 American Journal of Plant Sciences, 2013, 4, 1375-1386 http://dx.doi.org/10.4236/ajps.2013.47168 Published Online July 2013 (http://www.scirp.org/journal/ajps) Molecular Genetic Diversity in Iranian Populations of Puccinia triticina, the Causal Agent of Wheat Leaf Rust Seyed Taha Dadrezaie1,2, Samer Lababidi3, Kumarse Nazari3, Ebrahim Mohammadi Goltapeh1, Farzad Afshari2, Fida Alo3, Masoud Shams-Bakhsh1, Naser Safaie1 1College of Agriculture, Tarbiat Modres University, Tehran, Iran; 2Seed and Plant Improvement Institute, Karaj, Iran; 3International Center for Agricultural Research in the Dry Areas (ICARDA), Aleppo, Syria. Email: tahareza2000@yahoo.com Received April 16th, 2013; revised May 17th, 2013; accepted June 10th, 2013 Copyright © 2013 Syed Taha Dadrezaie et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Wheat leaf rust caused by Puccinia triticina, is the most common and widely distributed wheat rust in the world. In order to study the genetic structure of leaf rust population 14 pairs of AFLP and 6 pairs of FAFLP primers evaluated on 86 isolates samples collected in Iran during spring of 2009. Results showed that almost all investigated isolates were genetically different and special pattern of AFLP allele’s that confirm high genetic diversity within leaf rust population was observed. Analyses showed, all provinces were classified into three major groups particularly similar clusters were found between then neighboring provinces. Rust spore can follow the migration pattern in short and long distances to neighbor in provinces. Results indicated that the greatest variability was revealed by 97% of genetic differentiation within leaf rust populations and the lesser variation of 3% was observed between the rust populations. These results suggested that each population was not completely identical and high gene flow has occurred among the leaf rust popu- lation of different provinces. The highest differentiation and genetic distance among the Iranian leaf rust populations was detected between leaf rust population in Sistan and Baluchistan and highest similarity was observed between in Ardabil provinces. The high pathogenic variability of leaf rust races in Ardabil and Northern Khorasan may be an indi- cation that these two regions are the center of origin of pathogenic arability. Present study shows that leaf rust popula- tion in Iran is highly dynamic and variable. Keywords: Leaf Rust; Gene Resistance; Genetic Diversity; Puccinia triticina; AFLP and FAFLP Markers 1. Introduction Wheat leaf rust caused by Puccinia triticina Eriks (Pt), is the most common and widely distributed wheat rust in the world. Leaf rust damage is less than yellow and stem rust, but level of brown rust damage causes greater an- nual losses due to its more frequent and widespread oc- currence [1]. Despite extensive studies and scientific re- markable success, in reduingce damage in leaf rust in the twentieth century due to changes races still heavy losses to this valuable product is being imported. Damage the disease has been reported from different parts of the world. In 2007, leaf rust in Kansans’ winter wheat was decreased by 14% product, which affected production. In susceptible cultivars loss yield was estimated at more than 50% [2]. Wheat leaf rust is present in all wheat growing areas in Iran and a large number of races were found in a recent study of pathogenic variability of Pt population in Iran [3,4]. There are so many ways to control the disease, of the genetic resistance a preferred method for reducing dam- age leaf rust, evaluation of genetic diversity and popula- tion structure within species of fungi plant pathology in order to achieve resistance and its management is very essential. Therefore, understanding of the genetic struc- ture of pathogens and identification of pathogenic viru- lent factors in disease is primarily important. On the other hand ability to produce new races, proliferation and their ability to reproduce rapidly and the epidemic of the important features of the rust, compared with other dis- eases that cause them to be more important and it is nec- essary to constantly change pathogen races. Characteristics of virulent on resistance genes is more important application of, races analysis in the world is still more influential, Because these virulent properties are under strong selection. It is likely that these pheno- Copyright © 2013 SciRes. AJPS  Molecular Genetic Diversity in Iranian Populations of Puccinia triticina, the Causal Agent of Wheat Leaf Rust 1376 typic characteristics provide incorrect estimate of the potential genetic variation of pathogen populations. Va- riety of molecular markers based on the DNA that in plant pathogens are used for the study population. Using AFLP markers for segregation in the P. triticina has been conducted in different countries. On the other hand ure- dospores migration to new areas occurred by wind and air currents. In fact the important feature is that rust are travel over the continent and uredospores can spread by wind hundreds kilometers away from the infected plants source. Those factors are important for genetic diversity and occurrence of new virulence to the region. Therfore the investigate relationship pathogen races of in prov- inces with air flow path was the main objective of this study. A study on American virulence phenotype and ampli- fied fragment length polymorphism (AFLP) markers were used to examine 42 isolates collected between 1960 and 2004. The data indicated that the contemporary iso- lates (collected since 2000) were very distinct from older isolates (collected before 2000) based on virulence and AFLP markers, and that the old population prevalent before 2000 may have been replaced by the contempo- rary population. The old and new populations appear to be genetically distinct and may represent an exotic in- troduction rather than a mutation in isolates of the old population [5]. Kolmer et al., [6] tested 64 isolates for randomly amplified polymorphic DNA (RAPD). Fifteen different RAPD phenotypes of the fungus were distin- guished using 10 polymorphic DNA bands among 64 isolates. However, there were 37 virulence phenotypes of the fungus as determined by 19 near-isogonics differen- tial lines of wheat. RAPD markers have been useful for characterizing variation between regional groups of P. triticina virulence phenotypes but less so for detecting variation between closely related virulence phenotypes within or between regions [7]. A comparison, to study the population genetics of yellow rust in Gansu and Yunnan two China Provinces, with AFLP method was applied. Forty one AFLP genotypes were obtained from 150 isolates. Genotypic diversity in Gansu Province was higher than that in Yunnan Province. A free recombina- tion signature was detected in Gansu Province but not in Yunnan Province [8]. Effective breeding for disease resistance requires ex- tensive information on the incidence and virulence of endemic pathogens; which is of high importance for rust fungi as they are able to rapidly evolve new virulent races. Knowledge on the diversity of the leaf rust fungus in Iran is limited; and, information on the diversity of P. triticina isolates regularly occurring in Iran is also rare; to study the molecular diversity of Iran P. triticina iso- lates, amplified fragment length polymorphism (AFLP) markers have been successfully used in mycology and plant pathology for the differentiation of species within genera [9-11]. Because these markers are not neutral and under selection in contrast to resistance-specific viru- lence, which is subject to strong host selection are as more accurate tools for genetic structure study [12-14]. The FAFLP (Fluorescent AFLP) method, a large number of cut pieces of genome replication was labeled then these fragments were separate and distinct parts in a ma- chine sequencer [15]. AFLP and FAFLP markers were used for this study to investigate the genetic variability of leaf rust isolates collected from 14 province of Iran. Our general hypothesis was that genetic similarity within populations of 14 provinces would be considerably higher than between populations. This hypothesis might have been nullified by the existence of isolates of P. triticina. Our objective was, however, to study the gen- eral molecular diversity of P. triticina isolates collected from different part of Iran. No study has been done on molecular diversity in leaf rust in Iran before. This study to assess the genetic diversity between and within leaf rust populations in various provinces of the country using the AFLP and FAFLP molecular markers was performed in 2010 at ICARDA. These markers can be easily searched in whole genomes and this was attempts to ge- netically related fungi based clear geographical distribu- tion of air flows. 2. Materials and Methods 2.1. Leaf Samples Collection, Purified and Amplified Samples Collected About 100 samples of leaf rust-infected leaves were ran- domly collected from 14 provinces of Iran in early spring of 2009. The samples were dried in open air and then were kept in a refrigerator at 4˚C. The isolate were gradually regenerated on the susceptible Bolany cultivar and the spores were amplified. The initial proliferation was considered as a spores bulk or mass of the popula- tion. From each spores masses 5 to 7 pots were inocu- lated and from each pots 3 to 5 single pustule were ran- domly selected and amplified. To ensure purity all single pustules were single pustulated 2 or 3 times. 8 to 10 old seedlings of susceptible Bolany cultivar were used for proliferation. 2.2. Determination of Virulence Phenotypes Pathotype identification based on the formula Virulence/ Avirulence 38 differentials lines of Thatcher in seedling stage were tested. Thatcher wheat lines with single resis- tance gene were used as follows: Lr22b, Lr1, Lr2a, Lr2b, Lr2c, Lr3, Lr3ka, Lr3bg, Lr9, Lr10, Lr11, Lr12, Lr13, Lr14a, Lr14b, Lr15, Lr16, Lr17, Lr18, Lr19, Lr20, Lr21, Lr22a, Lr23, Lr24, Lr25, Lr26, Lr10, 27+31, Lr28, Lr29, Lr 30, Lr32, Lr 33, Lr34, Lr35, Lr36, Copyright © 2013 SciRes. AJPS  Molecular Genetic Diversity in Iranian Populations of Puccinia triticina, the Causal Agent of Wheat Leaf Rust 1377 Lr37, Lrb all isolates were evaluated on all the above genes and also physiological races of leaf rust were de- termined based on modified uniform nomenclature sys- tem. Using the results of above 20 lines which mostly isogenic in 5 tetramerous groups based on Ordonez et al. 2010 [2]. Bolany was as a susceptible check. To evaluate the seedling stage in inoculated plants, types of consid- ered infection were created on the lines and recorded 12 to 24 days after inoculation according to McIntosh et al (1995) method [16]. Infection types 0 to 2+ were classi- fied as avirulent and infection types 3 to 4 were classified as virulent. Pathogenesis frequency for resistance gene was calculated. 2.3. Determination of Molecular Phenotypes 2.3.1. D NA Extracti on DNA extracted from fresh urediniospores by using a modified CTAB procedure (Hexadecyl trimethyl ammo- nium bromide) as follows steps: 1) Weight 50 mg spores, 50 mg small ball and two small balls (derided spore in freeze drier for 6 - 8 days). 2) Place tubes into liquid ni- trogen for at least 30 seconds before continuing to step three. 3) Put the tube in amalgamator and tape tightly into place turn on for 40 seconds (30 shaking in each seconds). 4) Repeat steps 2 and 3 two time again (in to- tally three time). 5) Add heat (65˚C) CTAB 2% 1200 µl and place them in the water bath (65˚C) for 30 - 45 min- utes (during the incubation, mix each tube, one at a time, by inverting perform tube mixing three times during the incubation period and placed tubes in ice for 5 minutes. 6) Add 600 µl chloroform/isoamyl (24:1) mix tubes by gen- tle inversion for 2 minute (100 - 120 inversion). 7) Cen- trifugation for 12 min at 13,000 rpm in 4 temperature transfer aqueous phase to 2 ml eppendorf tubes. 8) Add 100 µl R40 and incubate at 37˚C for 60 minutes. 9) Add 600 µl chloroform/isoamyl (24:1) mix tubes by gentle inversion for 2 minute (100 - 120 inversion). 10) Cen- trifugation for 12 min at 13000 rpm in 4 temperature transfer aqueous phase to 2ml eppendorf tubes. 11) Add 100 µl of 3M sodium acetate PH 4.8. 12) Add 600 µl cold Isopropanol and then mix gently by hands keep it in freezer −20˚C for min. 13) Centrifugation for 12 min at 13,000 rpm in 4 temperature transfer aqueous phase to 2 ml eppendorf tubes. 14) Wash with cold ethanol 75% with 1000 µ. 15) Centrifugation for 12 min at 13,000 rpm in 4 temperature transfer aqueous phase to 2 ml eppen- dorf tubes. 16) Dry the pellet well on paper towel for 20 minutes. 17) Add 100 µl of TE buffer to resuspend and keep in Freezer. 2.3.2. AFLP For analysis of genetic diversity, AFLP markers were applied on population samples based on Vos et al., 1995 [17]. Restrictive digestion enzyme and PCR method were used in this method. AFLP protocol was followed as de- scribed in CIMMYT applied molecular protocol [18]. 2.3.3. Selectiv e PCR For use in stage of selective amplification initial repli- cating stage of the samples were diluted 5 times. In this study, Initially based on the results of previous work re- lated to the rust and in other cases more than 40 pairs combination of leaf rust on four selective sample (Safi- Abad Dezful, Mehran, Bayea kola and Ajab sheir) were tested. Then created based on polymorphism 14 pairs combination were selected and evaluated on all samples were analyzed on. All samples were about 100, because of limitations in the gel sample was reduced to 86 (Table 1) AFLP primers combinations used in this study: 1 = P02 + M51, 2 = P10 + M183, 3 = P11 + M50, 4 = P32 + M50, 5 = P11 + M47, 6 = P24 + M17, 7 = P24 + M54, 8 = P37 + M50, 9 = P16 + M47, 10 = P13 + M301, 11 = P16 + M183, 12 = P16 + M183, 13 = P16 + M88, 14 = P16 + M17. 2.4. Evaluation 6 Pairs of FAFLP Primer in ABI Electrophoresis System To complete study in order to accuracy of the previous results, six pairs primers of AFLP markers labeled with fluorescent dye (FAFLP) were used, then separated the dye-labeled fragments by capillary electrophoresis, this method is currently one of the most suitable methods for fragment analysis in automated ABI Prism 3100/3100- Avant sequencer (construction company, Applied Bio- systems). Just one of the primer combinations fluores- cent-labeled was used. Primers were labeled by three kinds of fluorescent dye, FAM blue, Ned yellow and green Vic in this study. During the reaction, fluorescent dye is not involved in amplification process and it's only used for peak observation (bands) in the ABI. For this purpose, eight P primers (P10, P11, P14, P15, P16, P19, P32, P40, and P41) which by various fluorescent were labeled and with three M primers (M54, M55, M54), make 24 combinations of primers. Based on experimen- tal results, six primer pairs were selected, this primers including (P11/M54; P11/M55; P10/M54; P10/M55; P14/M54; P19/M55). PCR program for testing the la- beled AFLP markers were used exactly similar to con- ventional PCR in the AFLP that previously described. After the primary amplification, of the three pairs of samples of different combinations with different color fluorescent mix (ABI electrophoresis system due to dif- ferent fluorescent, three primers in each well will be de- tected) was diluted in water. From this mixture diluted and uniform, only one micro liter were taken and to new platform are that each well contains 5 μl standard for- mamide (Hi-Di formamide) was transferred and pipette. Formamide standard (Hi-Di formamide) containing Rox Copyright © 2013 SciRes. AJPS  Molecular Genetic Diversity in Iranian Populations of Puccinia triticina, the Causal Agent of Wheat Leaf Rust Copyright © 2013 SciRes. AJPS 1378 Table 1. Characteristics of leaf rust isolates collected form differant parts of Iran in spring of 2009. Isolates No. Location Race Isolates No. Location Race 1 Ahvaz MGHSS 44 Moghan (Ardabil) FKTRS 2 Ahvaz MGMSN 45 Moghan (Ardabil) BJKFJ 3 Ahvaz DBGTJ 46 Moghan (Ardabil) CJHCS 4 Susangerd MBMTJ 47 Moghan (Ardabil) PGRKS 5 Lali MBHNB 48 Karaj PJTRS 6 Safi-Abad (Dezful) MJHTS 49 Karaj PJTTS 7 Safi-Abad (Dezful) BBJNG 50 Karaj FDRRS 8 Safi-Abad (Dezful) CBBTJ 51 Karaj FFTTS 9 Mehran CGTRS 52 Karaj FJQRS 10 Mehran BBGSG 53 Boroujerd FJRSS 11 Mehran DBGTJ 54 Boroujerd PKRTS 12 Mehran DBGTJ 55 Boroujerd FKRRS 13 Safi-Abad (Dezful) FHQSS 56 Boroujerd FFTTS 14 Baykla (Sari) FKTMS 57 Neishabour FKTRS 15 Sari MJTRS 58 Neishabour FKTRS 16 Qrakhyl (Sari) CJRCS 59 Zarghan FJRRS 17 Kelardasht FJRMS 60 Zarghan FKTTS 18 Mashhad FKTRS 61 Zarghan FKTRS 19 Qrakhyl (Sari) MHSTS 62 Mashhad FKTTS 20 Baykla (Sari) MKSTS 63 Ardabil FJRRS 21 Baykla (Sari) MKSKS 64 Ardabil FJTRS 22 Baykla (Sari) FKTHS 65 Ardabil PKTRS 23 Sari FHTTS 66 Gorgan CGRMQ 24 Gorgan FKTMS 67 Gorgan FGRRQ 25 Gorgan MKTTS 68 Gorgan FGRRQ 26 Gorgan PKTTS 69 Ajabshir FGHMQ 27 Gorgan FKTSS 70 Bonab FGHLQ 28 Islamabad FKRKS 71 Kelardasht DHRTS 29 Islamabad FKRKS 72 North Khorasan FGRRQ 30 Moghan (Ardabil) MDSKS 73 North Khorasan FGRRQ 31 Moghan (Ardabil) MKSJS 74 North Khorasan PGTRS 32 Moghan (Ardabil) MKKTS 75 North Khorasan FGTMQ 33 Moghan (Ardabil) MJTHS 76 North Khorasan FGRMQ 34 Moghan (Ardabil) FKTRS 77 North Khorasan FGRMQ 35 Moghan (Ardabil) FKTRS 78 North Khorasan PJTTS 36 Eshtehard CHRMN 79 North Khorasan PJRTS 37 Marivan FKTKS 80 Moghan (Ardabil) FHRRS 38 Marivan FKRHS 81 Moghan (Ardabil) FHTRS 39 Marivan CHQKS 82 Zabul BJRRS 40 Marivan FKRHS 83 Zabul FJRRQ 41 Islamabad FKRMS 84 Ahvaz CJTQQ 42 Moghan (Ardabil) CJRRS 85 Anchusa (Moghan) FJRRS 43 Moghan (Ardabil) MFTTS 86 Barley leaf rust PHRRQ 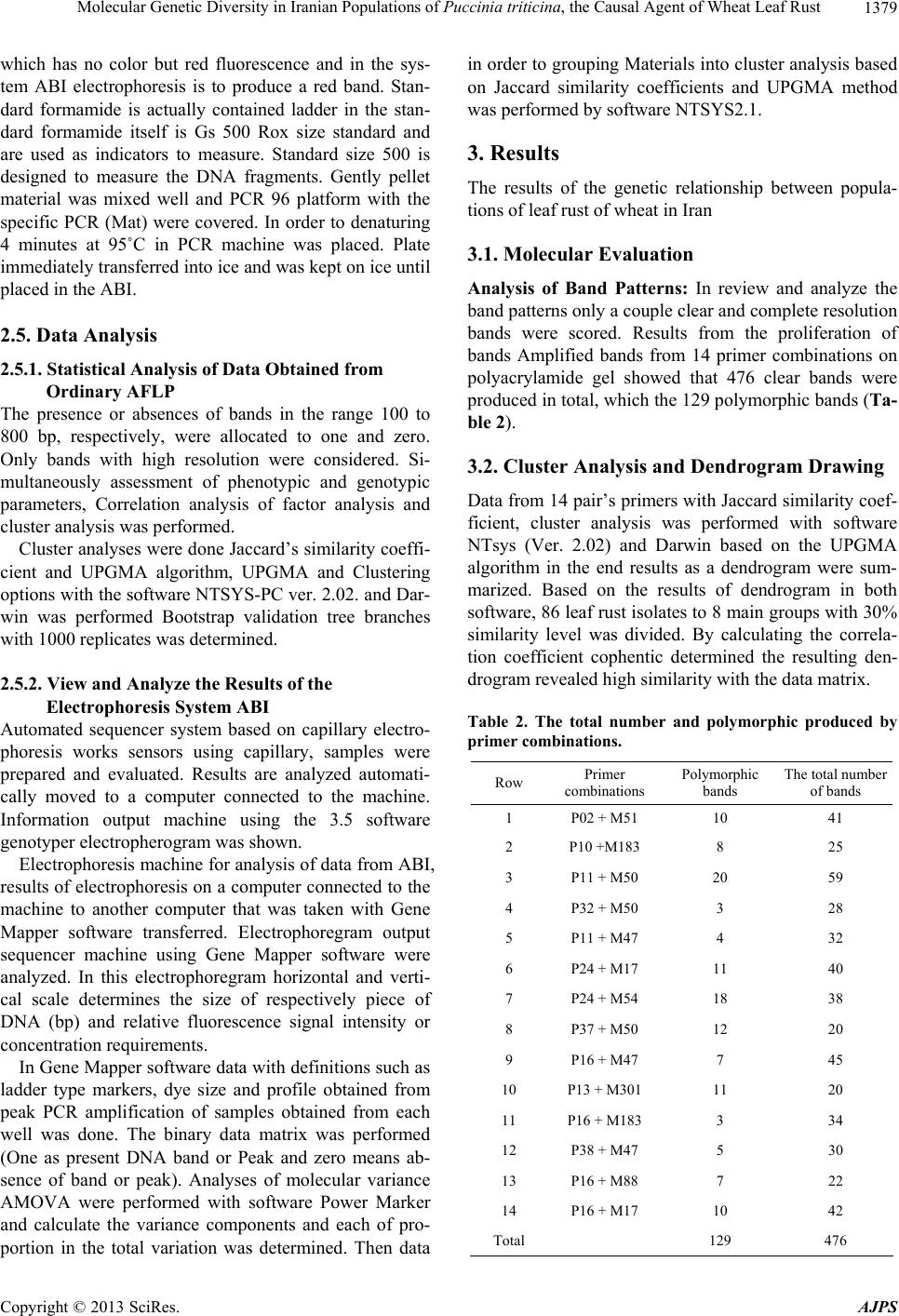 Molecular Genetic Diversity in Iranian Populations of Puccinia triticina, the Causal Agent of Wheat Leaf Rust 1379 which has no color but red fluorescence and in the sys- tem ABI electrophoresis is to produce a red band. Stan- dard formamide is actually contained ladder in the stan- dard formamide itself is Gs 500 Rox size standard and are used as indicators to measure. Standard size 500 is designed to measure the DNA fragments. Gently pellet material was mixed well and PCR 96 platform with the specific PCR (Mat) were covered. In order to denaturing 4 minutes at 95˚C in PCR machine was placed. Plate immediately transferred into ice and was kept on ice until placed in the ABI. 2.5. Data Analysis 2.5.1. Stati s ti cal Analysis of Data Obtained from Ordinary AFLP The presence or absences of bands in the range 100 to 800 bp, respectively, were allocated to one and zero. Only bands with high resolution were considered. Si- multaneously assessment of phenotypic and genotypic parameters, Correlation analysis of factor analysis and cluster analysis was performed. Cluster analyses were done Jaccard’s similarity coeffi- cient and UPGMA algorithm, UPGMA and Clustering options with the software NTSYS-PC ver. 2.02. and Dar- win was performed Bootstrap validation tree branches with 1000 replicates was determined. 2.5.2. View and Analyze the Results of the Electrophoresis System ABI Automated sequencer system based on capillary electro- phoresis works sensors using capillary, samples were prepared and evaluated. Results are analyzed automati- cally moved to a computer connected to the machine. Information output machine using the 3.5 software genotyper electropherogram was shown. Electrophoresis machine for analysis of data from ABI, results of electrophoresis on a computer connected to the machine to another computer that was taken with Gene Mapper software transferred. Electrophoregram output sequencer machine using Gene Mapper software were analyzed. In this electrophoregram horizontal and verti- cal scale determines the size of respectively piece of DNA (bp) and relative fluorescence signal intensity or concentration requirements. In Gene Mapper software data with definitions such as ladder type markers, dye size and profile obtained from peak PCR amplification of samples obtained from each well was done. The binary data matrix was performed (One as present DNA band or Peak and zero means ab- sence of band or peak). Analyses of molecular variance AMOVA were performed with software Power Marker and calculate the variance components and each of pro- portion in the total variation was determined. Then data in order to grouping Materials into cluster analysis based on Jaccard similarity coefficients and UPGMA method was performed by software NTSYS2.1. 3. Results The results of the genetic relationship between popula- tions of leaf rust of wheat in Iran 3.1. Molecular Evaluation Analysis of Band Patterns: In review and analyze the band patterns only a couple clear and complete resolution bands were scored. Results from the proliferation of bands Amplified bands from 14 primer combinations on polyacrylamide gel showed that 476 clear bands were produced in total, which the 129 polymorphic bands (Ta- ble 2). 3.2. Cluster Analysis and Dendrogram Drawing Data from 14 pair’s primers with Jaccard similarity coef- ficient, cluster analysis was performed with software NTsys (Ver. 2.02) and Darwin based on the UPGMA algorithm in the end results as a dendrogram were sum- marized. Based on the results of dendrogram in both software, 86 leaf rust isolates to 8 main groups with 30% similarity level was divided. By calculating the correla- tion coefficient cophentic determined the resulting den- drogram revealed high similarity with the data matrix. Table 2. The total number and polymorphic produced by primer combinations. Row Primer combinations Polymorphic bands The total number of bands 1 P02 + M51 10 41 2 P10 +M183 8 25 3 P11 + M50 20 59 4 P32 + M50 3 28 5 P11 + M47 4 32 6 P24 + M17 11 40 7 P24 + M54 18 38 8 P37 + M50 12 20 9 P16 + M47 7 45 10 P13 + M301 11 20 11 P16 + M183 3 34 12 P38 + M47 5 30 13 P16 + M88 7 22 14 P16 + M17 10 42 Total 129 476 Copyright © 2013 SciRes. AJPS  Molecular Genetic Diversity in Iranian Populations of Puccinia triticina, the Causal Agent of Wheat Leaf Rust 1380 Also, separately data from six primer pairs labeled with software NTsys (Ver. 2.02) and Darwin based on UPGMA algorithm cluster analysis was performed. The isolates were grouped with the Jaccard similarity coeffi- cient and was determined and by calculating the correla- tion coefficient cophentic that the data matrix has a high similarity (r = 0.82) with this dendrogram. Based on re- sults of the data labeled primers the isolates were divided into nine groups. Often groups in both cluster had overlapping together but more importantly, the provinces that have been iden- tified as important areas of brown rust such as Ardebil, Khuzestan, Mazandaran, and Golestan North Khorasan were present in most groups and Indicates that diversity was found within the province. Both data merged to- gether and again perform cluster analysis, and its Den- drogram was drawn. Eleven groups were formed. Those eleven separate groups were formed in 28% similarity and indicate a large diversity in groups. The results based on molecular data showed that diversity of leaf rust iso- lates in Iran is very high. The first groups were included 33 isolates where from all the provinces apart of Sistan- Baluchistan province, there were one or more isolates. The second group was consisted of five isolates from the four provinces of Fars, Sistan and Baluchistan, Khuzestan and Khorasan. The third group included one isolate from the Lorestan and the eleventh group was Moghan isolates from Anchusa. italica in a single group was formed a sister group with other groups again. AFLP data on polyacrylamide gel and sequencer also showed very high diversity. For a more complete deter- mined genetic relationship between isolates and similar- ity and better closeness between them and also investi- gate probability of molecular markers associated with virulent isolates, virulence and virulence data of 86 iso- lates of leaf rust on 38 genes resistance to leaf rust was analyzed with software NTsys (Ver. 2.02) and Darwin. This analysis was based on UPGMA algorithm but iso- lates with the simple similarity coefficient were grouped. Dendrogram obtained by calculating the correlation co- efficient cophentic was found to the data matrix has a high similarity (r = 0.82). Simple coefficient is used in phenotypic characters (Figure 1). Based on the results of the data virulence and a viru- lence isolates were in thirteen groups. The first group included 16 isolates; the second group included 5 iso- lates. Direct and individually correlation from virulent data groups with groups derived from molecular data was not obtained, but all data and analysis indicate that diversity is very high in the samples investigated and confirmed the correlation between neighboring provinces. More than 65% of isolates were virulent on 17 to 23 genes. One isolate was virulent on 26 genes and one iso- late was also virulent just on six genes. This isolates ei- ther in terms of the on which genes resistance are viru- lent and both can be pathogenic on number of resistance genes have been varied. Regarding that which terms of the genes that are pathogenic and on which genes not virulent in 64 races were placed and regarding can be pathogenic to a number resistance genes were classified in 19 groups (Figure 2). In this results are not a significant relationship be- tween populations and geographical distribution that one particular group is belongs to one particular region or a province. But there were several different groups in provinces and in large groups are present all provinces, which suggests that the in provinces population diversity is high and from this views are the same population. In fact the provinces were grouped together. This was most closely related to neighboring provinces (Table 3, Fig- ure 3). 3.3. Diversity and Genetic Distance between Populations Based on data from the markers labeled in sequencer Table 3. Number of ineffective genes and Number of iso- lates. Number of ineffective genes Number of isolates 26 1 25 1 24 3 23 9 22 9 21 8 20 11 19 5 18 7 17 7 16 5 15 4 14 2 13 5 12 3 11 2 10 1 7 2 6 1 Copyright © 2013 SciRes. AJPS  Molecular Genetic Diversity in Iranian Populations of Puccinia triticina, the Causal Agent of Wheat Leaf Rust Copyright © 2013 SciRes. AJPS 1381 Figure 1. Dendrogram derived from analysis of AFLP bands on polyacrylamide gel and ABI machine of 86 leaf rust isolates using UPGMA method and Jaccard’s similarity coefficient.  Molecular Genetic Diversity in Iranian Populations of Puccinia triticina, the Causal Agent of Wheat Leaf Rust 1382 Figure 2. Dendrogram based on data from 86 leaf rust isolates virulent on 38 gene leaf rust resistance. 1003 the polymorphic alleles are identified, analysis of molecular variance and genetic distance on this data was calculated by the software Power Marker (Table 4). Results of Analysis Molecular of variance showed that the genetic differences within populations are 97% and difference between populations is 3% and very high gene flows between populations exist. Genotypic diversity among the populations studied was different so that the Ardebil population with 81% and Fars population with 50% respectively had the highest and lowest genotypic diversity. Maximum of the genetic distance between populations of leaf rust isolates in Iran’s was belonging to Sistan-Baluchistan province, After that Golestan and Kermanshah provinces had the highest genetic distance with the other (Figure 4). Leaf rust population of Ardebil Isolates was more near with other populations of leaf rust in Iran, which North Khorasan was with the lowest dis- tance. After the North Khorasan Ardebil had the highest similarity with other province. It seems these two areas are centers of leaf rust diversity and distribution in Iran. Copyright © 2013 SciRes. AJPS  Molecular Genetic Diversity in Iranian Populations of Puccinia triticina, the Causal Agent of Wheat Leaf Rust 1383 Figure 3. Pathogenic potential of different isolates studied on resistance genes. Figure 4. Dendrogram obtained from cluster analysis of leaf rust populations using Nei genetic distance and the UP- GMA algorithm. Table 4. The calculated values for genetic diversity betwee n and within populations of leaf rust. Source Df SS MS Est. Var. % Among Pops 12 1105.98392.165 2.681 3% Within Pops 73 5488.08775.179 75.179 97% Total 85 6594.070 77.860 100% 4. Discussion The results showed that almost all the studied isolates were genetically different, all studied P. triticina isolates were produced unique AFLP alleles patterns, which the high genetic diversity within population of the leaf rust fungus was described by McDonald and Linde 2002 con- firmed [19]. Also this research confirmed results of pathotypes and physiological races of P. triticina in Iran conducted by Dadrezaei et al., 2012 that there was great diversity in Iranian races of leaf rust. Their results showed that among 234 single, 177 different virulence classes (races) were identified [4]. In Canada Kolmer 69 isolates collected, and were evaluated by 10 pair’s combination of AFLP. Only 37 distinct phenotypes virulent with Thatcher lines were detected, whereas 69 Molecular phenotypes with assis- tance 164 AFLP markers were detected [7]. In another study in Morocco form four different farming-ecological areas leaf rust isolates collected and were evaluated with 5 pairs AFLP primer combinations. Analysis of molecu- lar variance of AFLP pattern showed no significant dif- ferences among populations. But diversity within popu- lation was very high. Patterns of genetic diversity among isolates collected indicate high gene flow and a complex system of reproduction. Leaf rust isolates in Morocco have sexual and asexual production stages and sexual stage is on Anchusa. italica [20]. Reproductive system and gene flow are important fac- tors that determine their genetic structure. The wheat rusts are heteroecious, and therefore requires a telial/ uredinial host (usually wheat) and an alternative (pycnial/ aecial) host (for leaf rust are) Thalictrum speciosissimum, Isopyrum fumaroides or Anchusa italica to complete the full life cycle. Anchusa and Thalictrum have been re- ported in Iran, but no study or report on sexuality pro- duction in Iran exists. Probably sexuality form leaf rust is prevalent in Iran so probably part of the genetic diversity observed in population of P. triticina may be due to re- combination. This study showed that the contrary to Torabi et al. 2001 [21] which declared that genetic diver- sity of leaf rust in Iran was not very high, genetic diver- sity leaf rust in Iranian isolates is very high and accord- ing to research results in different world. In this study to separation and analysis of the different races of leaf rust, both characteristics of virulent on resistance genes and molecular markers were used. Both results indicate and confirmed very high variation in pathogenicity and ge- netic diversity in pathogen. Characteristics virulent on resistance genes the importance, more applicable, more useful in races analyses and are still in use, But because virulent properties are under strong selection it is likely provide incorrect estimate the potential genetic variations pathogen populations. But variety of DNA-based mo- Copyright © 2013 SciRes. AJPS  Molecular Genetic Diversity in Iranian Populations of Puccinia triticina, the Causal Agent of Wheat Leaf Rust 1384 lecular markers have been used to study populations of plant pathogens, because these markers that are selec- tively neutral, highly informative more accurate tool and it is best to use for studying the genetic structure and because analyses are based on allele frequencies, it is vital adequate to make reliable estimates [12]. Pattern of pathogenicity in the province showed that there are the common races in different province of countries exist. Also on the air streams flowing in spring and at track races in this trace, probably has race connec- tion between the southern regions, South West, West, North West, North and North East of country. This grouping is based on the relationship between patho- genicity and non pathogenicity on 38 Thatcher isolines that had been discussed previously was also confirmed in these results. But the relationship more clearly and pre- cisely by dendrogram was created from the molecular markers. By exact examining dendrogram and its nu- merous subgroups can be detected three adjacent prov- inces that were most similar or near together could ob- serve. In better words three similarities can be identified in three domains South West to North West, North West to North East and North-East to the South East, and that most isolates in the adjacent provinces in the dendrogram are located together. When carefully observed to these pathways they are affected by air mass that Iran is fully compatible correspond and way they are affected by the Zagros and Alborz Mountains range. The main air masses during the wheat growing season will affect Iran include Mediterranean air masses that enter the country from the West and North West. Iran is out of the East and most parts of the country will affect its precipitation. The flow direction before entering Iran will pass Leba- non, Syria, Turkey, and Iraq. The second is related to the Sudanese air mass that form Africa, Yemen and Saudi Arabia and crossed over into the Persian Gulf will be entering in Iran. These two air masses have very important role to transferring urediniospores in wheat growing areas. This direction is the same direction that in the 1990s makes transferring of yellow rust spores had virulence on gene Yr9. The Yr9 virulence detected in Ethiopia in 1986 migrated to Yemen in 1991 then to Egypt and West Asia such as Syria and Iran (1991-1992) and reached Indian sub-continent (1996) over a period of 10 years riding on wind currents from west to east. This caused severe crop losses in widely grown cultivars cov- ering more than 20 million hectares. These air masses will cause yellow rust epidemics in the CWANA region and heavy damage to wheat. For example estimated grain losses were in the order of 1.5 million tons in 1993 and one million tons in 1995 [22]. Such new reaces introduc- tions of leaf rust and yellow rust in this area is probable Third air mass affected Iran is Siberian air mass. It is entered from the north of Iran. This air mass past to the countries of the Caucasus, Kazakhstan, Uzbekistan and Turkmenistan across the Caspian Sea and causes the highest rainfall in the north of the country. Existence of the identical races especially in the adjacent provinces along with the existence of air flows on this way rein- forced the reason of linking of these provinces through air flows. This relationship was both in grouping based on being virulent or non-virulent on the seedling of wheat leaf rust near isogenic lines and in the abundance of the races. The aggressive isolates in rather similar groups showed the relation between the provinces. Al- most most of the provinces were related to each other. This suggests the gene flow and vast and effective trans- mission of spores through air flows [4]. There are nu- merous examples of mutations and migration that cause appearance and rise of non-indigenous races form un- known areas. Non-native races durum wheat in the last decade in Europe and North America has been attacked. In Rabbani Nasab studies genetic similarity of most Iranian wheat yellow rust populations with West and North West yellow rust populations (including the prov- inces of Elam, Hamadan, Kermanshah and Ardebil) more than 65 percent. He caused the occurrence of gene flow due to genetic similarity population to each other’s [23]. Genetic diversity analysis of yellow rust isolates in Denmark, England, Germany and France use to AFLP showed that great Yellow rust spores immigration are occurrence between Denmark, England, Germany and France [24]. Phenotypic and genotypic results of differ- ent studies, including traps nursery and various molecu- lar markers studies showed that leaf rust spores can be exchanged between the America and Mexico and dem- onstrate transfer of spores moving from America to Mexico and probably from Mexico or the Southwest Pa- cific to America [1,2,25,26]. Analysis of molecular variance using the AFLP data indicated that the greatest variability was revealed by 97% of genetic differentiation within leaf rust popula- tions and the lesser variation of 3% was observed be- tween the rust populations. Although the high molecular variability within the rust population may indicate the colonial behavior of leaf rust in distinct provinces, but similarity between rust populations indicates the gene flow. These results suggested that each the investigated populations were not completely identical and high gene flow has occurred among the leaf rust population of dif- ferent provinces. The highest differentiation and genetic distance among the Iranian leaf rust populations was de- tected between leaf rust population in Sistan and Balu- chistan in South east followed by Golestan and Kerman- shah provinces in North and Southwest, respectively. Highest similar virulence pattern of physiological races was observed between the rust population between Ardabil province and other studied provinces followed Copyright © 2013 SciRes. AJPS  Molecular Genetic Diversity in Iranian Populations of Puccinia triticina, the Causal Agent of Wheat Leaf Rust 1385 by the leaf rust population in Northern Khorasan. The maximum number of virulence factor for 31 Lr genes was found for P. t races TKTTN and TFTTN that were collected form Ardabil and North Khorasan, respectively [4]. The high pathogenic variability of leaf rust races in Ardabil and Northern Khorasan may be indication that these two regions are the center of origin of pathogenic ariability and also the source of race distribution to the other regions. Present and befor study [4] shows that leaf rust population in Iran is highly dynamic and variable and is subjected to frequent pathogenic shifts and there- fore as long term strategy the wheat breeding programs in Iran need to deploy combination of effective resistance genes such as Lr9, Lr28, Lr25, Lr19, Lr29, and Lr2a with non-race specific and adult-plant resistance genes of which Lr34, Lr46, and Lr67 are slow rusting leaf rust resis- tance genes widely used in breeding program worldwide. During this process which may not be achieved in short time, other control measures such as gene deployment, and chemical control might need to be considered as short term strategy. The consistent results of molecular studies based on phenotypic variation with pathogenicity in a very high genetic diversity in Iranian leaf rust populations seems to Iran is one of the centers of leaf rust diversity in the world. Race diversity in Iran could be because the wheat pathogen evolution probably in Iran is very old, since Iran and its neighbors that are located in the Fertile Crescent that be wheat Origins in the world and adjacent with other regions of the world that wheat have been identified as the primary origins and related to air flow between the these regions, as well as very large popula- tion, short cycle generation especially in epidemics con- ditions that cause widespread epidemics in Iran and pri- mary and alternative hosts are in different regions of Iran probably all of the reasons for the racial diversity in Iran be, so that they may be genetic structure of this fungus is different from other populations in other parts of the world. Oliveira and Samborski believed that the P. triticina centre of origin is the Fertile Crescent region of the Middle East, where the natural range of the primary and alternative hosts overlaps [27]. It is believed that origin of any plant where most of the existing genetic diversity and so plants have evolved with disease. 5. Conclusion Based on the results of leaf rust in Iran is very diverse and has very high evolutionary potential, Thus stability of single-gene resistance probably be short. The strategy for breeding for resistance to this disease should be es- tablished based on the use of quantitative genes (QTL) with other resistance genes or race-non specific with race specific resistance genes and Cultivars rotation, chemical control and even rotation of use chemical fungicides. Main strategy to control leaf rust disease in the country should be use of a combination of genetic strategies and fungicides. Effective resistance genes such as Lr9, Lr28, Lr25, Lr19, Lr29 and Lr2a in combination with nonspecific genes such as Lr34 and Lr46 or Lr67 could create a more effective and stability resistance. Cultivars which have different gene combinations are replaced and interchange before the appearance of new pathogenicity phenotypes will located, as rotation used in the resistance genes will to stable and also cause long duration of the resistance genes REFERENCES [1] J. Huerta-Espino, R. P. Singh, S. S. Germán, B. D. McCallum, R. F. Park, W. Q. Chen, S. C. Bhardwaj and H. Goyeau, “Global Status of Wheat Leaf Rust Caused by Puccinia triticina,” Euphytica, Vol. 179, No. 1, 2011, pp. 143-160. doi:10.1007/s10681-011-0361-x [2] M. E. Ordonez, S. E. German and J. A. Kolmer, “Genetic Differentiation within the Puccinia triticina Population in South America and Comparison with the North American Population Suggests Common Ancestry and Inters Con- tinental Migration,” Phytopathology, Vol. 100, No. 4, 2010, pp. 376-383. doi:10.1094/PHYTO-100-4-0376 [3] M. Torabi, V. Mardoukhi, A. Froutan, M. Aliramaei, S. T. Dadrezaie, H. A. Moghaddam, S. Rajaei and H. Azimi, “Virulence Genes of Puccinia recondita f. sp. Tritici, the Causal Agent of Wheat Leaf Rust in Some Regions of Iran during 1995-1999,” Seed and Plant, Vol. 18, 2003, pp. 432-449. [4] S. T. Dadrezaei, E. M. Goltapeh, F. Afshari and K. Nazari, “Identification of Pathotypes and Physiological Races of Puccinia triticina Eriks, the Casual Agent of Wheat Leaf Rust in the Iran in 2009-2010,” Seed and Plant Improve- ment Journal, Vol. 28, No. 4, 2012, pp. 685-715. [5] S. G. Markell and E. A. Milus, “Emergence of a Novel Population of Puccinia striiformis f. sp. Tritici in Eastern United States,” Phytopathology, Vol. 98, No. 6, 2008, pp. 632-639. doi:10.1094/PHYTO-98-6-0632 [6] J. A. Kolmer, J. Q. Liu and M. Sies, “Virulence and Mo- lecular Polymorphism in Puccinia recondita f. sp. Tritici in Canada,” Phytopathology, Vol. 85, No. 3, 1995, pp. 276-285. doi:10.1094/Phyto-85-276 [7] J. A. Kolmer, “Molecular Polymorphism and Virulence Phenotypes of the Wheat Leaf Rust Fungus Puccinia triticina in Canada,” Canadian Journal of Botany, Vol. 79, No. 8, 2001, pp. 917-926. [8] X. Liu, C. Huang, Z. Sun, J. Liang, Y. Luo and Z. Ma, “Analysis of Population Structure of Puccinia striiformis in Yunnan Province of China by Using AFLP,” European Journal of Plant Pathology, Vol. 129, No. 1, 2011, pp. 43-55. doi:10.1007/s10658-010-9688-8 [9] F. J. Keiper, M. J. Hayden, R. F. Park and C. R. Wellings, “Molecular Genetic Variability of Australian Isolates of Five Cereal Rust Pathogens,” Mycological Research, Vol. 107, No. 5, 2003, pp. 545-556. doi:10.1017/S0953756203007809 Copyright © 2013 SciRes. AJPS  Molecular Genetic Diversity in Iranian Populations of Puccinia triticina, the Causal Agent of Wheat Leaf Rust Copyright © 2013 SciRes. AJPS 1386 [10] J. G. Menzies, G. Bakkeren, F. Matheson, J. D. Procunier and S. Woods, “Use of Inter-Simple Sequence Repeats and Amplified Fragment Length Polymorphisms to Ana- lyze Genetic Relationships among Small Grain-Infecting Species of Ustilago,” Phytopathology, Vol. 93, No. 2, 2003, pp. 167-175. doi:10.1094/PHYTO.2003.93.2.167 [11] H Schmidt, L Niessen and R. F. Vogel, “AFLP Analysis of Fusarium Species in the Section Sporotrichiella— Evidence for Fusarium Langsethiae as a New Species,” International Journal of Food Microbiology, Vol. 95, No. 3, 2004, pp. 297-304. doi:10.1016/j.ijfoodmicro.2003.12.008 [12] B. A. McDonald, “The Population Genetics of Fungi: Tools and Techniques,” Phytopathology, Vol. 87, No. 4, 1997, pp. 448-453. doi:10.1094/PHYTO.1997.87.4.448 [13] J. A. Kolmer, “Physiologic Specialization of Puccinia recondita f. sp. Tritici in Canada in 1991,” Canadian Journal of Plant Pathology, Vol. 15, No. 1, 1993, pp. 34-36. doi:10.1080/07060669309500847 [14] J. K. M. Brown, “The Choice of Molecular Marker Meth- ods for Population Genetic Studies of Plant Pathogens,” New Phytologist, Vol. 133, No. 1, 1996, pp. 183-195. doi:10.1111/j.1469-8137.1996.tb04353.x [15] J. N. Goulding, J. V. Hookey, J. Stanley, W. Olver, K. R., Neal, D. A. AlaAldeen and C. Arnold, “Fluorescent Am- plified-Fragment Length Polymorphism Genotyping of Neisseria meningitidis Identifies Clones Associated with Invasive Disease,” Journal of Clinical Microbiology, Vol. 38, No. 12, 2000, pp. 4580-4585. [16] R. A. McIntosh, C. R. Wellings and R. F. Park, “Wheat Rusts: An Atlas of Resistance Genes,” Kluwer Academic Publishers, Dordrecht, 1995. doi:10.1007/978-94-011-0083-0 [17] P. Vos, R. Hogers, M. Rijans, T. Vandelee, M. Horens, A. Fijters, J. Pot, J. Peleman, M. Kuiper and M. Zabeau, “AFLP: A New Technique for DNA Fingerprinting,” Nu- cleic Acides Research, Vol. 23, No. 21, 1995, pp. 4407- 4414. doi:10.1093/nar/23.21.4407 [18] CIMMYT, “Laboratory Protocols: CIMMYT Applied Molecular Genetics Laboratory,” 3rd Edition, CIMMYT, Mexico City, 2005. [19] B. A. McDonald and C. C. Linde, “Pathogen Population Genetics Evolutionary Potential and Durable Resistance,” Annual Review of Phytopathology, Vol. 40, 2002, pp. 349-379. doi:10.1146/annurev.phyto.40.120501.101443 [20] F. Bouftass, M. Labhilili, B. Ezzahiri and A. Ziouti, “Molecular Polymorphism of the Wheat Leaf Rust Fun- gus in Morocco Using Amplified Fragment Length Poly- morphism,” Phytopathology, Vol. 158, 2010, pp. 111- 116. doi:10.1111/j.1439-0434.2009.01573.x [21] M. Torabi, K. Nazari and F. Afshari, “Genetic of Patho- genicity of Puccinia recondita f. sp. Tritici, the Causal Agent of Leaf Rust of Wheat,” Iranian Journal of Agri- culture Science, Vol. 32, 2001, pp. 625-635. [22] M. Torabi, W. Mardouchi, K. Nazari, H. Golzar and A. S. Kashani, “Effectiveness of Wheat Yellow Rust Resis- tance Gene in Different Part of Iran,” Cereal Rust and Powdery Mildew Bulletin, Vol. 23, 1995, pp. 9-12. [23] H. Rabaninasab, M. Okhovat, M.Torabi, M. Abbasi and J. Mozaffari, “Virulence and Molecular Diversity in Puc- cinia striiformis f. sp. Tritici form Iran,” Journal of Plant Protection, Vol. 22, 2008, pp. 47- 60. [24] M. S. Hovmøllera, A. F Justesena and J. K. M. Brown, “Clonality and Long-Distance Migration of Puccinia stri- iformis f.sp. Tritici in North-West Europe,” Plant Pa- thology, Vol. 51, No. 1, 2002, pp. 24-32. doi:10.1046/j.1365-3059.2002.00652.x [25] J. A. Kolmer and M. E. Ordonez, “Genetic Differentiation of Puccinia triticina Populations in Central Asia and the Caucasus,” Phytopathology, Vol. 97, No. 9, 2007, pp. 1141-1149. doi:10.1094/PHYTO-97-9-1141 [26] M. E. Ordonez and J. A. Kolmer, “Simple Sequences Repeat Diversity of a World-Wide Collection of Puccinia triticina from Durum Wheat,” Phytopathology, Vol. 97, No. 5, 2007, pp. 574-83. doi:10.1094/PHYTO-97-5-0574 [27] B. D. D’Oliveira and D. J. Samborski, “Aecial Stage of Puccinia recondita on Ranunculaceae and Boraginaceae in Portugal,” In: R. C. Macer and M. S. Wolfe, Eds., Pro- ceedings of the First European Brown Rust Conference, Cambridge, 1966, pp. 133-150.
|