Paper Menu >>
Journal Menu >>
 Journal of Environmental Protection, 2010, 1, 420-425 doi:10.4236/jep.2010.14048 Published Online December 2010 (http://www.SciRP.org/journal/jep) Copyright © 2010 SciRes. JEP Biodegradation of Tritium Labeled Polychlorinated Biphenyls (PCBs) by Local Salt Tolerant Mesophylic Bacillus Strains Rasulov Bakhtiyor Abdughafurovich1, Kim Andrey Andreevich2, Lorenz Adrian3, Yadgarov Khojiakbar Tashpulatovich1 1Institute of Genetics and Plant Experimental Biology, Uzbekistan Academy of Sciences, Tashkent, Uzbekistan; 2Institute of Nuclear Physics, Uzbekistan Academy of Sciences, Tashkent, Uzbekistan; 3Department Isotope Biogeochemistry, Helmholtz Centre for Environmental Research, Leipzig, Germany. Email: bakhtiyor_1980@mail.ru Received August 13th, 2010; revised September 8th, 2010; accepted September 13th, 2010. ABSTRACT Salt-resistant Bacillus strains, isolated from agricultural soils in Uzbekistan, were tested for degradation activity to- wards a mixture of polychlorinated biphenyls (PCBs) under aerobic conditions. The study employed the use of tritium labeled PCB congeners and traced the tritium label in cultures with high salt content. The experiments show that most of the selected strains were able to adsorb a part of the radioactivity, indicating transformation of the added PCBs. Gas chromatography demonstrated transformation of PCBs. The radioactive label was removed from several cultures by up to 91%, indicating also mineralization of PCBs. The study suggests that the isolated strains might be good candidates for the bioremediation of contaminated high-salt soils in Uzbekistan and other Central-Asian countries. Keywords: Bacillus, Biodegradation, PCBs, Salinity, Tritium Label 1. Introduction Excessive use of fertilisers and pesticides in agriculture, primarily in the cotton and wheat plantations over the past decades, has resulted in significant contamination of soils not only in Uzbekistan, but also in a number of other Central Asian countries. Huge former cotton and wheat plantations now contain many different types of these chemicals. One important example for these chemicals is the polychlorinated biphenyls (PCBs that have been widely used for numerous open and closed industrial applica- tions. Commercial PCB mixtures were sold under the names Aroclor (Monsanto, USA), Phenoclor (Caffaro, Italy), Clophen (Bayer, Germany), Kanechlor (Kanega- fuchi, Japan), and Sovol (former USSR). Due to their widespread use and their thermal and chemical stability they have also found their way to agricultural soil. PCBs are organic chemicals belonging to a class of hydrocar- bons that are among the most hazardous environmental contaminants, as their physical and chemical properties especially their low water solubility and high lipophilic- ity lead to a high persistence in the environment and to bioaccumulation in the food chains [1,2]. Several bacteria have been described to degrade PCBs under aerobic conditions. Most of these bacteria also degrade biphenyls as a growth substrate. Such PCB de- graders are mainly obligate aerobes, motile, gram nega- tive rods. They can be enriched by cultivation with bi- phenyl or PCBs as a growth substrate on agar plates and are identified on agar plates by detecting clearing of bi- phenyl or PCB films around colonies. Biphenyl is mostly degraded by the initial action of a 2, 3-dioxygenase at- tack on the 2, 3-carbons and meta-cleavage, producing benzoate. Similarly, there are many bacterial strains from the genera Pseudomonas [3], Arthrobacter [4], Vibrio, Aeromonas, Micrococcus, Acinetobacter, Bacillus, Rho- dococcus [5], and Streptomyces that degrade mono-, di-, tri-, and several tetrachlorinated PCBs by meta-cleavage of non-chlorinated 2,3-carbons. Soils in Uzbekistan vary considerably in their chemi- cal composition and their salinity. The average soil salin- ity exceeds 40-60 mM [6]. However, in some provinces, particularly in Karakalpakstan, and Khorezm, Syrdarya provinces the degree of salinity is several times higher than this. Overall climate and specific weather patterns  Biodegradation of Tritium Labeled Polychlorinated Biphenyls (PCBs) by Local Salt Tolerant Mesophylic Bacillus Strains Copyright © 2010 SciRes. JEP 421 play a major role in the impacts that soil salinity will have with regard to the different regional soils of Uz- bekistan. Microbial biodegradation is the major process leading to transformation of organic chemicals in soil, and hence bioremediation is viewed as the most attrac- tive approach to the ultimate destruction of exogenous agricultural chemicals added to cultivated soils. However, the efficiency of bioremediation of agrochemicals in soils depends on the soil conditions. Therefore, under the giv- en climate and soil conditions in Uzbekistan, it appears necessary to use salt tolerant strains for bioaugmentation approaches. The application of microorganisms encoding contaminant-specific biodegradative pathways may pro- vide the most cost-effective technology for the remedia- tion of pollutant-contaminated sites. This type of tech- nology may also be the most acceptable for the public because it represents a natural process that is essentially taking advantage of nature’s recycling abilities. The most effective and acceptable bioremediation systems strive for complete mineralization of pollutants to inorganic products. The aim of our research was to investigate the biodeg- radation of PCBs in the presence of sodium chloride – the main source of salinity in the soil. Therefore, we used salt tolerant bacterial strains of the genus Bacillus from our strain collection and tested them for PCB degradation capability. 2. Materials and Methods 2.1. Strains We used a bacterial strain collection that was set up in a number of state projects on biodegradation of hazardous organic pesticides, such as PCBs, HCCH (hexachlorecy- clohexane), DDD (1,1-di(4’-chlorophenyl)-2,2 dichloro- ethane) and DDT (1,1-di(4’-chlorophenyl)-2,2,2 trichlo- roethane). 2.2. Culture Medium The strains were cultured in synthetic mineral medium with the following additions (g/l): NaCl – 0.5; KH2PO4 – 0.7; (NH4)2HPO4 – 1.5; MgSO4·7 H2O – 0.5; pH 7.3. As the sole source of carbon and energy, PCBs were added in a concentration of 20 μg/ml. Incubation was carried out on a shaker at 28℃ for 60 days. 2.3. Labeling of PCBs Before labeling, the labeling system and the capture was washed with nitrogen and a mixture of hexane:acetone (1:1). PCBs were labeled by thermal activation of tritium with the help of a labeling tool developed in the labora- tory of biological radiochemistry [7]. A solution of non- labeled PCBs was inserted to the surface of the reaction retort, cooled with liquid nitrogen and vacuumized to 5 × 10-3 mm mercury. Labeling was carried out in five cycles, with five times filling the system with gaseous tritium up to a pressure of 8 × 10-3 mm mercury. Tritium was acti- vated with a heated tungsten spiral three times with a 20 minute time interval between heating the spiral. The mixture of PCBs was analysed before and after the la- beling via HPLC. The labeled PCBs were purified on silica gel in hexane (Rf = 0.78). 2.4. GC Analyses GC analysis was done using an electron capture detector. A Chromaton N-Super column (2 m × 4 mm) (Chemapol, Czech Republic) was used, with a corn size of 0.125-0.16 mm, column temperature 200℃, injector temperature 230℃, detector temperature 260℃; carrier gas 30 ml/ min, 5% DS-200 (Chemapol, Czech Republic). 2.5. Analysis of Accumulation of PCBs by the Strains Strains cultured on synthetic agar-solidified medium were transferred to liquid medium. After one day of incubation 300 µl of culture (108 cells/ml) were transferred to a test tube containing 1 ml synthetic medium and 20 µl of the prepared solution of tritium-labeled PCBs in hexane. The tritium-labeled PCB was the sole carbon and energy source. The strains were incubated from several days up to two months at 28℃ on a shaker. After incubation bac- terial cells were separated by centrifugation at 6500 rpm for 30 min. Cells and supernatant were dried and PCBs were extracted with hexane. 2.6. Analysis of PCBs Degradation by the Selected Strains Bacterial cells, cultured on the agar-solidified mineral medium, were transferred to liquid medium. After one day, 300 μl (108 cells/ml) of this culture were transferred into test tubes with 5 ml liquid medium containing pep- tone (1%) and 100 μg of PCBs. In the controls, cells were killed by autoclaving for 30 min. The strains were incubated in a thermostat at 28℃ for 60 days. Then, cells were harvested by centrifugation. Pellets and super- natants were dried at 60℃ and PCBs were extracted with a mixture of hexane and acetone (1:1, vol/vol). The ex- tracts were then analyzed by GC as described above. 2.7. Analysis of Biodegradation of PCBs in the Soil Autoclaved soil samples (15 g each) were mixed with 100 μg of PCBs in 5 ml of hexane. Then, soil samples were dried to remove hexane from the samples. Each soil sample was inoculated with 300 μl from one of the liq-  Biodegradation of Tritium Labeled Polychlorinated Biphenyls (PCBs) by Local Salt Tolerant Mesophylic Bacillus Strains Copyright © 2010 SciRes. JEP 422 uid-medium cultures (108 cells/ml). The control consisted of a PCBs containing soil sample without bacteria. Dur- ing the two month incubation period all samples were kept humid by the addition of sterile water. After incuba- tion, samples were dried at 60℃ and PCBs were ex- tracted with a mixture of hexane and acetone (1:1, vol/vol). The extracts were analyzed by GC as described above. 3. Results We investigated the capability of selected pure Bacillus strains (denominated №№ 20, 22, 23, 24, 27, 28, 29, 30, 31, and 32) to utilize tritium-labeled PCBs in liquid cul- ture and soil. The strains were isolated from soils in Uz- bekistan that were heavily polluted since more than 40 years. We added 0.1 mg tritium-labeled PCBs solved in 5 ml of hexane to the bottom of the tubes. PCBs were then dried by complete evaporation of hexane. The applied radioactivity inserted with 0.1 mg of tritium labeled PCBs amounted to 13,647 imp/10 seconds. Finally, the tubes were inoculated with 5 ml of bacterial cultures. The control sample consisted of sterilized medium. The tubes, containing the culture liquids of the strains, were incu- bated in the thermostat at 28℃ for 10 days. After incuba- tion the cultures were transferred to centrifuge tubes, and bacterial cell were pelleted at 8000 rpm for 30 min. The supernatant was then transferred to fresh culture tubes. The pellets, containing bacterial cells were dried and weighed. Supernatants (culture liquid) were also dried at 80℃ until they were completely dry. From the dried samples of bacterial strains and culture liquid PCBs were extracted using a mixture of hexane and aceton (1:1, vol/vol) three times. The combined PCB extracts were again dried, dissolved in 100 µl of hexane and analyzed for radioactivity. Radioactivity was measured by liq- uid-scintillation counting in РЖС-05 (Liquid Scintilla- tion Radiometer) in a toluene scintillate cocktail (1 l to- luene containing 4 g of РРО and 0.05 g of РОРОР). For standardization a volume of 25 µl of a control solution was taken. Obtained data were then recalculated to ac- count for the whole volume. The results are given in the Figure 1. It was observed that a large part of the tritium label was incorporated into cell mass, showing that the PCBs were transformed and used for cell growth. Only a minor part was still found in the medium as non-evaporable substances. Another part of the tritium label was lost from the tubes either by the production of volatile trit- ium-labeled intermediates or by the transfer of tritium from the PCBs into water and successive evaporation during the analysis procedure. In the strains B. mycoides 0 2000 4000 6000 8000 10000 12000 14000 16000 2022 23242728 293031 32Control Bacterial strain number Radioactivity in 100 µl (imp/10 sec) Culture medium Pelleted bacteria Figure 1. Radioactivity originating from tritium-labeled PCBs detected associated to the bacteria and the culture medium after incubation for 60 days. 20, B. subtilis 28 and Bacillus sp. 30 the percentage of the remaining radioactivity was between 53 and 75% (in the strain of Bacillus sp. 23 up to 91%). In these strains (B. mycoides 20, Bacillus sp. 23, B. subtilis 28 and Ba- cillus sp. 30) between 86 and 95% of the remaining trit- ium was incorporated into the bacteria. In other strains the remaining radioactivity was 16-30%, while the rest was lost. In the control sample no decrease of the radio- activity was observed. Therefore, we can exclude the possibility that there was an isotope exchange of tritium fixed in the added PCBs with hydrogen from the water phase. The tritium-labeled PCBs remaining in the medium were analyzed by GC (Figure 2). It was observed that the spectra of PCB fractions changed during the incuba- tion when bacteria were present. This proves active transformation of PCBs by the strains in the presence of NaCl. The chromatogram of Sovol was also compared with the standard chromatograms of Aroclor (Aroclor 1016/1260 Standard, Aroclor 1221 Standard, Aroclor 1232 Standard, Aroclor 1242 Standard, Aroclor 1248 Standard, Aroclor 1254 Standard), available at the stan- dard library NIST02. The chlorination spectrum of Sovol considerably differs from that of the various Aroclors, which were used for calibration of the mass-selective detector Hewlett-Packard HP5972. That is why we de- veloped quantitative estimation of PCBs content in the tested samples. The obtained chromatograms from our incubations were reprocessed with MSD ChemStation Agilent Technologies in the overlay chromatogram re- gime. Chromatographic analysis of soil samples showed that they were rich in low chlorinated biphenyls. The amount of tritium-labeled PCBs that was trans- formed and incorporated into the bacteria was recalcu- lated from the incorporated radioactivity and the dry weight of bacterial cells. The results of these calculations are given in the Table 1. Here, the high capacity of strains 20 and 23 to specifically transform PCBs be 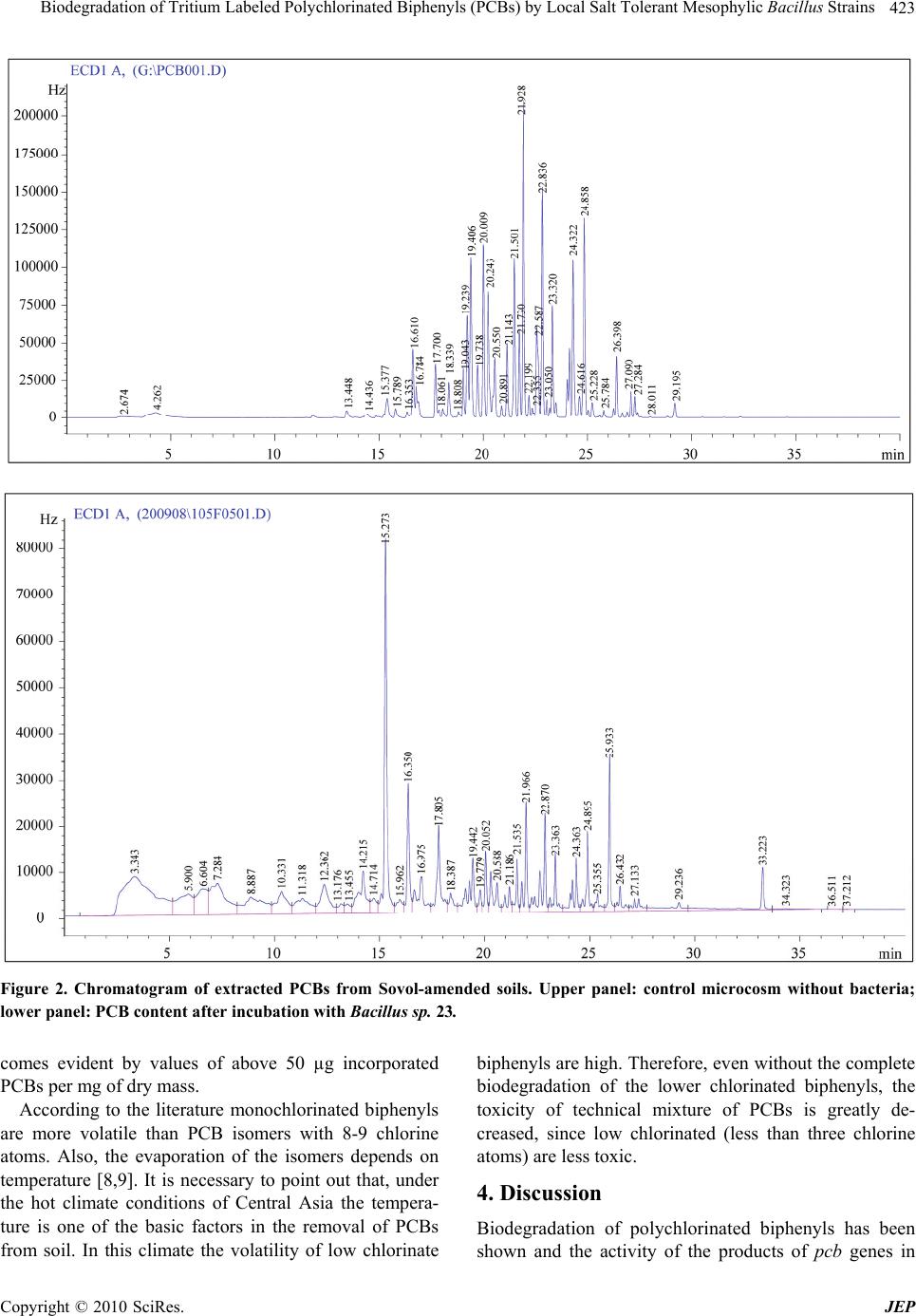 Biodegradation of Tritium Labeled Polychlorinated Biphenyls (PCBs) by Local Salt Tolerant Mesophylic Bacillus Strains Copyright © 2010 SciRes. JEP 423 Figure 2. Chromatogram of extracted PCBs from Sovol-amended soils. Upper panel: control microcosm without bacteria; lower panel: PCB content after incubation with Bacillus sp. 23. comes evident by values of above 50 µg incorporated PCBs per mg of dry mass. According to the literature monochlorinated biphenyls are more volatile than PCB isomers with 8-9 chlorine atoms. Also, the evaporation of the isomers depends on temperature [8,9]. It is necessary to point out that, under the hot climate conditions of Central Asia the tempera- ture is one of the basic factors in the removal of PCBs from soil. In this climate the volatility of low chlorinate biphenyls are high. Therefore, even without the complete biodegradation of the lower chlorinated biphenyls, the toxicity of technical mixture of PCBs is greatly de- creased, since low chlorinated (less than three chlorine atoms) are less toxic. 4. Discussion Biodegradation of polychlorinated biphenyls has been shown and the activity of the products of pcb genes in  Biodegradation of Tritium Labeled Polychlorinated Biphenyls (PCBs) by Local Salt Tolerant Mesophylic Bacillus Strains Copyright © 2010 SciRes. JEP 424 Table 1. Determination of tritium-labeled PCBs bound to the bacteria. Strain Radioactivity bound to the bacteria, imp/10 sec Dry weight of bacterial cells Bound PCBs (% out of exposed radioactivity) Bound PCBs per mg dry weight of bacterial cells B. mycoides 20 8,853 1.2 mg 64.87 µg (64.87%) 54.06 µg Bacillus sp. 22 984 2.3 mg 7.21 µg (7.21%) 3.13 µg sBacillus sp. 23 11,064 1.2 mg 81.07 µg (81.07%) 67.56 µg B. megaterium 24 1,208 1.1 mg 8.85 µg (8.85%) 8.05 µg B. subtilis 27 2,148 4.6 mg 15.74 µg (15.74%) 3.42 µg B. subtilis 28 7,768 10.3 mg 56.92 µg (56.92%) 5.54 µg B. megaterium 29 640 1.5 mg 4.69 µg (4.69%) 3.13 µg Bacillus sp. 30 6,216 3.8 mg 45.55 µg (45.55%) 11.99 µg B. megaterium 31 3,728 2.7 mg 27.32 µg (27.32%) 10.12 µg B. subtilis 32 2,396 20.9 mg 17.56 µg (17.56%) 0.84 µg different extreme environments has been documented. However, a strong effect of temperature on PCBs degra- dation has been found [10,11], and biphenyl dioxygenase was induced in psychorotolerant, polychlorinated biphe- nyl (PCBs) degrading Pseudomonas spp. [10,11]. How- ever, biodegradation of polychlorinated biphenyls in salt affected environments still needs to be better clarified. There is no detailed data on salinity influence on biodeg- radation activity of catabolic strains, expression of pcb genes, or synthesis and functioning of biphenyl dioxy- genase(s). Therefore, in the present study we examined the biodegradative activity of catabolic strains, isolated from salt-rich, pesticide-polluted soils in Uzbekistan. Former pollutants included DDT, and HCCH. The final concentration of NaCl in our study was 4%, in which the tested strains survived in previous experiments. We ob- served degradation of the PCBs by several catabolic strains within 4 months of incubation, whereas other re- searchers incubated for 1 year in the temperature range from 4 to 66℃ for microbial dechlorination of 2,3,4,6- tetrachlorobiphenyl [8]. In all tested samples with salt-tolerant catabolic strains, PCB removal (up to 85-90%) was much higher than with salt-intolerant catabolic strains of the same genus. That again proved normal catabolic functioning of the tested strains. It is important to note, that despite all strains be- longed to the same genus, the products of biodegradation slightly varied in respect to the number of chlorine atoms and their positions in the ring. Especially, it was specific to the intermediates with penta- and hexachlorinated rings in the early stages of incubation. The chroma- tographic analysis of a technical mixture of PCBs show- ed abundant presence of highly chlorinated biphenyls, such as heptachlorobiphenyls (2,2’,3,4,4’,5’,6-, 2,2’, 3,3’,4,- 6,6’-, and 2,2’,3,4’,5,5’,6-heptachlorbiphenyl), hexachlorobiphenyls (2,2’,3,4,4’,5’-, 2,2’4,4’,5,5’-, and 2,2’,3,-4,4’,5’- hexachlorobiphenyls), and 2,2’,4,5,5’- pentachlorobi-phenyl. Also tri- and tetrachlorobiphenyls were detected in the mixture. After two and four months of incubation the amount of highly chlorinated biphenyls was strongly reduced in all inoculated samples. The chromatographic analysis of two month samples revealed that the amount of heptachlorobiphenyls and hexachlore- biphenyls was sharply reduced. It is known that some bacterial strains can loose their biodegradation ability after introducing them into soil. Therefore, we performed additional tests on the func- tioning of the tested strains in soil. The strains were grown under model conditions in the soil with tritium labeled PCBs as the sole electron and carbon source. We found that most strains effectively mineralized PCBs resulting in decreasing level of tritium label in the soil with time (Figure 1). Based on the obtained data we can conclude that the tested strains effectively utilize tritium-labeled PCBs and different PCBs metabolism intermediates in the presence of high concentrations of sodium chloride, which is the main contributor to salinity. In the NaCl containing mi- crocosms the strains Bacillus sp. 23, B. mycoides 20, B. subtilis 28 and Bacillus sp. 30 transformed PCBs mainly to cell mass. Other strains most probably produced vola- tile compounds with low numbers of chlorine atoms, which evaporated during incubation and the analysis procedure. Herewith, we can suppose that practically all tritium labeled PCBs and their intermediates are incor- porated into the strains, and the degree of biodegradation under model conditions in the soil was lower than that of cultures without soil.  Biodegradation of Tritium Labeled Polychlorinated Biphenyls (PCBs) by Local Salt Tolerant Mesophylic Bacillus Strains Copyright © 2010 SciRes. JEP 425 Based on our results we can recommend the use of all our investigated Ba cillus strains to different extends as new biopreparations for the biodegradation of PCBs in the salt-affected soils in the Republic of Uzbekistan, and other Central Asian countries, including Kazakhstan, Turkmenistan, Tajikistan, and Kyrgyzstan, where tech- nical PCBs were heavily used in industrial and agricul- tural processes. REFERENCES [1] J. F. Quensen, J. M. Tiedje and S. A. Boyd, “Reductive Dechlorination of Polychlorinated Biphenyls by Anaero- bic Microorganisms from Sediment,” Science, Vol. 242, No. 4879, 1988, pp. 752-754. [2] V. A. McFarland and J. U. Clarke, “Environmental Oc- currence, Abundance, and Potential Toxicity of Poly- chlorinated Biphenyl Congeners: Considerations for a Congener-Specific Analysis,” Environment Health Per- spect, Vol. 81, 1989, pp. 225-239. [3] B. D. Erickson and F. J. Mondello, “Enhanced Biodegra- dation of Polychlorinated Biphenyls after Site-Directed Mutagenesis of a Biphenyl Dioxygenase Gene,” Applied and Environmental Microbiology, Vol. 59, No. 11, 1993, pp. 3858-3862. [4] E. S. Gilbert and D. E. Crowley, “Plant Compounds that Induce Polychlorinated Biphenyl Biodegradation by Ar- throbacter sp. Strain B1B,” Applied and Environmental Microbiology, Vol. 63, No. 5, 1997, pp. 1933-1938. [5] A. W. Boyle, C. J. Silvin, J. P. Hassett, J. P. Nakas and S.W. Tanenbaum, “Bacterial PCB Biodegradation,” Bio- degradation, Vol. 3, No. 2-3, 1992, pp. 285-298. [6] A. Abdullayev, “Azospirillum in Saline Soils of Uzbeki- stan,” Ph.D Dissertation, Institute of Microbiology of Uzbekistan Academy of Sciences, Tashkent, 2006. [7] A. A Kim, G. T. Djuraeva, P. V. Zinovev, I. I. Sadikov, A. A. Rylov, “A New Technique for Tritium Labeling of Complex Technical Mixture of PCB Congeners,” Journal of Radioanalytical and Nuclear Chemistry, Vol. 272, No. 3, 2007, pp. 483-489. [8] E. R. Master and W. W. Mohn, “Induction of bpha, En- coding Biphenyl Dioxygenase, in Two Polychlorinated Biphenyl-Degrading Bacteria, Psychrotolerant Pseudo- monas Strain Cam-1 and Mesophilic Burkholderia Strain lb400,” Applied and Environmental Microbiology, Vol. 67, No. 6, 2001, pp. 2669-2676. [9] E. R. Master, N. Y. R. A. Leticia Gómez-Gil, J. B. Pow- lowski, W. W. Mohn and L. D. Eltis, “Biphenyl Dioxy- genase from an Arctic Isolate is not Cold Adapted,” Ap- plied and Environmental Microbiology, Vol. 74, No. 12, 2008, pp. 3908-3911. [10] Q. Wu, D. L. Bedard and J. Wiegel, “Effect of Incubation Temperature on the Route of Microbial Reductive Dech- lorination of 2,3,4,6-Tetrachlorobiphenyl in Polychlori- nated Biphenyl (PCB)-Contaminated and PCB-Free Fre- shwater Sediments,” Applied and Environmental Micro- biology, Vol. 63, No. 7, 1997, pp. 2836-2843. [11] E. R. Master and W. W. Mohn, “Psychrotolerant Bacteria Isolated from Arctic Soil that Degrade Polychlorinated Biphenyls at Low Temperatures,” Applied and Environ- mental Microbiology, Vol. 64, No. 12, 1998, pp. 4823- -4829. |

