Paper Menu >>
Journal Menu >>
 Journal of Environmental Protection, 2010, 1, 431-437 doi:10.4236/jep.2010.14050 Published Online December 2010 (http://www.SciRP.org/journal/jep) Copyright © 2010 SciRes. JEP 431 Phytoextraction of Metal Contaminants by Typha angustifolia: Interaction of Lead and Cadmium in Soil-Water Microcosms Thanawan Panich-Pat1*, Suchart Upatham2, Prayad Pokethitiyook3, Maleeya Kruatrachue3, Guy R. Lanza4 1Faculty of Liberal Arts and Science, Kasetsart University, Nakhon Pathom, Thailand; 2Faculty of Science, Burapha University, Chonburi, Thailand; 3Faculty of Science, Mahidol University, Bangkok, Thailand; 4Environmental Science Program, University of Massachusetts, Amherst, USA. Email: faastwp@ku.ac.th Received September 6th, 2010; revised October 16th, 2010; accepted October 20th, 2010. ABSTRACT A greenhouse study was conducted on phytoextraction and accumulation of lead (Pb) and cadmium (Cd) from con- taminated soil – water microcosms by the narrow-leaved cattail, Typha angustifolia. The plants were grown in sandy loam soil containing 1,666 and 38.5 mg/L of Pb(NO3)2 and Cd(NO3)2 respectively. The trends of lead and cadmium by T. angustifolia for all soil – water microcosms suggested interaction effects as decreased soil lead concentrations and increased water cadmium concentrations over time. T. angustifolia expressed trends as increased biomass in all con- taminated shoots and roots examined. Cadmium uptake in shoot and root biomass slightly decreased when lead was initially added to the soil but cadmium uptake in root biomass increased after 30 days. Data suggested an interaction between lead and cadmium and that lead uptake was inhibited when cadmium was present. Keywords: Phytoextraction, Contaminant Interaction, Lead, Cadmium, Microcosm, Typha angustifolia 1. Introduction Lead and cadmium are elements that are highly toxic to plants [1]. Both heavy metals are capable of interacting in biological systems and are persistent contaminants that can change their speciation, but do not biodegrade. Cad- mium is the heavy metal of greatest concern in most ag- ricultural soils. It is loosely held by soil constituents and is readily available to plant roots and transported through the xylem to the vegetative and reproductive organs, negatively affecting the crops [2]. In general, broadleaf plants such as lettuce and swiss chard accumulate more cadmium than grasses. Plant leaves and stems can also accumulate more than seeds. Nevertheless, lead and cadmium can be toxic and inhibit DNA synthesis, mitosis, cell division and germination [3]. Iqbal et al. [3] also investigated the effect of lead and cadmium individually and in combination on the germination and seedling growth of Leucaena leucocephala and Delonix regia. They found reduced germination and seedling growth, and that both species showed more tolerance to lead than cadmium. Kastori et al. [4] found that the treatment of sunflower plants with lead diminished the concentrations of chlorophylls and carotenes, while Larsson et al. [5] observed a reduction in the chlorophyll concentrations of plants exposed to cadmium. Mohan and Hosetti [6] sug- gested that both cadmium and lead drastically depressed catalase activity but stimulated peroxidase activity. They reported that the interaction between lead and cadmium on growth of other plants e.g., the root of Juncus acustus, was strongly inhibited by lead nitrate. Iqbal et al. [3] reported that the toxicity of lead and cadmium to young trees of Fagus silvatica is higher when both are com- bined. Other reports indicated that these two metals can bind to the cell wall, thus weakening their toxic effects to plants [7]. Phytoremediation is the use of plants to remove con- taminants from soil, water and air. One promising phy- toremediation process is the phytoextraction of heavy metal contaminants from soil. Typha spp has been stud- ied for their ability to phytoextract metals from soil, but most research to date reports the phytoextraction of indi- vidual heavy metal contaminants rather than mixtures of metals that commonly occur at contaminated sites. This  Phytoextraction of Metal Contaminants by Typha angustifolia: Interaction of Lead and Cadmium in Soil-Water Microcosms Copyright © 2010 SciRes. JEP 432 study examined the interaction of lead and cadmium during metal phytoextraction from soil-water micro- cosms by Typha angustifolia. 2. Materials and Methods 2.1. Plant Microcosm T. angustifolia were obtained from New England Wet- land Plant Company and planted in plastic bucket mi- crocosms (50 cm height, 30 cm diameter) containing 10 kg of sandy loam soil, collected from Orchard Hill, Massachusetts, USA. Sixteen buckets of microcosms were prepared, each with 2 plants in 10 kg of soil with a 5 liter layer of surface water. The microcosms were maintained in a greenhouse at a temperature of 24 1℃ under a full spectrum of 400 W light source providing a 16 hour per day photoperiod. The plants in microcosms were allowed to grow for 30 days until the plants reached an average height of 30 cm and then received the heavy metal treatments. 2.2. Soil Characterization One to three gram samples of soil were dried overnight in an oven at 105℃. Soil samples were sieved through 2mm and 0.5 mm screen, thoroughly mixed and ground in a mortar and pestle to obtain a uniform texture. Soil subsamples of 0.1-0.3 g were digested in 3 ml of concen- trated HNO3 using microwave digestion (CEM model MDS – 2100) for approximately 30 minutes. Samples were then evaporated to near dryness, filtered, and ad- justed to a volume of 25 ml in distilled water. Soil sam- ples were analyzed for lead and cadmium concentration before and after the addition of lead and cadmium mix- tures using Flame Atomic Absorption Spectrophotometer (FAAS), Graphite Furnace Atomic Absorption Spectro- photometer (GFAAS), and Hydride Generation (HG). 2.3. Preparation of Stock Solutions and Application of Lead and Cadmium Lead as Pb(NO3)2 and cadmium as Cd(NO3)2 were dis- solved in distilled water to prepare a stock solutions for treatment groups at concentrations of 10,000 mg/L and 1,000 mg/L, respectively. One liter of the lead stock so- lution was added to 5 L of distilled water to obtain a concentration of 1,666 mg/L for application to each mi- crocosm. Two hundred ml of the cadmium stock solution was added into 5 L to obtain a concentration of 38.5 mg/L. The sixteen microcosms were randomly divided into 4 treatment groups, each group with four replicates as follows: Group 1 served as the controls (no additions of lead and cadmium), Group 2 received cadmium solutions at a concentra- tion of 38.5 mg/L, Group 3 received lead solutions at a concentration of 1,666 mg/L, Group 4 received a mixture of lead at 1,666 mg/L and cadmium at 38.5 mg/L. 2.4. Plant Analysis Plant samples were harvested every fifteen days after heavy metal treatment. The whole plant was washed thoroughly with running tap water, divided into shoots and roots, and weighed, dried in an oven at 80℃ for two days and weighed again. Dried plant tissues were cut into small pieces, and homogenized. Approximately 0.1-0.3 g of shoot and root material were digested in a 3 ml con- centration of HNO3 and microwave digestion (CEM model MDS – 2100) for 30 minutes. Samples were eva- porated to near dryness, filtered, and adjusted to a vol- ume of 25 ml distilled water. The sample solutions were collected in polypropylene bottles and measured using FAAS, GFAAS, and HG. 2.5. Water Analysis After fifteen days of treatment, 5 ml water samples were collected from the control and the treatment groups. The water samples were adjusted to a volume of 25 ml, col- lected in polypropylene bottles, and analyzed for lead and cadmium using FAAS, GFAAS, and HG. 2.6. Data Analysis Data were analyzed using Excel analysis of variance (ANOVA) for significant differences (P 0.05). Dun- can’s New Multiple Range Test was used to determine significant differences (P 0.05). 3. Results 3.1. Soil and Water Characteristics The basic characteristics of the soil used in the soil-water microcosms are presented in Table 1. The results indi- cate that the soil is a sandy loam typical of many ecosys- tems. At the end of the experiment, the soil and water were examined in each microcosm for total lead and cadmium. Table 2 and Figures 1 and 2 summarized the lead and cadmium concentrations for all soil – water microcosms after 15 days. The results indicated that soil lead levels of Group 4 microcosms were higher than Group 3 micro- cosms (1061 476.6 as compared to 841.7 39.82 mg/kg), but the water lead levels were slightly different, 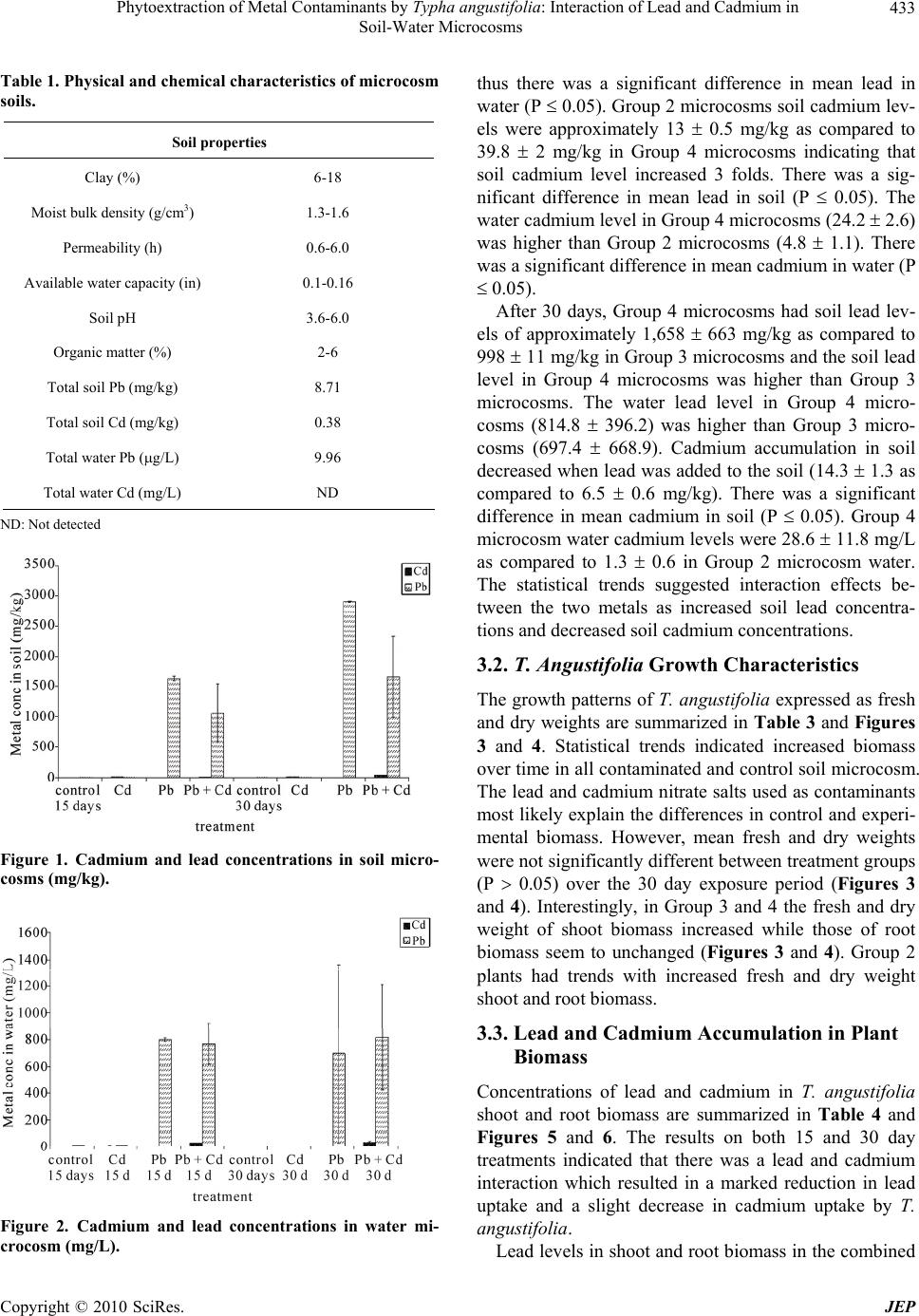 Phytoextraction of Metal Contaminants by Typha angustifolia: Interaction of Lead and Cadmium in Soil-Water Microcosms Copyright © 2010 SciRes. JEP 433 Table 1. Physical and chemical characteristics of microcosm soils. Soil properties Clay (%) 6-18 Moist bulk density (g/cm3) 1.3-1.6 Permeability (h) 0.6-6.0 Available water capacity (in) 0.1-0.16 Soil pH 3.6-6.0 Organic matter (%) 2-6 Total soil Pb (mg/kg) 8.71 Total soil Cd (mg/kg) 0.38 Total water Pb (g/L) 9.96 Total water Cd (mg/L) ND ND: Not detected Figure 1. Cadmium and lead concentrations in soil micro- cosms (mg/kg). Figure 2. Cadmium and lead concentrations in water mi- crocosm (mg/L). thus there was a significant difference in mean lead in water (P 0.05). Group 2 microcosms soil cadmium lev- els were approximately 13 0.5 mg/kg as compared to 39.8 2 mg/kg in Group 4 microcosms indicating that soil cadmium level increased 3 folds. There was a sig- nificant difference in mean lead in soil (P 0.05). The water cadmium level in Group 4 microcosms (24.2 2.6) was higher than Group 2 microcosms (4.8 1.1). There was a significant difference in mean cadmium in water (P 0.05). After 30 days, Group 4 microcosms had soil lead lev- els of approximately 1,658 663 mg/kg as compared to 998 11 mg/kg in Group 3 microcosms and the soil lead level in Group 4 microcosms was higher than Group 3 microcosms. The water lead level in Group 4 micro- cosms (814.8 396.2) was higher than Group 3 micro- cosms (697.4 668.9). Cadmium accumulation in soil decreased when lead was added to the soil (14.3 1.3 as compared to 6.5 0.6 mg/kg). There was a significant difference in mean cadmium in soil (P 0.05). Group 4 microcosm water cadmium levels were 28.6 11.8 mg/L as compared to 1.3 0.6 in Group 2 microcosm water. The statistical trends suggested interaction effects be- tween the two metals as increased soil lead concentra- tions and decreased soil cadmium concentrations. 3.2. T. Angustifolia Growth Characteristics The growth patterns of T. angustifolia expressed as fresh and dry weights are summarized in Table 3 and Figures 3 and 4. Statistical trends indicated increased biomass over time in all contaminated and control soil microcosm. The lead and cadmium nitrate salts used as contaminants most likely explain the differences in control and experi- mental biomass. However, mean fresh and dry weights were not significantly different between treatment groups (P 0.05) over the 30 day exposure period (Figures 3 and 4). Interestingly, in Group 3 and 4 the fresh and dry weight of shoot biomass increased while those of root biomass seem to unchanged (Figures 3 and 4). Group 2 plants had trends with increased fresh and dry weight shoot and root biomass. 3.3. Lead and Cadmium Accumulation in Plant Biomass Concentrations of lead and cadmium in T. angustifolia shoot and root biomass are summarized in Table 4 and Figures 5 and 6. The results on both 15 and 30 day treatments indicated that there was a lead and cadmium interaction which resulted in a marked reduction in lead uptake and a slight decrease in cadmium uptake by T. angustifolia. Lead levels in shoot and root biomass in the combined 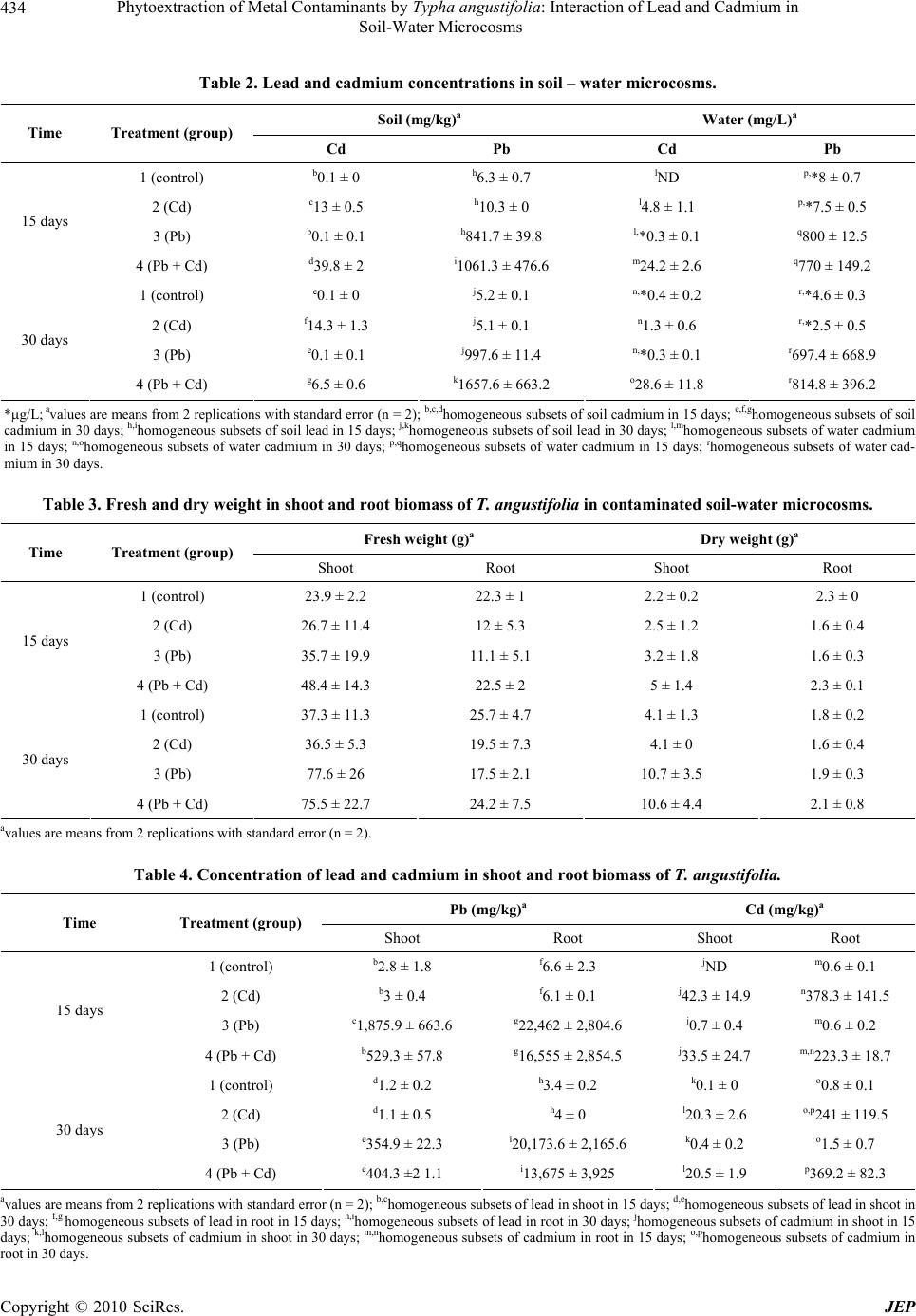 Phytoextraction of Metal Contaminants by Typha angustifolia: Interaction of Lead and Cadmium in Soil-Water Microcosms Copyright © 2010 SciRes. JEP 434 Table 2. Lead and cadmium concentrations in soil – water microcosms. Soil (mg/kg)a Water (mg/L)a Time Treatment (group) Cd Pb Cd Pb 1 (control) b0.1 ± 0 h6.3 ± 0.7 lND p,*8 ± 0.7 2 (Cd) c13 ± 0.5 h10.3 ± 0 l4.8 ± 1.1 p,*7.5 ± 0.5 3 (Pb) b0.1 ± 0.1 h841.7 ± 39.8 l,*0.3 ± 0.1 q800 ± 12.5 15 days 4 (Pb + Cd) d39.8 ± 2 i1061.3 ± 476.6 m24.2 ± 2.6 q770 ± 149.2 1 (control) e0.1 ± 0 j5.2 ± 0.1 n,*0.4 ± 0.2 r,*4.6 ± 0.3 2 (Cd) f14.3 ± 1.3 j5.1 ± 0.1 n1.3 ± 0.6 r,*2.5 ± 0.5 3 (Pb) e0.1 ± 0.1 j997.6 ± 11.4 n,*0.3 ± 0.1 r697.4 ± 668.9 30 days 4 (Pb + Cd) g6.5 ± 0.6 k1657.6 ± 663.2 o28.6 ± 11.8 r814.8 ± 396.2 *g/L; avalues are means from 2 replications with standard error (n = 2); b,c,dhomogeneous subsets of soil cadmium in 15 days; e,f,ghomogeneous subsets of soil cadmium in 30 days; h,ihomogeneous subsets of soil lead in 15 days; j,khomogeneous subsets of soil lead in 30 days; l,mhomogeneous subsets of water cadmium in 15 days; n,ohomogeneous subsets of water cadmium in 30 days; p,qhomogeneous subsets of water cadmium in 15 days; rhomogeneous subsets of water cad- mium in 30 days. Table 3. Fresh and dry weight in shoot and root biomass of T. angustifolia in contaminated soil-water microcosms. Fresh weight (g)a Dry weight (g)a Time Treatment (group) Shoot Root Shoot Root 1 (control) 23.9 ± 2.2 22.3 ± 1 2.2 ± 0.2 2.3 ± 0 2 (Cd) 26.7 ± 11.4 12 ± 5.3 2.5 ± 1.2 1.6 ± 0.4 3 (Pb) 35.7 ± 19.9 11.1 ± 5.1 3.2 ± 1.8 1.6 ± 0.3 15 days 4 (Pb + Cd) 48.4 ± 14.3 22.5 ± 2 5 ± 1.4 2.3 ± 0.1 1 (control) 37.3 ± 11.3 25.7 ± 4.7 4.1 ± 1.3 1.8 ± 0.2 2 (Cd) 36.5 ± 5.3 19.5 ± 7.3 4.1 ± 0 1.6 ± 0.4 3 (Pb) 77.6 ± 26 17.5 ± 2.1 10.7 ± 3.5 1.9 ± 0.3 30 days 4 (Pb + Cd) 75.5 ± 22.7 24.2 ± 7.5 10.6 ± 4.4 2.1 ± 0.8 avalues are means from 2 replications with standard error (n = 2). Table 4. Concentration of lead and cadmium in shoot and root biomass of T. angustifolia. Pb (mg/kg)a Cd (mg/kg)a Time Treatment (group) Shoot Root Shoot Root 1 (control) b2.8 ± 1.8 f6.6 ± 2.3 jND m0.6 ± 0.1 2 (Cd) b3 ± 0.4 f6.1 ± 0.1 j42.3 ± 14.9 n378.3 ± 141.5 3 (Pb) c1,875.9 ± 663.6 g22,462 ± 2,804.6 j0.7 ± 0.4 m0.6 ± 0.2 15 days 4 (Pb + Cd) b529.3 ± 57.8 g16,555 ± 2,854.5 j33.5 ± 24.7 m,n223.3 ± 18.7 1 (control) d1.2 ± 0.2 h3.4 ± 0.2 k0.1 ± 0 o0.8 ± 0.1 2 (Cd) d1.1 ± 0.5 h4 ± 0 l20.3 ± 2.6 o,p241 ± 119.5 3 (Pb) e354.9 ± 22.3 i20,173.6 ± 2,165.6 k0.4 ± 0.2 o1.5 ± 0.7 30 days 4 (Pb + Cd) e404.3 ±2 1.1 i13,675 ± 3,925 l20.5 ± 1.9 p369.2 ± 82.3 avalues are means from 2 replications with standard error (n = 2); b,chomogeneous subsets of lead in shoot in 15 days; d,ehomogeneous subsets of lead in shoot in 30 days; f,g homogeneous subsets of lead in root in 15 days; h,ihomogeneous subsets of lead in root in 30 days; jhomogeneous subsets of cadmium in shoot in 15 days; k,lhomogeneous subsets of cadmium in shoot in 30 days; m,nhomogeneous subsets of cadmium in root in 15 days; o,phomogeneous subsets of cadmium in root in 30 days. 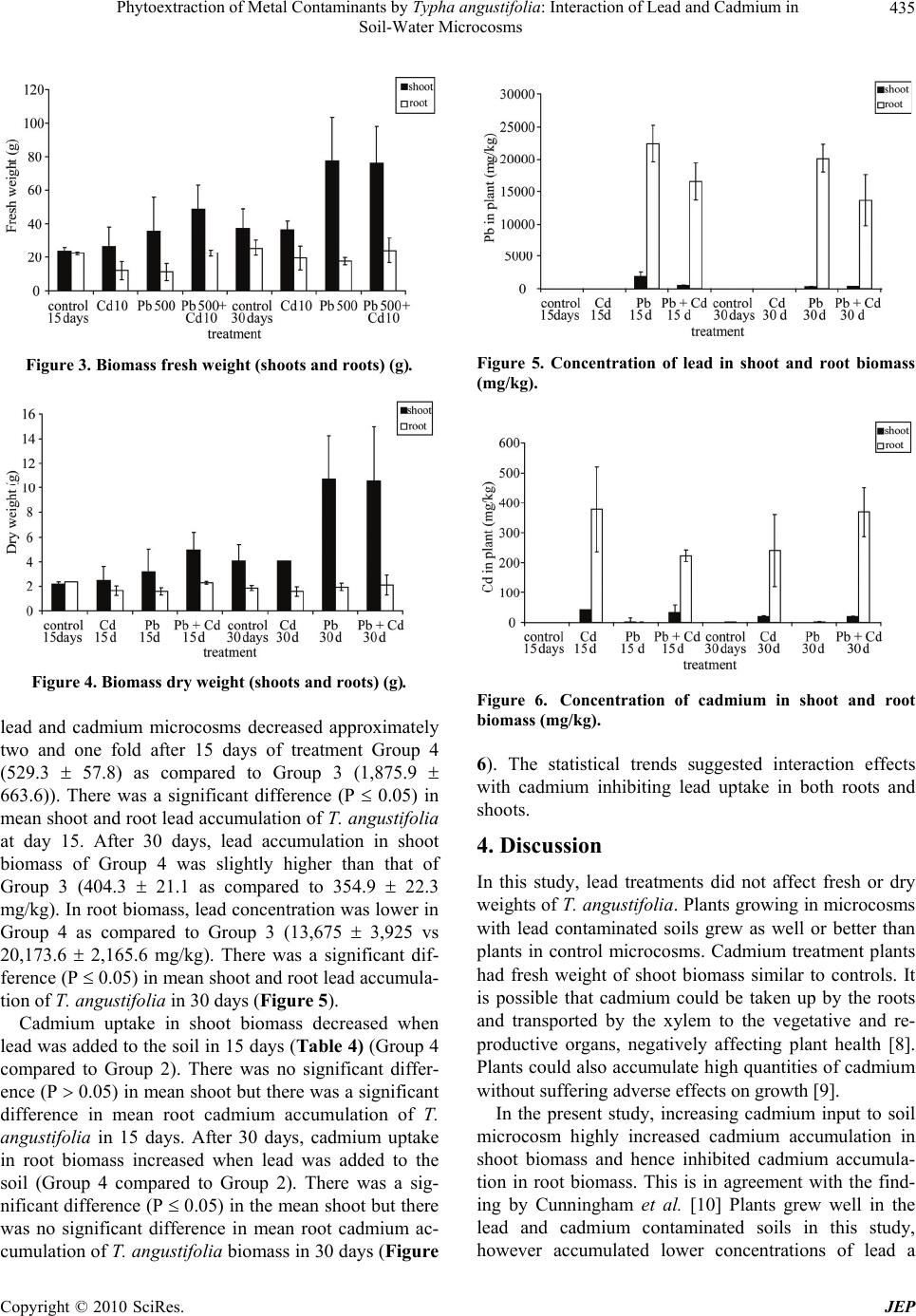 Phytoextraction of Metal Contaminants by Typha angustifolia: Interaction of Lead and Cadmium in Soil-Water Microcosms Copyright © 2010 SciRes. JEP 435 Figure 3. Biomass fresh weight (shoots and roots) (g). Figure 4. Biomass dry weight (shoots and roots) (g). lead and cadmium microcosms decreased approximately two and one fold after 15 days of treatment Group 4 (529.3 57.8) as compared to Group 3 (1,875.9 663.6)). There was a significant difference (P 0.05) in mean shoot and root lead accumulation of T. angustifolia at day 15. After 30 days, lead accumulation in shoot biomass of Group 4 was slightly higher than that of Group 3 (404.3 21.1 as compared to 354.9 22.3 mg/kg). In root biomass, lead concentration was lower in Group 4 as compared to Group 3 (13,675 3,925 vs 20,173.6 2,165.6 mg/kg). There was a significant dif- ference (P 0.05) in mean shoot and root lead accumula- tion of T. angustifolia in 30 days (Figure 5). Cadmium uptake in shoot biomass decreased when lead was added to the soil in 15 days (Table 4) (Group 4 compared to Group 2). There was no significant differ- ence (P 0.05) in mean shoot but there was a significant difference in mean root cadmium accumulation of T. angustifolia in 15 days. After 30 days, cadmium uptake in root biomass increased when lead was added to the soil (Group 4 compared to Group 2). There was a sig- nificant difference (P 0.05) in the mean shoot but there was no significant difference in mean root cadmium ac- cumulation of T. angustifolia biomass in 30 days (Figure Figure 5. Concentration of lead in shoot and root biomass (mg/kg). Figure 6. Concentration of cadmium in shoot and root biomass (mg/kg). 6). The statistical trends suggested interaction effects with cadmium inhibiting lead uptake in both roots and shoots. 4. Discussion In this study, lead treatments did not affect fresh or dry weights of T. angustifolia. Plants growing in microcosms with lead contaminated soils grew as well or better than plants in control microcosms. Cadmium treatment plants had fresh weight of shoot biomass similar to controls. It is possible that cadmium could be taken up by the roots and transported by the xylem to the vegetative and re- productive organs, negatively affecting plant health [8]. Plants could also accumulate high quantities of cadmium without suffering adverse effects on growth [9]. In the present study, increasing cadmium input to soil microcosm highly increased cadmium accumulation in shoot biomass and hence inhibited cadmium accumula- tion in root biomass. This is in agreement with the find- ing by Cunningham et al. [10] Plants grew well in the lead and cadmium contaminated soils in this study, however accumulated lower concentrations of lead a  Phytoextraction of Metal Contaminants by Typha angustifolia: Interaction of Lead and Cadmium in Soil-Water Microcosms Copyright © 2010 SciRes. JEP 436 30-day growth period. Coughtrey and Martin [11] studied Midger plant (Holcus lanatus) uptake of cadmium, lead, and zinc in solution culture and showed interactions on lead concen- trations in roots. Interestingly, the significant effects in shoots of this population were positive and tend towards increased metal concentrations. Miller et al. [12] reported positive interactions between lead and cadmium on lead and cadmium uptake in corn roots. Moshe et al. [13]. demonstrated that low levels ( 1 mg/L) of lead in- creased the toxicity of cadmium (0.1 mg/L) in phyto- plankton. Antagonism occured when the concentration of lead exceeded that of cadmium but no synergistic effects were noted in Chlorella when cadmium, copper, chro- mium, and nickel were added to the culture media. Pre- treatment of algae with nickel and mercury reduced cad- mium toxicity; this may reflect competition among met- als for cellular binding sites. The results of this study provide evidence for phytoex- traction interactions of lead and cadmium in soil-water microcosms. The statistical trends suggested that the in- teraction is complex and produced, decreased lead in root biomass and increased cadmium in shoot biomass al- though there were no significant variations in biomass production. Chukwuma [14] compared the accumulation of cadmium, lead, and zinc in cultivated and wild plant species in a derelict lead - zinc mine and found an overall reduction in the potential toxicity of cadmium by zinc through simple mass action effects specific for cultivated plants, they noted that other additional tolerant or adap- tive mechanisms might be operative in the wild plants. McKena et al. [15] reported the interactions between zinc and cadmium in nutrient solution and their effects on the accumulation of both metals in plant roots and leaves and also reported higher cadmium concentrations in older compared to younger leaves of lettuce and spinach. Many other studies involving several metals and nu- trients have been reported. Lagerwerff and Biersdorf [16] reported that cadmium and zinc were competitive cations. Similarly, Robert et al. [17] showed that cadmium can functionally substitute for zinc. The changes in the iron and zinc concentrations induced by increased cadmium levels resulted in alterations of the iron/zinc ratio and the alteration was more pronounced in roots than shoots, with both tissues exhibiting increases in the iron/zinc ratio with increased concentrations of cadmium. On the contrary, the toxicity of cadmium has been linked to the fact that cadmium competes for similar active sites but does not functionally substitute for zinc [18]. Cadmium and lead are toxic heavy metals and zinc an essential element makes this association interesting as it raises the possibility that the toxic effects of cadmium may be preventable or treatable by zinc. Hinesly et al. [19] indicated that both cadmium and zinc uptake by plants were dependent on the pH of the growing media. Ravera [20] showed that cadmium had toxic effects in plants on photosynthesis and also indicated various changes in biological activities. Subsequent studies have confirmed these findings and extended the interaction to other toxic effects of cadmium like inhibition of cell pro- liferation, and cytotoxic action [21] and growth suppres- sion in plants [22]. The biochemical mechanisms of cadmium - zinc in- teraction are unknown, but various cellular and subcellu- lar processes like the ratio of the cadmium to zinc in the tissues, induction of synthesis of different types of met- allothionein, alteration of absorption and tissue distribu- tion of one metal by another, and competition at the level of zinc containing metalloenzymes are known to be in- volved in the interaction [23]. Minnie et al. [24] studied phytoextraction of soil cobalt using hyperaccumulator plants and found interaction and decreased uptake of nickel in the presence of cobalt. Homer et al. [25] also reported that the uptake of cobalt may suppress nickel uptake, indicating a possible synergistic or antagonistic relationship between the elements. 5. Conclusions T. angustifolia exhibited very good potential for the phytoextraction of mixtures of lead and cadmium con- taminants from soil-water microcosms. Although cad- mium interaction appeared to reduce the uptake of lead in soil-water microcosms contaminated with both metals, uptake of lead into T. angustifolia roots was high, reach- ing levels of 13,675 3,925 mg/kg after 30 days growth. 6. Acknowledgements This work was supported by the Thailand Research Fund. We thank Dr. Wipharat Chuachuad for analyzing the samples. REFERENCES [1] M. Baghour, A. M. Diego, V. Gemma, H. Joaquin, C. Nicolas and R. Luis, “Phytoextraction of Cadmium and Lead and Physiological Effects in Potato Plants (Solanum tuberosum var. Spunta): Importance of Root Tempera- ture,” Journal of Agriculture Food Chemistry, Vol. 49, No. 11, 2001, pp. 5356-5363. [2] F. Fodor, E. Cseh, A. Varga and G. Zaray, “Lead Uptake, Distribution, Remobilization in Cucumber,” Journal of Plant Nutrition, Vol. 21, No. 7, 1998, pp. 1363-1373. [3] M. Z. Iqbal, M. Rukhsana and S. Muhammad, “The Ef- fect of Lead and Cadmium on Trees,” Journal of Soc. Munic. Arb., Vol. 36 No. 1, 2000, pp. 1-2.  Phytoextraction of Metal Contaminants by Typha angustifolia: Interaction of Lead and Cadmium in Soil-Water Microcosms Copyright © 2010 SciRes. JEP 437 [4] R. Kastori, M. Plesnicar, Z. Sakac, D. Pankovic and I. Arsenijevicmaksimovic, “Effect of Excess Lead on Sun- flower Growth and Photosynthesis,” Journal of Plant Nu- trition, Vol. 21, No. 1, 1998, pp. 75-85. [5] E. H. Larron, J. F. Bornman and H. Asp, “Influence of UV B Radiation and Cd2+ an Chlorophyll Fluorescence, Growth and Nutrient Content in Brassica napus,” Journal of Experimental Botany, Vol. 49, No. 323, 1998, pp. 1031- 1039. [6] B. S. Mohan and B. B. Hosetti, “Potential Phytotoxicity of Lead and Cadmium to Lemna Minor Grown in Sewage Stabilization Ponds,” Environmental Pollution, Vol. 98, No. 3, 1997, pp. 233-238. [7] M. Wierzbicka, “Lead in the Apoplast of Allium Cepa L. root Tips-Ultrastructural Studies,” Plant Science, Vol. 133, No. 1, 1998, pp. 105-119. [8] A. Stroinski, “Some Physiological and Biochemical As- pects of Plant Resistance to Cadmium Effect. I. Antioxi- dative System,” Acta Psychologica, Vol. 21, No. 1, 1999, pp. 175-188. [9] A. Kabata-Pendias and H. Pendias, “Trace Elements in Soil and Plants,” CRC Press, Boca Raton, 1992. [10] L. M. Cunningham, F. W. Collins and T. C. Hutchinson, “Physiological and Biochemical Aspects of Cadmium Toxicity in Soybean,” In: Proceedings of International Conference Heavy Metals in the Environment, Toronto, 1975. [11] P. J. Coughtrey and M. H. Martin, “Cadmium, Lead, and Zinc Interactions and Tolerance in Two Populations of Holcus lanatus L. Grown in Solution Culture,” Environ- mental and Experimental Botany, Vol. 19, 1979, pp. 285- -290. [12] J. E. Miller, J. J. Hassett and D. E. Koeppe, “Interactions of Lead and Cadmium on Metal Uptake and Growth of Corn Plants,” Journal of Environmental Quality, Vol. 6, No. 3, 1977, pp. 18-20. [13] M. Moshe, N. Betzer and Y. Kott, “Effect of Industrial Wastes on Oxidation Pond Performance,” Water Res- ources, Vol. 6, 1972, pp. 1165-1171. [14] C. Chukwuma Sr, “Comparison of the Accumulation of Cadmium, Lead and Zinc in Cultivated and Wild Plant Species in the Derelict Enyigba Lead-zinc Mine,” Toxi- cological and Environmental Chemistry, Vol. 38, No. 3- -4, 1993, pp. 167-173. [15] I. M. McKenna, R. L. Chaney and F. M. Williams, “The Effects of Cadmium and Zinc Interactions on the Accu- mulation and Tissue Distribution of Zinc and Cadmium in Lettuce and Spinach,” Environment Pollution, Vol. 79, No. 2, 1993, pp. 113-120. [16] J. V. Lagerwerff and G. T. Biersdorf, “Interaction of Zinc with Uptake and Translocation of Cadmium in Radish,” Proceedings 5th Annual Conference on Trace Substances on Environmental Health, University of Missouri, Co- lumbia, 1972, pp. 515-522. [17] A. R. Robert, J. M. Raymond and D. E. Koeppe, “Uptake of Cadmium – Its Toxicity, and Effect on the Iron Ratio in Hydroponically Grown Corn,” Journal of Enviromen- tal Quality, Vol. 4, No. 1, 1975, pp. 473-476. [18] R. E. Vallee and D. D. Ulmer, “Biochemical Effects of Mercury, Cadmium, and Lead,” Annual Review of Bio- chemistry, Vol. 40, No. 10, 1972, pp. 91-128. [19] T. D. Hinesly, K. E. Redberg, R. I. Pietz and E. L. Ziegler, “Cadmium and Zinc Uptake by Corn (Zea mays L.) with Repeated Applications of Sewage Sludge,” Journal of Agricultural and Food Chemistry, Vol. 32, 1984, pp. 155-163. [20] O. Ravera, “Cadmium in Freshwater Ecosystems,” Ex- perientia, Vol. 40, No. 5, 1984, pp. 1-14. [21] I. Rosas, M. E. Carbajal, S. Gomez-Arroyo, R. Belmont and R. Villalogos-Pietrini, “Cytogenetic Effects on Cad- mium Accumulation on Water Hyacinth (Eichornia cras- sipes),” Environment Resarch, Vol. 33, No. 3, 1984, pp. 386-395. [22] R. L. Chaney, M. C. White and P. W. Simon, “Plant Up- take of Heavy Metals from Sewage Sludge Applied to Land,” Proceeding of Iind National Conference Munici- pal Sludge Management, Rockville, 1975, pp. 167-178. [23] P. Das, S. Samantaray and G. R. Rout, “Studies on Cad- mium Toxicity in Plants: a Review,” Environmental Pol- lution, Vol. 98, No. l, 1997, pp. 29-36. [24] M. Minnie, L. C. Rufus, P. B. Eric, Y.-M. Li and J. S. Angle, “Phytoextraction of Soil Cobalt Using Hyperac- cumulator Plants,” International Journal of Phytorem, Vol. 2, No. 4, 2000, pp. 319-329. [25] F. A. Homer, R. S. Morrison, R. R. Brook, J. Clemens and R. D. Reeves, “Comparative Studies of Ni, Co, and Cu Uptake by Some Ni Hyperaccumulators of the Genus Alyssum,” Plant Soil, Vol. 138, 1999, pp. 195-205. |

