amino acids and peptides, Laboratory of toxicology and chemotherapy IFOC (Institute of Fine Organic Chemistry), Yerevan, Armenia Received 25 September 2010; revised 26 October 2010; accepted 30 October 2010. ABSTRACT Zinc oxide nanocomposites in the form of coatings and composite films with antitumor activity were obtained by deposition of ZnO nanofilms on surfaces of ethyl ether Salicyli- dene DL-tyrosine (S1) and ethyl ether Salicyli- dene DL-tyrosine Cu (II) chelate (S2) by magne- tron sputtering of Zn target. Ethyl ether Sali- cylidene DL-tyrosine, Cu (II) chelate of ethyl ether salicylidene DL-tyrosine reveal some anticancer properties. Their zinc oxide nano- composites were obtained in the form of coat- ings (S1 + ZnO, S2 + ZnO) and composite films presenting a mixture of polyvinyl alcohol (PVA) with S1, S2 (S1 + PVA + ZnO, S2 + PVA + ZnO), for the purpose of increasing anticancer activity. Considerable increase in antitumor activity re- veal ZnO nanocomposites with salicylidene amino acid chelates (as distinct from their ethers) in the form of S2 + ZnO (47%) and S2 + PVA + ZnO (48%) in comparison with S2 (20%). Structural, spectral properties of the salicylidene amino acids and their ZnO nanocomposites were studied. Keywords: Zinc Oxide; Nanofilm; Magnetron Sputtering; Nanocompo sites; Antitumor Activity 1. INTRODUCTION It is known that biologically active materials formed by deposition of metal oxide nanostructures on the or- ganic and polymeric systems surfaces reveal an ability to depress and kill pathogenic biological flora, and their introduction to organisms prevents “fixing” of viruses and bacteria to cell walls, improves antibacterial and antiviral immunity [1-3]. Here, very important is both the choice of inorganic material for modification of the biologically active ma- terials surface and the deposition procedure. Thin films or nanoscale coatings of ZnO nanoparticles on suitable substrates have excellent electrical, optical, and medical properties with broad range of applications for functional coatings, sensors, in optical devices, transparent electrodes, solar cells and antibacterial, anti- tumor activity [4-10]. Various methods like chemical, thermal, spray pyroly- sis, pulsed laser deposition etc. have been developed to coat ZnO nanoparticles in the form of thin films on solid supports such as metal, metal oxides, glass or thermally stable substrates [6,8,11-14]. Among the known methods of obtaining nanocompo- sitions, magnetron deposition of metal oxide nanofilms on the organic and polymeric systems surfaces at rela- tively low temperatures holds a unique position. Stand-out distinguishing features of the method are ample oppor- tunities for controlling the nanocomposition formation process, low deposition temperature (20ºС T ≤ 100ºС) and simplified technique of layer-by-layer deposition of coatings, allowing growth of nanocomposites with pre- determined structural parameters. Development of new nanotechnologies is of great importance for anticancer chemotherapy as the modern methods of anticancer drug administration in therapeutic (and slightly hither) doses are accompanied, as a rule, by development of serious side effects that frequently are the reasons of termination of the further course of treat- ment. It is known that amino acid Schiff’s bases form stable neutral lipophilic complexes with divalent ions of d-family metals. Some of them, for example, copper complexes reveal radioprotective, cytostatic action, in- hibiting reversibly DNA synthesis [15-19]. It is also known that in organisms, basic amino acids are binding agents for metal oxides, in particular for ZnO. Adjusting and immune effect of ZnO on DNA synthesis, produc-  E. Arakelova et al. / Natural Science 2 (2010) 1341-1348 Copyright © 2010 SciRes. Openly accessible at http:// www.scirp.org/journal/NS/ 1342 tion of interleukin (IL-2, IL-6, IL-10) and normalization of cytokine concentration was studied at the patients with chronic liver disease, in particular, hepatic cirrhosis [20]. On this basis, formation of thin films ZnO on the sur- faces of biologically active compounds (aimed at the surface modification) by magnetron deposition of Zn target is of special interest. This work is devoted to deposition of zinc oxide films on biologically active coatings and composite films of ethyl ether salicylidene DL-tyrosine, ethyl ether Sali- cylidene DL-tyrosine Cu (II) chelate with PVA aimed at modification of their surfaces and obtaining coatings and composite films with antitumor activity. The paper discusses the following problems: 1) Obtaining of zinc oxide nanocomposites by deposi- tion of zinc oxide nanofilms on coatings, composite films of ethyl ether salicylidene DL-tyrosine and ethyl ether salicylidene DL-tyrosine Cu (II) chelate by DC- magnetron deposition of zinc targets. 2) Study of anticancer activity of ethyl ether Sali- cylidene DL-tyrosine, ethyl ether salicylidene DL-tyrosine Cu (II) chelate and zinc oxide nanocomposites in the form of coatings and composite films 3) Structural, spectral properties of salicylidene amino acid and their chelate compounds, as well as ZnO nano- composites coatings of S1 + ZnO, S2 + ZnO and PVA composite films (S1 + PVA + ZnO, S2 + PVA + ZnO). 2. EXPERIMENTAL DETAILS/ METHODOLOGY (SELECT THE MORE APPROPRIATE) The paper is aimed at obtaining of zinc oxide nano- composite coatings and polymer composite films with anticancer activity by deposition of ZnO nanofilms on surfaces of ethyl ether salicylidene DL-tyrosine, S1 and their Cu (II) chelate, S2 by magnetron sputtering of Zn target. Ethyl ether salicylidene DL-tyrosine and ethyl ether salicylidene DL-tyrosine Cu (II) chelate are new compounds for the first time synthesized in the Institute of Fine Organic Chemistry of National Academy of Sci- ences of the Republic of Armenia. They were used as model compounds for obtaining zinc oxide nanocompo- sitions. 2.1. Formation of Coatings and Composite Films with PVA from Ethyl Ether Salicylidene DL-Tyrosine and Ethyl Ether Salicylidene DL-Tyrosine Cu (II) Chelate Coatings from ethyl ether salicylidene DL-tyrosine and ethyl ether salicylidene DL-tyrosine Cu (II) chelate were obtained on glass substrates as a paste with di- methyl sulfoxide. As solvents, olive oil, ethylene glycol (EG), polyeth- ylene glycol (PEG), dimethyl sulfoxide (DMSO) were tested. DMSO was chosen as a low-toxic substance with antiinflammatory, antimicrobial action, high permeabil- ity through biological membranes. Pastes from S1, S2 were prepared taking into account their daily maximum permissible doses for the animals. Several samples of polyvinyl alcohol differed in mo- lecular mass and functional composition were used to create needed polymeric matrix and choose the condi- tions of obtaining the films prospectively appropriate for their combination with S1,S2 and zinc oxide. The PVA samples were: PVA-1, MM approximately 20000, mass fraction of acetate group 1.3%, PVA-2, MM approxi- mately 21000, mass fraction of acetate group 10.6%, PVA-4, MM = 50000-60000, mass fraction of acetate group 5.3%. PVA-4 differs from PVA-1 and PVA-2 in higher MM, and its mass fraction of acetate group is intermediate between PVA-1 and PVA-2. The composition films from S1 and S2 with PVA were obtained from 0.5 ml of 5% PVA solution with a meas- ured amount of S1 and S2 corresponding to therapeutic doses of S1 and S2 for white nondescript mice. The film diameter was 2.8 cm (surface area was 6.1 cm2). On the composite films of S1 and S2 with PVA the ZnO nano- films were deposited by magnetron sputtering of zinc target. 2.2. DC-Magnetron Deposition of ZnO Nanofilms on Surfaces of Salicylidene Amino Acids and Their Chelates To deposit nanosize ZnO films on surfaces of coatings and composite films of ethyl ether salicylidene DL-tyrosine, ethyl ether salicylidene DL-tyrosine Cu (II) chelate, a modified UVN-71P3 device, DC-magnetron intended for sputtering of metal targets was used. UVN-71P3 device was equipped with a system of working gas flow measurement and control consisting of supply and indication unit PR4000F as well as two gas flow regulators MFC 1179 for Аr and О2 gases. The substrates from optic glass for deposition of coatings or composite films from the investigated com- pounds were fixed inside the device using special clamps ensuring their movement within the vacuum chamber. ZnO nanofilm deposition process was carried out by magnetron sputtering of Zn target at parallel arrange- ment of the target and substrate. The deposition device is powered from an energy supply source. To carry out experiments on ZnO nanofilm deposition within the in- vestigated temperature interval, a system based on Peltier element or resistance heater ensuring decrease  E. Arakelova et al. / Natural Science 2 (2010) 1341-1348 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/NS/ 134 1343 and stabilization of the substrate temperature was used. ZnO nanofilms were deposited on rotating substrates (to ensure uniform thickness) at the substrate temperature range from 20ºС to 100ºС. When selecting technological regimes of ZnO nano- film deposition on the substrate (the working gases О2 and Аr ratio, operating current of the magnetron source, target-to-substrate distance, substrate temperature), the conditions were considered to eliminate undesirable ex- traneous transformations of salicylidene amino acids and their chelates. To form zinc oxide nanocompositions of the investi- gated compounds S1, S2 in the form of coatings and composite films, a technique of ZnO nanofilm deposi- tion on glass substrates was preliminary developed. ZnO nanofilms were deposited on glass substrates by magnetron sputtering of Zn targets (55 mm in diameter) at the operating current from 80 mA to 500 mA, pressure of 10-2 mm Hg, and target-to-substrate distance from 7 cm to 13 cm. As working gases, Аr and О2 were used in the ratio of 70%-30%. To keep clean the deposition process, the vacuum chamber was exhausted to 2 × 10-6 mm Hg before the film deposition. 2.3. Technique of Identification of Antitumor Activity of Ethyl Ether Salicylidene DL-Tyrosine, Their Cu (II) Chelate and Zinc Oxide Nanocomposites on Strains of Inoculate Tumors The study was carried out in toxicology and chemo- therapy laboratory of Scientific Technological Centre of Organic and Pharmaceutical Chemistry of the National Academy of Sciences of the Republic of Armenia under conditions close to GLP principles and European stan- dards. Antitumor coatings and composite films of zinc oxide nanocomposites of ethyl ether salicylidene DL-tyrosine, ethyl ether salicylidene DL-tyrosine Cu (II) chelate and initial compounds were tested on the strains of sarcoma 180 tumors inoculated to white nondescript mice. Pre- liminary, maximum permissible doses (MPD) were de- termined by vivisections, and on their basis-therapeutic doses of the substances, which made as a rule 20% of the single MPD. For subinoculation, as a transplant the peaces of tumor tissues not subject to necrosis and crushed to homoge- neous mass were used. Physiological salt solution was added to the tumor in 1:3–1:4 ratio, and the obtained suspension was introduced to the animals (with initial weight 22-26 g) subcutaneously by a syringe at a rate of 0.3 ml. Nanocomposites in the form of ZnO + (S1, S2) coat- ings were introduced to the animals subcutaneously as a suspension in DMSO 48 hours after the tumor subin- oculation on a daily basis during 6 days. Zinc oxide nanocomposites and initial compounds in the form of composite films were deposited operation- ally in sterile conditions (box) by subcutaneous applica- tions for 6 days through a 5-7 mm in section in spinal and scapular parts of the animals. 48 hours after the experiment the animals were killed, the tumors were withdrawn and weighed. Antitumor activity was determined by the percentage of the tumor inhibition with respect to the control animals. 2.4. Сharacterization of Ethyl Ether Salicylidene DL-Tyrosine, Their Cu (II) Chelates and Zinc Oxide Nanocomposites X-ray diffraction (XRD) patterns were obtained on CAD4, Enraf-Nonius and DRON-2.0 X-ray diffracto- meters. NMR spectra were recorded on Varian Mercury 300VX spectrometer with all-round frequency 300 MHz. IR spectra were recorded on NEXUS FT-IR spec- trometer. Mass spectra were recorded on МХ-1321А. 3. RESULTS & DISCUSSION 3.1. Selection of Optimum Surface Areas for S1, S2 The ZnO nanofilm surface coatings are S1, S2 deposi- tions over a certain surface area of object glass. Thick- ness and optimum ZnO nanofilm surface area was cal- culated for the case, when its content in the film was 5-10% of that of S1, S2. It is known from publications that at addition of 5% ZnO to plasma of rabbit with in- culcated Staphylococcus, antibacterial activity increases in comparison with that for control samples without ZnO [20]. On this basis, ZnO content in the film (5-10% of S1, S2 weight) was taken for rough estimate, with a view to obtain reliable optimum results. To ensure techno- logical effectiveness of the deposition process, the sur- face area was calculated taking into account the amount of S1 and S2 for daily dose of 6 mice. Suspension of S1 + ZnO nanocomposite did not reveal reliable antitumor action (10%) at subcutaneous introduction of m1 = 1.4 mg/mouse of ethyl ether salicylidene DL-tyrosine to mice, when ZnO deposition area on S1 was s1 = 2.54 cm2. However at the same daily dose, when ZnO deposi- tion area on S1 coatings were s1 = 9.62 cm2 and s2 = 15.9 cm2, antitumor activity was 30%-32% for d = 100 nm thick ZnO nanofilms (Table 1). At subcutaneous introduction of S2 + ZnO, when the content of ethyl ether salicylidene DL-tyrosine Cu (II) chelate was m = 1.2 mg/mouse and s = 2.54 cm2, the 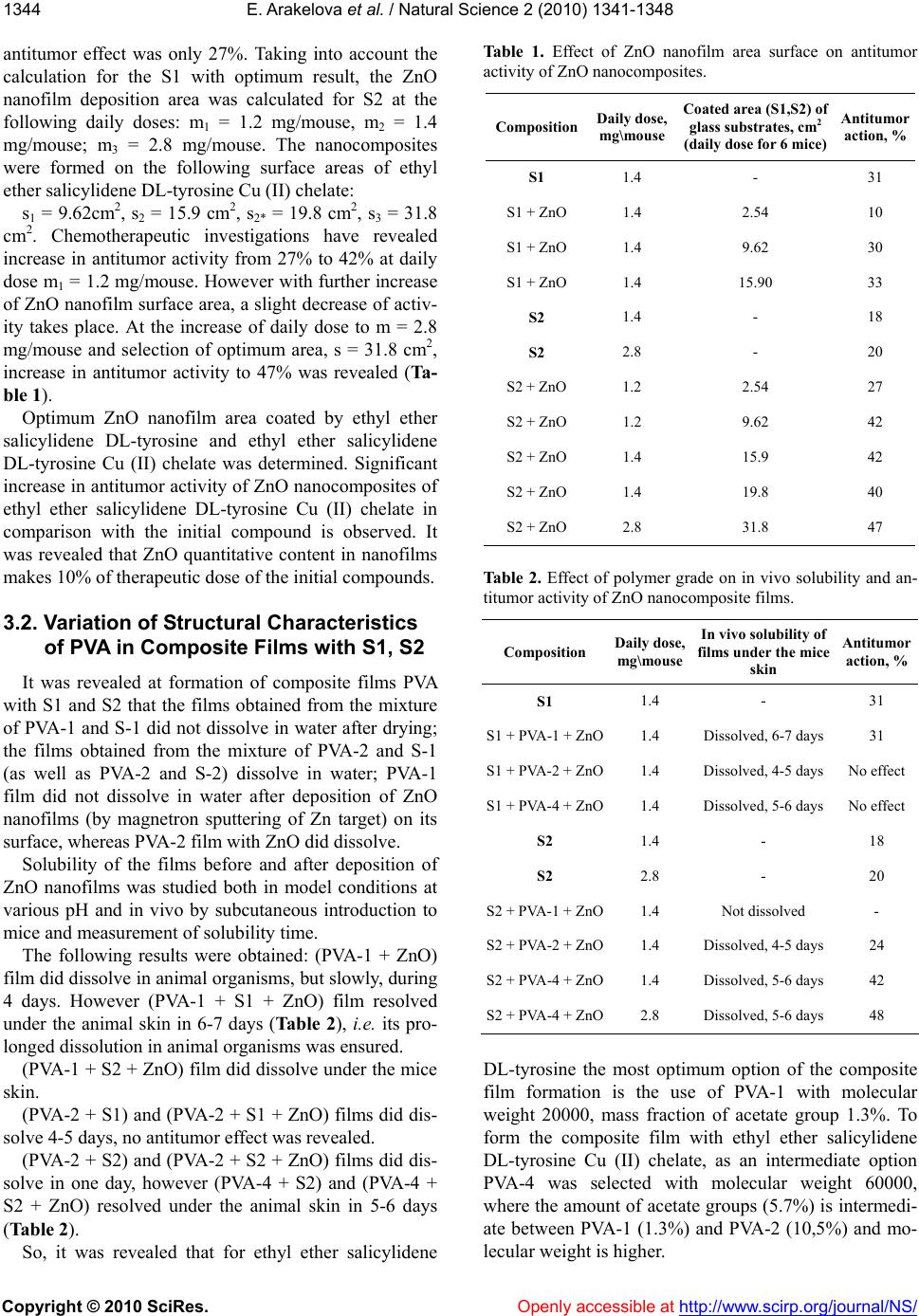 E. Arakelova et al. / Natural Science 2 (2010) 1341-1348 Copyright © 2010 SciRes. Openly accessible at http:// www.scirp.org/journal/NS/ 1344 antitumor effect was only 27%. Taking into account the calculation for the S1 with optimum result, the ZnO nanofilm deposition area was calculated for S2 at the following daily doses: m1 = 1.2 mg/mouse, m2 = 1.4 mg/mouse; m3 = 2.8 mg/mouse. The nanocomposites were formed on the following surface areas of ethyl ether salicylidene DL-tyrosine Cu (II) chelate: s1 = 9.62cm2, s2 = 15.9 cm2, s2* = 19.8 cm2, s3 = 31.8 cm2. Chemotherapeutic investigations have revealed increase in antitumor activity from 27% to 42% at daily dose m1 = 1.2 mg/mouse. However with further increase of ZnO nanofilm surface area, a slight decrease of activ- ity takes place. At the increase of daily dose to m = 2.8 mg/mouse and selection of optimum area, s = 31.8 cm2, increase in antitumor activity to 47% was revealed (Ta- ble 1). Optimum ZnO nanofilm area coated by ethyl ether salicylidene DL-tyrosine and ethyl ether salicylidene DL-tyrosine Cu (II) chelate was determined. Significant increase in antitumor activity of ZnO nanocomposites of ethyl ether salicylidene DL-tyrosine Cu (II) chelate in comparison with the initial compound is observed. It was revealed that ZnO quantitative content in nanofilms makes 10% of therapeutic dose of the initial compounds. 3.2. Variation of Structural Characteristics of PVA in Composite Films with S1, S2 It was revealed at formation of composite films PVA with S1 and S2 that the films obtained from the mixture of PVA-1 and S-1 did not dissolve in water after drying; the films obtained from the mixture of PVA-2 and S-1 (as well as PVA-2 and S-2) dissolve in water; PVA-1 film did not dissolve in water after deposition of ZnO nanofilms (by magnetron sputtering of Zn target) on its surface, whereas PVA-2 film with ZnO did dissolve. Solubility of the films before and after deposition of ZnO nanofilms was studied both in model conditions at various pH and in vivo by subcutaneous introduction to mice and measurement of solubility time. The following results were obtained: (PVA-1 + ZnO) film did dissolve in animal organisms, but slowly, during 4 days. However (PVA-1 + S1 + ZnO) film resolved under the animal skin in 6-7 days (Table 2), i.e. its pro- longed dissolution in animal organisms was ensured. (PVA-1 + S2 + ZnO) film did dissolve under the mice skin. (PVA-2 + S1) and (PVA-2 + S1 + ZnO) films did dis- solve 4-5 days, no antitumor effect was revealed. (PVA-2 + S2) and (PVA-2 + S2 + ZnO) films did dis- solve in one day, however (PVA-4 + S2) and (PVA-4 + S2 + ZnO) resolved under the animal skin in 5-6 days (Table 2). So, it was revealed that for ethyl ether salicylidene Table 1. Effect of ZnO nanofilm area surface on antitumor activity of ZnO nanocomposites. Composition Daily dose, mg\mouse Coated area (S1,S2) of glass substrates, cm2 (daily dose for 6 mice) Antitumor action, % S1 1.4 - 31 S1 + ZnO 1.4 2.54 10 S1 + ZnO 1.4 9.62 30 S1 + ZnO 1.4 15.90 33 S2 1.4 - 18 S2 2.8 - 20 S2 + ZnO 1.2 2.54 27 S2 + ZnO 1.2 9.62 42 S2 + ZnO 1.4 15.9 42 S2 + ZnO 1.4 19.8 40 S2 + ZnO 2.8 31.8 47 Table 2. Effect of polymer grade on in vivo solubility and an- titumor activity of ZnO nanocomposite films. Composition Daily dose, mg\mouse In vivo solubility of films under the mice skin Antitumor action, % S1 1.4 - 31 S1 + PVA-1 + ZnO1.4 Dissolved, 6-7 days 31 S1 + PVA-2 + ZnO1.4 Dissolved, 4-5 days No effect S1 + PVA-4 + ZnO1.4 Dissolved, 5-6 days No effect S2 1.4 - 18 S2 2.8 - 20 S2 + PVA-1 + ZnO1.4 Not dissolved - S2 + PVA-2 + ZnO1.4 Dissolved, 4-5 days 24 S2 + PVA-4 + ZnO1.4 Dissolved, 5-6 days 42 S2 + PVA-4 + ZnO2.8 Dissolved, 5-6 days 48 DL-tyrosine the most optimum option of the composite film formation is the use of PVA-1 with molecular weight 20000, mass fraction of acetate group 1.3%. To form the composite film with ethyl ether salicylidene DL-tyrosine Cu (II) chelate, as an intermediate option PVA-4 was selected with molecular weight 60000, where the amount of acetate groups (5.7%) is intermedi- ate between PVA-1 (1.3%) and PVA-2 (10,5%) and mo- lecular weight is higher.  E. Arakelova et al. / Natural Science 2 (2010) 1341-1348 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/NS/ 134 1345 3.3. Optimum Technological Parameters of ZnO Nanofilm Deposition on Coatings and S1, S2 Composite Films Optimum technological parameters of zinc oxide nan- ofilm magnetron deposition on the surface of ethyl ether salicylidene DL-tyrosine, ethyl ether salicylidene DL- tyrosine Cu (II) chelate in the form of coatings (S1, S2) and composite films (PVA + S1, PVA + S2) were deter- mined. The substrate material temperature was measured de- pending on the changes of the magnetron source operat- ing current at various target-to-substrate distances (Fig- ure 1(а)). It follows from this dependence that within the investigated range of operating currents from 80 mA to 500 mA, the substrate surface temperature was below 100˚С. At such temperatures, there were no undesirable extraneous transformations of the surface of biologically active substances S1 and S2. X-ray investigations were carried out and experimental results on antitumor active- ity were obtained for ZnO compositions formed at vari- ous technological regimes of ZnO deposition on the re- searched biologically active compounds. It was revealed that the structure of 100 nm thick ZnO nanofilms with optimum size of nanoparticles (15-30 nm) is appropriate for interaction with the researched biologically active compounds in form of both coatings and composite films. Such nanosize ZnO films were obtained at the following technological regimes: operating current from 400 mA, operating voltage 240 V and operating pressure 10-2 mm Hg. For these regimes, the ZnO nanofilm deposition rate was studied depending on target-to-substrate distance (Figure 1(b)). Optimum target-to-substrate distance, 10 cm was selected taking into account the obtained de- pendences and uniformity of the ZnO films deposited on the S1, S2 sample surfaces. At such target-to-substrate distance the film growth rate made approximately 6 nm/min. Composites on the basis of ZnO nanofilms and bio- logically active substances S1 and S2 were obtained by deposition of approximately 100 nm thick zinc oxide nanofilm (using Zn target) on S1 and S2 deposited in the form of coatings or composite films, in the following technological regime: operating current from 400 mA, gas mixture Ar and O2 at 70:30 ratio; operating pressure 10-2 mm Hg, target-to-substrate distance 10 cm. 3.4. Antitumor Activity of S1, S2 and Zinc Oxide Nanocomposites It was revealed as a result of study of acute toxicity that MPD values for S1 and S2 are 50 mg/kg and 250 mg/kg, correspondingly. In view of these data, as pre- liminary therapeutic dose for S1 and S2 were used 15 (a) (b) Figure 1. (a) Dependence of the substrate material temperature upon the magnetron source current at various target-to-substrate distances: 1) 7 cm; 2) 10 cm; (b) Dependence of ZnO nanofilm deposition rate upon the target-to-substrate distances at various magnetron source currents: 1) 200 mA; 2) 400 mA. mg/kg and 50 mg/kg, correspondingly. In chemotherapeutic experiments S1 at 0.3 mg/mouse (15 mg/kg) dose did not reveal antitumor effect and general toxic action, so in subsequent experiment the dose was increased to 1.5 mg/mouse. It was revealed that at such dose S1 inhibited sarcoma 180 growth by 31% and did not reveal toxic action on the animal or- ganism. S2 at therapeutic dose of 1 mg/mouse did not cause significant inhibition of sarcoma 180 (18%), and with the dose increase to 2.8 mg/mouse no increase in therapeutic action was determined (20%). S1 + ZnO nanocomposite in the form of coatings and composite films S1 + PVA-1 + ZnO revealed the same activity as S1: 33% and 31%. However S2 + ZnO nano- composites where S2 content was 1 or 2.8 mg/mouse, revealed at subcutaneous injection to animals and film  E. Arakelova et al. / Natural Science 2 (2010) 1341-1348 Copyright © 2010 SciRes. Openly accessible at http:// www.scirp.org/journal/NS/ 1346 application much higher antitumor activity (in form of both S2 + ZnO coating, 47%, and S2 + PVA-4 + ZnO composite films, 48%) than S2(20%). So, as distinct from nanocomposites with S1 + ZnO, S2 + ZnO in form of both coatings and composite films revealed relatively higher therapeutic effectiveness than at using S2 only. 3.5. Structural, Spectral Characteristics of S1, S2 and Zinc Oxide Nanocomposites Complete X-ray diffraction study of ethyl ether Sali- cylidene DL-tyrosine was carried out using CAD4, En- raf-Nonius diffractometer. The compound was synthe- sized for the first time in the Institute of Fine Organic Chemistry of National Academy of Sciences of the Re- public of Armenia. Figure 2 present the molecular structure (Figure 2(a)) and crystal packing on plane ac for three molecules (for cleanness) (Figure 2(b)) of ethyl ether salicylidene DL-tyrosine. In the compound’s crystalline structure, bond lengths (N (2)-C (3): 1.296Å, C (1)-N (2): 1.448 Å) are typical for C = N Schiff base bond and C-N single bond, respectively [21]. The compound structure con- tains intramolecular hydrogen bonds between N (2) and O (10) atoms, N (2)-H (2)…O (10), 2.565 Å in length and intermolecular hydrogen bonds O (18)-H (18)…O (10), 2.623 Å in length. IR study was carried out on ethyl ether salicylidene DL-tyrosine Cu (II) chelate and has shown that the compound includes free hydroxyl group of a salicylidene fragment in the valence vibration region 3309 cm-1. In the metal complex, shift of COO- absorption band was occurred to 1668 cm-1 as distinct from S1 with 1733 cm-1, and a shift –CH = N to 1651 cm-1 due to interact- tion between Cu and nitrogen on account of free electron pair. Also, there were differences in the region of deforma- tion vibrations: C-O, C-O-H and C-O-C, Me-O, Me-N. Also, IR study was carried out on ethyl ether Sali- cylidene DL-tyrosine zinc oxide nanocomposites. The study has revealed identity of ethyl ether salicylidene DL-tyrosine zinc oxide nanocomposite and initial S1 spectra. They did not contain expected shifts of absorp- tion bands of C = O, C-O-H, C-O-C functional groups in 1206-1065 cm-1 region (deformation vibrations) and in the valence vibration region of OH group (3000-3500 cm-1). ZnO presumably presents in the form of mechanical mixture with ethyl ether salicylidene DL-tyrosine. Comparison of the absorption spectra of metal com- plex and ethyl ether salicylidene DL-tyrosine Cu (II) chelate zinc oxide nanocomposite has revealed a shift of absorption band in the valence vibration region of OH group from 3309 cm-1 in the initial metal complex to (a) (b) Figure 2. Molecular structure (a) and crystal packing on the plane ac (b) of ethyl ether salicylidene DL-tyrosine.  E. Arakelova et al. / Natural Science 2 (2010) 1341-1348 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/NS/ 134 1347 3335 cm-1 in metal oxide composite. It is likely that this shift is related to interaction between ZnO and OH group of the salicylidene fragment. In case of ZnO nanocomposite films (S1 + PVA-1 + ZnO) there is interaction of PVA-1 functional groups with ZnO and absence of ZnO-S1 interaction. In case of (S2 + PVA-4 + ZnO) films interactions of ZnO with functional groups of both PVA-4 and S2 were revealed. Study of nanocomposite films has revealed the de- pendence of the interactions with formation of bonds in the composite film components upon the structure of S1and S2 compounds. X-ray investigations of ethyl ether salicylidene DL- tyrosine have detected presence of intramolecular hy- drogen bond N-H…O and intermolecular hydrogen bonds of OH hydroxyl group and carbonyl group, which form chains in compounds. IR spectral investigations of ZnO nanocomposite coatings (S1 + ZnO) did not reveal ZnO-S1 bonds. Spectral investigations of ZnO nano- composite films (S1 + PVA + ZnO) have revealed inter- action of PVA functional groups with ZnO and absence of ZnO-S1 interaction. IR spectral investigations of ethyl ether salicylidene DL-tyrosine Cu (II) chelate did not reveal intramolecular or intermolecular hydrogen bonds in the structure; however ZnO nanocomposite coatings, S2 + ZnO, reveal obvious formation of intermolecular hydrogen bond of ZnO...НO type. ZnO nanocomposite films from PVA with S2 reveal interaction of ZnO with functional groups of both PVA and S2. Thus, the surface modification of ethyl ether Sali- cylidene DL-tyrosine Cu (II) chelate coatings as well of composite films from these compounds by deposition of ZnO nanofilms using magnetron sputtering results in increase of antitumoral activity of the obtained nano- composites, apparently due to formation of intermolecu- lar hydrogen bond between ZnO and hydroxyl groups of these compounds. 4. CONCLUSIONS 1) Zinc oxide nanocomposites of ethyl ether salicyli- dene DL-tyrosine and ethyl ether salicylidene DL-tyrosine Cu (II) chelate were obtained by deposition of approxi- mately 100 nm thick zinc oxide nanofilms on coatings and composite S1, S2 films using magnetron sputtering of Zn targets in the temperature range of 20˚С T ≤ 100˚С at operating pressure of 10-2 mm Hg. 2) Optimum ZnO nanofilm area coated by ethyl ether salicylidene DL-tyrosine and ethyl ether salicylidene DL-tyrosine Cu (II) chelate was determined. Significant increase in antitumor activity of ZnO nanocomposites of ethyl ether salicylidene DL-tyrosine Cu (II) chelate in comparison with the initial compound is observed. It was revealed that ZnO quantitative content in nanofilms makes 10% of therapeutic dose of the initial compounds. 3) Zinc oxide composite films of ethyl ether salicyli- dene DL-tyrosine and ethyl ether salicylidene DL-tyrosine Cu (II) chelate with polyvinyl alcohol are obtained en- suring their prolongation in animals (mice) organizm. It is shown that for ethyl ether salicylidene DL-tyrosine the most optimum option of the composite film formation is the use of PVA with molecular weight 20000 and mass fraction of acetate group 1.3% To form the composite film with ethyl ether salicylidene DL-tyrosine Cu (II) chelate, the optimum option is PVA with molecular weight 60000 and amount of acetate groups of 5.7%. 4) Chemotherapeutic investigations have shown that antitumor activity of zinc oxide nanocomposites of ethyl ether salicylidene DL-tyrosine in the form of both S1 + ZnO coatings (33%) and S1 + ПВС + ZnO composite films (31%) was approximately the same as that of S1(31%). 5) ZnO nanocomposites with Cu (II) chelate of ethyl ether salicylidene DL-tyrosine (as distinct from their ester) in the form of S2 + ZnO (47%) coatings and S2 + PVA + ZnO (48%) composite films have revealed con- siderable increase in antitumoral activity in comparison with S2 (20%). 6) X-ray investigations of ethyl ether salicylidene DL-tyrosine have detected presence of intramolecular hydrogen bonds and intermolecular hydrogen bonds, which form chains in the compounds. IR spectral invest- tigations of ZnO nanocomposite coatings (S1 + ZnO) did not reveal ZnO-S1 bonds. Spectral investigations of ZnO nanocomposite films (S1 + PVA + ZnO) have re- vealed interaction of PVA functional groups with ZnO and absence of ZnO-S1 interaction. 7) IR spectral investigations of ethyl ether salicylidene DL-tyrosine Cu (II) chelate did not reveal intramolecular or intermolecular hydrogen bonds in the structure, how- ever ZnO nanocomposite coatings, S2 + ZnO, reveal (judging from the deviation in valence vibrations of OH group) obvious formation of intermolecular hydrogen bond of ZnO-H…O type. ZnO nanocomposite films from PVA with S2 reveal interaction of ZnO with func- tional groups of both PVA and S2. 8) Surface modification of ethyl ether salicylidene DL-tyrosine Cu (II) chelate coatings as well as compos- ite films based on these compounds, by deposition of ZnO nanofilms using magnetron sputtering resilts in increase of antitumor activity of the obtained nanocom- posites, apparently due to formation of intermolecular hydrogen bonds between ZnO and hydroxyl groups of these compounds. 5. ACKNOWLEDGEMENTS This work was supported by Grant А-1563 awarded by the Interna-  E. Arakelova et al. / Natural Science 2 (2010) 1341-1348 Copyright © 2010 SciRes. http://www.scirp.org/journal/NS/ 1348 tional Science and Technology Center. Openly accessible at REFERENCES [1] Brown, S. (1992) Engineered iron oxide-adhesion mu- tants of the Escherichia coli phage lambda receptor. Proceedings of the National Academy of Sciences, USA, 1992, 89, 8651-8655. [2] Burrell, R.E. (1997) Anti–microbial coating for medical devices. Patent Application , 1203. [3] Chule, K., Chule, A.V., Chen, B.-J. and Ling, Y.-C. (2006) Preparation and characterization of ZnO nanoparticles coated paper and its antibacterial activity study. Green Chemistry, 8, 1034-1041. [4] Wang, Z.L. (2004) Zinc oxide nanostructures: growth, properties and applications. Journal of Physics: Con- densed Matter, 16, 829. [5] Wang, X.D., Summers, C.J. and Wang, Z.L. (2004) Large-scale hexagonal-patterned growth of aligned ZnO nanorods for nano-optoelectronics and nanosensor arrays. Nano Letters, 4, 423. [6] Shim, E.S., Kang, H.S., Kang, J.S., Kim, J.H. and Lee, S. Y. (2002) Effect of the variation of film thickness on the structural and optical properties of ZnO thin films depos- ited on sapphire substrate using PLD. Applied Surface Science, 186, 474-476. [7] Chen, P., Ma, X. and Yang, D., (2006) Fairly pure ultra- violet electroluminescence from ZnO-based light-emit- ting devices. Applied Physics Letters. Applied Physics Letters, 89, 111-112. [8] Jin, B.J., Woo, H.S., Im, S., Base, S.H. and Lee, S.Y. (2001) Applied Surface Sci ence, 169-170, 521-524. [9] Hill, G.M., Mahan, D.C., Carter, S.D., Cromwell, G.L., Ewan, R.C., Harrold, R.L., Lewis, A.J., Miller, P.S., Shurson, G.C. and Veum, T.L. (2001) Effect of pharmacological concentrations of zinc oxide with or without the inclusion of an antibacterial agent on nursery pig performance. Journal of Animal Science, 79, 34-41. [10] Hanley, C., Layne J., Punnoose, A., Reddy K.M., Isaac C., Andrew C., Kevin F. and Denise W. (2008) Increase in Concentration of a Zn-Containing Volatile Complex by UV Irradiation of a Target for ZnO Films Synthesis. Nanotechnology, 19, 10. [11] Arakelova, E., Grigoryan F., Parvanyan, V., Asatryan, G. and Antonio, S. (2006) Increase in concentration of a Zn-containing volatile complex by UV irradiation of a target for ZnO films synthesis. Texas. EPD Congress 2006, Texas, 2006, 813-818. [12] Arakelova, E., Parvanyan, F.G.V., Asatryan, G. and Anto- nio, S. (2006) Study of decomposition regularities for a Zn-containing volatile complex used in ZnO films syn- thesis. Texas EPD Congress 2006, Texas, 2006, 883-888. [13] Xu, W.Z., Ye, Z.Z., Zeng, Y.J., Zhu, L.P., Zhao, B.H., Jiang, L., et al., (2006) ZnO light-emitting diode grown by plasma-assisted metal organic chemical vapor deposi- tion. Applied Physics Letters, 88, 112-116. [14] Banerjee, A.N., Ghosh, C.K., Chattopadhyay, K.K., Mi- noura, H., Sarkar, A.K., Akiba, A. and Kamiya, A. (2006) Low-temperature deposition of ZnO thin films on PET and glass substrates by DC-sputtering technique. Thin Solid Films, 496, 112-116. [15] Ghazaryan, S.H., Grigoryan, K.P., Ghevondyan, A.I., Minasyan, S.H., Tonoyan, V.C., Bajinyan, S.A., Pogho- syan A.S., Malakyan, M.H. and Sorensen, J. R. S. (2001) The synthesis of new derivatives of amino acids and pep- tides: Study of the radioprotective activity. 14th. Interna- tional Conference on Radiopharmaceutical Chemistry, Switzerland, 2001, 10-15. [16] Minasyan, S.H., Ghazaryan, S.H., Tonoyan, V.C., Bajin- yan, S.A., Grigoryan, K.P., Sorensen, J.R.S. and Greena- way F.T. (2006) Synthesis, characterization, and meas- urement of antioxidant reactivity of Salicylidene- D,L-Tyrosine Ethyl Ester and Copper (II)(Salicylidene-D, LTyrosine Ethyl Ester)2 in a linoleic acid peroxidation reaction system. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry, 36, 425-434. [17] Parashar, R.K., Sharma, R.C. and Mohab, G. (1987) Syn- thesis and biological activity of some copper tridentate Schiff Buses. In: Sorenson J.R.J. Ed., Biology of copper complexes. Humana Press, Clifton NJ, 533-540. [18] Nath, M., Kamaluddin, L. and Cheema, J. (1993) Copper (II) complexes of salicylidene amino acid Schiff bases as models. Peroxidase and catalase. Indian Journal of Chemistry, 32, 108-113. [19] Picart, L., Goodwin, W.H., Murphy, T.B. and Johnson D.K. (1982) Zinc regulates DNA synthesis and IL-2, IL-6, and IL-10 production of PWM-stimulated PBMC and normalizes the periphere cytokine concentration in chronic liver disease. Journal of Cell Biochemistry, 6, 172-173. [20] Akiyama, H., Yamasaki, O. Kanzaki, H., Tada, J. and Arata, J. (1998) Effect of zinc oxide on the attachment of Staphylococcus aureus strains. Journal of Dermatologi- cal Science, 17, 67-74. [21] Zhou, G.D. and Duan L.Y. (1995) Basis of structural chemistry. 2nd Edition, Perking University Publishing Corporation, Beijing, 249-250.
|