 Food and Nutrition Sciences, 2013, 4, 727-734 http://dx.doi.org/10.4236/fns.2013.47093 Published Online July 2013 (http://www.scirp.org/journal/fns) Purification and Partial Characterization of Polyphenol Oxidase from Sapodilla Plum (Achras sapota) Jorge Tamayo Cortez1, Carlos Hernán Herrera Méndez2*, Enrique Sauri Duch1, María de Lourdes Vargas y Vargas1, Sara Solís Pereira1 1Research and Postgraduate Division, Instituto Tecnológico de Mérida, Merida, Mexico; 2Agroindustrial Engineering Department, Campus Celaya-Salvatierra, Universidad de Guanajuato, Salvatierra, Mexico. Email: jtamayin@hotmail.com, *caherhe_23@hotmail.com, esauri@itmerida.mx, acras_99@yahoo.com, ssolis@itmerida.mx Received April 29th, 2013; revised May 29th, 2013; accepted June 6th, 2013 Copyright © 2013 Jorge Tamayo Cortez et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The browning of fruits can be considered as an enzymatic oxidation which is believed to be one of the main causes of quality loss during post-harvest handling. The enzymes responsible for this are the oxidoreductases; the polyphenol oxi- dase (PPO) (monophenol, o-diphenol, oxygen oxidoreductase; EC 1.14.18.1) belongs to this group. This enzyme, which is found in the sapodilla plum (Achras sapota), was purified using a phenylsepharose and a SephacrylS-200 columns. The molecular weight of the purified enzyme was estimated to be about 66 kDa by gel filtration and 29 kDa by SDS-PAGE. A single protein band was found using the latter system (SDS-PAGE), which shows that the PPO of the pulp of the sapodilla plum may be composed of two protein subunits with similar molecular weight. The optimum pH was 7.0 and the optimum temperature 60˚C. The most effective inhibitors tested were ascorbic acid, sodium metabisul- fite and acetic acid. Keywords: Enzymatic System; Oxidation; Polyphenol Oxidase; Browning Process; Nutritional Value 1. Introduction For nearly a century, fruits and vegetables have been recognized as a good source of vitamins, minerals and fiber. They are considered so important for our nutrition that five daily rations are suggested. Compared with peo- ple who consume a diet with only small amounts of fruits and vegetables, those who eat generous amounts of them as part of a healthful diet are likely to have reduced risk of chronic diseases, including stroke and perhaps other cardiovascular diseases, as well as certain cancers. The sapodilla plum (Achras sapota) is a tree from the tropical regions of the American continent, mainly from the South of Mexico and Central America. The fruit is climacteric, reaches commercial maturity around 6 - 8 days after harvest and enters senescence two days later; it is, therefore, a perishable fruit. The browning process is one of the main causes of the loss of quality and nutritional value in fruit and vegetable preservation [1]. This phenomenon can be considered as an enzymatic oxidation. Polyphenol oxidases (PPO) are responsible for the browning of fruits and vegetables by catalyzing the oxidation of phenolic compounds; these enzymes can be inactivated by heat or by the elimination of oxygen [2,3]. The sapodilla plum is a perishable fruit. Enzymatic browning, which is mainly caused by polyphenol oxidase (monophenol, o-diphenol, oxygen oxidoreductase; EC 1.14.18.1), makes its conservation and commercialization a difficult thing [4]. This enzyme is widely distributed in microorganisms, animals, and plants, being responsible not only for browning in plants but also for melanisation in animals. Previous studies have reported the purifica- tion and characterization of PPO from different sources: the enzyme from cabbage showed a molecular weight of 40 kDa, an optimal pH of 7.6 and a residual enzymatic activity of 25% after the protein was subjected to a 10 min thermal treatment at 100˚C [5]; the polyphenol oxi- dase from avocado was isolated and partially purified in the presence of TX-114, showing monophenolase/di- phenolase activity with an optimum pH of 7 for both [6]; the purification and characterization of polyphenol oxi- dase from banana pulp [7], purification and characteriza- tion of the polyphenol oxidase in beets [8]. The purifica- tion of PPO from sapodilla plum has not been reported *Corresponding author. Copyright © 2013 SciRes. FNS  Purification and Partial Characterization of Polyphenol Oxidase from Sapodilla Plum (Achras sapota) 728 yet. The commercial potential of the Sapodilla plummight be expanded if the enzymatic browning process is con- trolled. Therefore, our objective was to purify and par- tially characterize the polyphenol oxidase from sapodilla plum. This study could help understand the role of poly- phenol oxidase on the quality deterioration of the sapo- dilla plum, which could be useful in the search of effect- tive methods for inhibiting browning during storage. 2.Materials and Methods 2.1. Materials Sapodilla plum (Achras sapota), harvested at commercial maturity in the February-May season from an orchard in the Cansahcab town of the state of Yucatan, Mexico, stored at 4˚C. 2.2. Extraction Procedure Unless otherwise stated, all extraction procedures were carried out at 4˚C in order to reduce enzyme activity. Ripe fresh fruit (50 g after peeling and coring) were blended for 1 min with 100 ml of 0.1 M phosphate buffer, pH 7, and 0.2 g of ascorbic acid, different concentrations of polyvinylpolypyrrolidone (PVPP) and 0.1% of TX100 at 4˚C. The raw enzymatic extract (PPO) was obtained from the sapodilla plum using Triton TX100 detergent from Sigma; this detergent has been used for the extraction and solubilisation of polyphenol oxidase in several fruits [6-8]; ascorbic acid (Sigma) was used as reducer agent of endogenous phenolic compounds found in the sapodilla plum; finally, PVPP from Sigma was used to prevent quinone formation, as it reacts with the proteins that are present in the enzymatic extract. 2.3. Monitoring of Polyvinylpolypyrrolidone (PVPP) Activity in Collected Fractions, and Purification Procedure The presence of excessive quantities of impurities may interfere with bioassays. Phenolic compounds, which are widespread in plants and frequently occur in high con- centrations, seem to be a major source of impurities and to have possible inhibitory activity. Polyvinylpolypyr- rolidone (PVPP) has been shown to be reasonably spe- cific in separating a phenolic fraction from plant tissue extracts by hydrogen bond formation [9]. In order to evaluate the effect of PVPP on the process of obtaining the enzymatic extract, several PVPP concen- trations were analysed: 0%, 1%, 2%, 3%, 4%, and 5% in fresh fruit weight. We used 50 g of ripe fresh fruit, which were suspended in 100 ml of 0.1 M phosphate buffer, pH 7, for each of the above-mentioned PVPP concentrations, 0.2 g of ascorbic acid and 0.1% of TX100 at 4˚C. This mixture was homogenised for 1 min and filtered through 6 gauze-layers right after it was filtered through a N.1 filter-paper; it was then centrifuged at 10,000 g for 20 min at 4˚C. The sapodilla plums filtrate was treated with solid ammonium sulphate with a saturation of 20% - 80%. The precipitate was collected by centrifugation at 1200 × g for 40 min and redissolved in buffer (0.01 M phosphate buffer, pH 7.0). The fraction obtained through precipitate with ammonium sulphate (Sigma) with the highest activity of PPO was dialyzed overnight against 4 changes (4 × 1 L) of the same buffer, centrifuged and later applied to a phenylsepharose column. The (6 cm × 1 cm) column, with a 4 ml packed volume, was equili- brated with a 0.01 M phosphate buffer, pH 7, and eluted with a decreasing ammonium sulphate gradient (0.3% to 0%). The first eluted fraction that maintained the enzy- matic activity was introduced into another sephacryl S- 200 column, and the protein amount (280 nm) as well as the PPO enzymatic activity (420 nm) were determined. 2.4. Protein Determination Protein content was determined by the Bradford method [10], using immunoglobulin G (Sigma) as standard. Brad- ford reagent (1.5 ml) was added to 0.05 ml of sample. Absorbance at 595 nm was determined [11]. The en- richment process of the PPO fraction was determined according to the protein concentration method [12]. 2.5. Molecular Mass Determination by Size-Exclusion Chromatography The molecular weight of the native enzyme was deter- mined by gel filtration with a Sephacryl S-200 (30 cm × 1 cm) column, which was equilibrated with a 0.01 M phosphate buffer, pH 7. The column was pre-calibrated using molecular weight markers: cythocrome c (12.5 kDa), carbonic anhydrase (29 kDa), bovine albumin (66 kDa), alcohol dehydrogenase (150 kDa), alpha-amylase (200 kDa), apoferritin (443 kDa), thyroglobuline (669 kDa) and blue dextran (2000 kDa). Filtration was carried out following Andrews’s method [13]. 2.6. SDS-PAGE Electrophoresis was carried out in accordance with the Laemmli method [14] with the following characteristics: a 10% acrylamide resolution gel and a 6 cm high and 1.5 mm thick buffer Tris, pH 8.8; for the sample we used the Laemmli buffer 2×, and the run or electrode buffer was Tris-glycine, pH 6.8, with sodium dodecyl sulphate (SDS) from Sigma. Equal amounts of protein samples (10 mg) were loaded onto each lane. Protein bands were visual- ized by Coomassie blue staining according to the manu- Copyright © 2013 SciRes. FNS  Purification and Partial Characterization of Polyphenol Oxidase from Sapodilla Plum (Achras sapota) 729 facturer’s protocol (Sigma). 2.7. Enzyme ActivityAssay Enzymatic activity was measured spectrophotometrically by the method described by Oktay [15] using pyrocate- chol (J.T. Baker) as substrate. One unit of enzyme active- ity was taken as the increase in absorbance at 420 nm at 30˚C. The standard reaction mixture consisted of 0.1 ml of enzyme solution and 2.5 ml of pyrocatechol (0.06 M) in 0.1 M phosphate buffer (pH 7). Activity measurements were carried out in triplicate. In order to determine en- zyme activity in the presence of inhibitors, the enzyme was preincubated for about 15 min with these com- pounds. The kinetic characterization of the sapodilla plum’s PPO was carried out using a 0.1 M phosphate buffer, pH 7, at an incubation temperature of 30˚C, a 200 rpm shak- ing speed, 20 min enzyme-substrate reaction time, and, as substrate, pyrocatechol at several concentration levels (0.02, 0.04, 0.06, 0.08, 0.1 and 0.12 M). 2.8. Optimum pH PPO activity as a function of pH was determined within a pH range of 4.0 - 6.0 in 0.1 M acetate buffer and of 6.0 - 8.0 in 0.1 M phosphate buffer adjusted with 0.1 MNaOH and HNO3 [16]. PPO activity was assayed using the standard reaction mixture at 30˚C, but changing the buffer. PPO activity was calculated as percent residual activity at the optimum pH. 2.9. Effect of Temperature In order to determine the optimum temperature values of the enzyme, PPO activity was measured at different temperatures in the range of 30˚C - 70˚C using pyro- catechol as substrate. The effect of temperature on the activity of PPO was tested by heating the standard reac- tion solutions (buffer and substrate) to the appropriate temperatures before introducing the enzyme. Once tem- perature equilibrium was reached, the enzyme was added and the reaction followed spectrophotometrically at a constant temperature, given time intervals and a pH of 7. The standard reaction mixture consisted of 0.1 ml of enzyme solution and 2.5 ml of pyrocatechol in 0.1 M phosphate buffer (pH 7) [17]. 2.10. Monitoring the PPO Inhibitory Activity The inhibitors examined were ascorbic acid (Sigma), so- dium metabisulfite (Merk), sodium azide (Merk), acetic acid (Fermont), citric acid (Sigma), tartaric acid (Baker), oxalic acid (Baker) and honey. These inhibitors are re- ducing agents that play a role in preventing enzymatic browning either by reducing o-quinones to colorless di- phenols or by reacting irreversibly with o-quinones to form stable colorless products [18]. The reaction mixture contained 2.8 ml of pyrocatechol at a final concentration of 0.06 M in 0.1 M phosphate buffer (pH 7.0) and 0.2 ml of the enzymatic solution, plus the inhibitors solution in several concentration levels. Percentage inhibition was calculated using the following equation: Inhibition (%) = [(A0 − Ai)/A0)]100, where A0 is the initial PPO activity (without inhibitor) and Ai is the PPO activity with in- hibitor [19]. 3. Results and Discussion 3.1. Extraction of the Sapodilla Plum’s PPO It has been reported that some plant PPOs are mem- brane-bound. Therefore, using detergents is required in order to solubilise the enzyme [20]. Phenolic compounds interfere with the purification of proteins from plants. They cross-link proteins by hydrogen bonds and covalent interactions. Furthermore, the homogenization of the plant tissues initiates enzymatic browning, which results in the formation of quinones. Quinones may also form covalent linkages that may be irreversible. Phenol- ab- sorbing polymers such as PVPP and reducing agents such as ascorbic acid are commonly used in order to overcome these problems [20]. During the extraction of the sapodilla plum’s PPO we noticed (Figure 1) that when the polyvinylpolypyrrolidone (PVPP) concentra- tion increased from 0% to 5%, PPO activity in the extract increased; this can be a result of the PVPP effect on the sapodilla plum’s endogenous polyphenols; this means a greater enzymatic activity per mg protein: between 0.097 and 0.091 mg/ml in the analyzed conditions. It was also discovered that a PVPP concentration of 3% allowed to preserve a maximum activity of PPO. Values near 2.5% Figure 1. PVPP, the effect on polyphenol oxidase activity. PVPP concentrations of 0%, 1%, 2%, 3%, 4% and 5% of fresh fruit weight were analyzed. The reaction medium at 30˚C contained 0.1 M phosphate buffer, pH 7 for each PVPP concentration, 0.2 g of ascorbic acid and 0.1% of TX100. (■) Enzymatic activity; (●) specific activity. Copyright © 2013 SciRes. FNS  Purification and Partial Characterization of Polyphenol Oxidase from Sapodilla Plum (Achras sapota) Copyright © 2013 SciRes. FNS 730 were found when PPO was extracted from apple tissue [21]. 3.2. Enrichment of the Sapodilla Plum’s PPO Enzymatic Extract One of the most commonly used methods for protein enrichment and concentration is fractional precipitation with ammonium sulphate [22]. This method was applied to the sapodilla plum extract. Table 1 presents a summary of the results of PPO ex- traction and purification from Sapodilla plums and shows that PPO activity increased in the precipitate with the highest saturation percentage of ammonium sulphate in the sapodilla plum extract. We can also observe that when there is an ammonium sulphate saturation concen- tration of 80% (Table 1), the sapodilla plum’s PPO pre- cipitation is almost total, due to the fact that under these conditions a purification factor of approximately 4.8 was obtained, and, therefore, a specific activity factor of 762.7 UE factor was found. On the other hand, a recov- ery factor of almost 3 was reported by Jharna R. [23] in the purification and characterization of the pineapple’s PPO, and a similar value of 4.5 was obtained in the par- tial purification of persimmon by Nuñez, Sojo, García and Sánchez [24]. 3.3. Purification of the PPO Enzyme with Phenylsepharose and Sephacryl S-200 Chromatography The extract precipitate at 80% was resuspended in 1 ml of buffer; dialysed and passed through a phenylsepharose column. The first eluted fraction that maintained the en- zymatic activity was introduced into another sephacryl S-200 column, and the protein amount as well as the PPO enzymatic activity was determined. Two major fractions were obtained from the column (16 and 17 fractions), and one peak with PPO activity was separated by sephacrylS-200 chromatography (Figure 2). The major peak corresponded to a 51-fold increase in specific activ- ity over the crude extract and a recovery of 6.8%. Hydrophobic interaction chromatography has been utilized in the purification of PPO from various fruits, including peaches [25], strawberries [26] and pineapples [27]. Purification factors, elution profiles, and the per- centage of enzyme recovery varied between studies. Re- ported purification factors were 120-fold for PPO from Delicious cortex [28] and 13.8-fold for PPO from apricot [29]. Enzyme recoveries of 80% for PPO from apricot [29] and 40% for PPO from apple peel [30] have been reported. SDS-PAGE was performed in order to check the ho- mogeneity of the purified PPO. These results, according to the molecular weight patterns and the number of frac- tions 16 and 17, suggest that the native molecular weight of the sapodilla plum’s PPO is approximately 66 kDa, reaching a purification factor of approximately 38 times for the phenylsepharose column, and 51 times for the sephacryl S-200 column. A molecular weigh of 57 kDa was found in the characterization and purification of the artichoke’s polyphenoloxidase (Cynarascolymus L.) [31]. 3.4. Electrophoresis of the Purified Fractions (Phenylsepharose and Sephacryl S-200) in Polyacrylamide gel (PAGE) A denaturing gel electrophoresis (Figure 3) was per- formedin order to examine the purification degree of the PPO protein in the phenylsepharose and sephacryl S-200 columns. Figure 3 shows a typical molecular weight profile of fractions obtained at each purification step. A single band with the same molecular weight was noticed for each type of chromatography, suggesting that the fractions with the most activity and the most protein cor- respond to a single protein molecule. This protein band migrated, similarly to a protein with a molecular weight of 29 kDa, to a carbonic anhydrase molecular weight pattern. This suggests that the denaturalized molecular weight of the sapodilla plum’s PPO is close to 29 kDa. The results found by Jharna R. [23] in the purification and characterization of the Indian pineapple’s PPO showed that the molecular weight was 105 kDa with a Sephadex G-150 column; however, the SDS-PAGE gel indicated a single 25 kDa polypeptide band, suggesting a tetramer of identical units. Applying this principle to the sapodilla plum’s PPO, we think that, in this case, the polypeptide is a dimmer of identical units. The molecular weights of PPOs have been reported before: cabbage (39 kDa) [5], banana (41 kDa) [7]. Our results are in agreement with those reports in terms of the molecular weight of PPO. This purification process was repeated 3 times. Table 1. Activity of the sapodilla plum’s (Achras sapota) PPO, in precipitated fractions with ammonium sulphate. Satura. (%) Volume (ml) Total Activity (units) Total Prot. (mg/ml) Specific activity (units/mg protein) Purification factor (fold) Recovery (%) Crude extract 16 45.5 0.286 159.25 1 100 80% (NH2SO4) 13 104 0.137 762.7 4.8 4.8 Sephacryl S-200 2.5 159 0.0196 8112.24 51 6.8 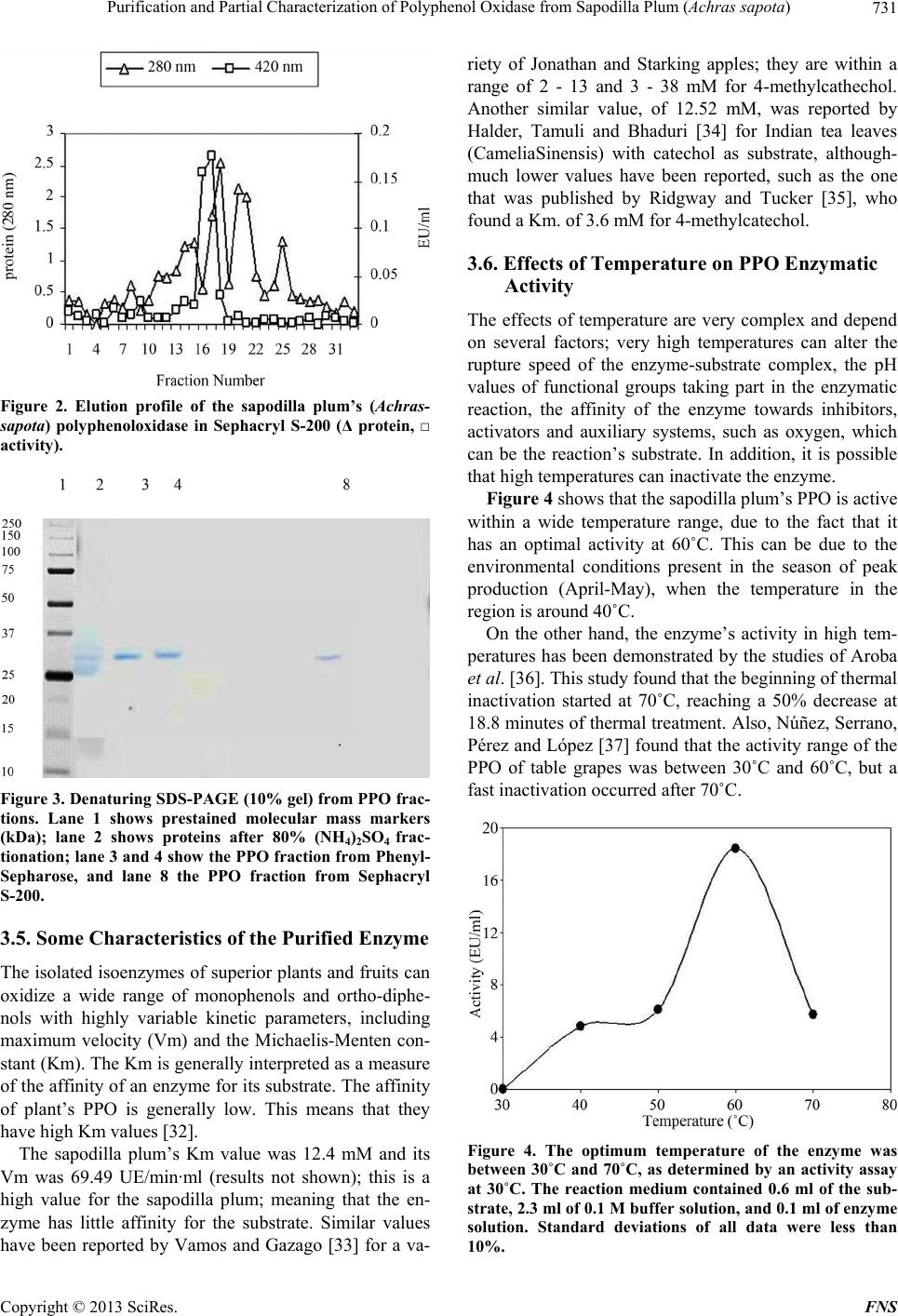 Purification and Partial Characterization of Polyphenol Oxidase from Sapodilla Plum (Achras sapota) 731 Figure 2. Elution profile of the sapodilla plum’s (Achras- sapota) polyphenoloxidase in Sephacryl S-200 (Δ protein, □ activity). Figure 3. Denaturing SDS-PAGE (10% gel) from PPO frac- tions. Lane 1 shows prestained molecular mass markers (kDa); lane 2 shows proteins after 80% (NH4)2SO4 frac- tionation; lane 3 and 4 show the PPO fraction from Phenyl- Sepharose, and lane 8 the PPO fraction from Sephacryl S-200. 3.5. Some Characteristics of the Purified Enzyme The isolated isoenzymes of superior plants and fruits can oxidize a wide range of monophenols and ortho-diphe- nols with highly variable kinetic parameters, including maximum velocity (Vm) and the Michaelis-Menten con- stant (Km). The Km is generally interpreted as a measure of the affinity of an enzyme for its substrate. The affinity of plant’s PPO is generally low. This means that they have high Km values [32]. The sapodilla plum’s Km value was 12.4 mM and its Vm was 69.49 UE/min·ml (results not shown); this is a high value for the sapodilla plum; meaning that the en- zyme has little affinity for the substrate. Similar values have been reported by Vamos and Gazago [33] for a va- riety of Jonathan and Starking apples; they are within a range of 2 - 13 and 3 - 38 mM for 4-methylcathechol. Another similar value, of 12.52 mM, was reported by Halder, Tamuli and Bhaduri [34] for Indian tea leaves (CameliaSinensis) with catechol as substrate, although- much lower values have been reported, such as the one that was published by Ridgway and Tucker [35], who found a Km. of 3.6 mM for 4-methylcatechol. 3.6. Effects of Temperature on PPO Enzymatic Activity The effects of temperature are very complex and depend on several factors; very high temperatures can alter the rupture speed of the enzyme-substrate complex, the pH values of functional groups taking part in the enzymatic reaction, the affinity of the enzyme towards inhibitors, activators and auxiliary systems, such as oxygen, which can be the reaction’s substrate. In addition, it is possible that high temperatures can inactivate the enzyme. Figure 4 shows that the sapodilla plum’s PPO is active within a wide temperature range, due to the fact that it has an optimal activity at 60˚C. This can be due to the environmental conditions present in the season of peak production (April-May), when the temperature in the region is around 40˚C. On the other hand, the enzyme’s activity in high tem- peratures has been demonstrated by the studies of Aroba et al. [36]. This study found that the beginning of thermal inactivation started at 70˚C, reaching a 50% decrease at 18.8 minutes of thermal treatment. Also, Núñez, Serrano, Pérez and López [37] found that the activity range of the PPO of table grapes was between 30˚C and 60˚C, but a fast inactivation occurred after 70˚C. Figure 4. The optimum temperature of the enzyme was between 30˚C and 70˚C, as determined by an activity assay at 30˚C. The reaction medium contained 0.6 ml of the sub- strate, 2.3 ml of 0.1 M buffer solution, and 0.1 ml of enzyme solution. Standard deviations of all data were less than 10%. Copyright © 2013 SciRes. FNS  Purification and Partial Characterization of Polyphenol Oxidase from Sapodilla Plum (Achras sapota) 732 3.7. Optimal pH of the Sapodilla Plum’s Polyphenol Oxidase Enzyme There is an optimal pH at which enzymes are most active. The optimum pH for the activity of the sapodilla plum’s PPO enzyme was determined using pyrocatechol as sub- strate; we can also see that there is a wide activity range at different pH values from 5 to 8. The optimum pH for the activity of PPO from sapodilla plum was found to be 7 (Figure 5). Optimal values of pH 6 - 7, with catechol as substrate, have also been reported for various fruits, including apple [10], pineapple [27], avocado, grape and pear [38]. However, at a pH below 4 the enzyme activity is low, suggesting that the enzyme can be controlled through the pH; similar values were found by Kavrayan et al. [39] in the purification and partial characterization of mint’s (Menthapiperita) polyphenoloxidase, with an optimal value of pH 7, using cathechol as substrate; low activity was found at pH values below 5. 3.8. Inhibition The browning process of fruits and vegetables can be prevented by removing the reactants, such as oxygen and the internal phenolic compounds, or by using PPO in- hibitors. The complete elimination of oxygen during fruit processing is very difficult, due to the fact that it is found in the environment. Several compounds were studied in order to detect the inhibitive action against the sapodilla plum’s PPO; Table 2 shows the values that were obtained with several com- pounds used as inhibitors and pyrocathechol as substrate. Of the inhibitors used, ascorbic acid was found to be the most effective, followed by sodium metabisulfite, so- dium azide, acetic acid and honey (Table 2). Honey was Figure 5. The optimum pH for polyphenoloxidase activity in the sapodilla plum. The buffers used were 0.1 M acetate (pH 4.0 - 6.0) and 0.1 M phosphate (pH 6.0 - 8.0) adjusted with 0.1 MNaOH and HNO3. Standard deviations of all data were less than 10%. Table 2. Effect of some inhibitors on the PPO activity from sapodilla plum (Achras sapota). Compound Concentration (%) Inhibition (%) Ascorbic acid 0.02 98 Sodium metabisulfite 0.050 97 Sodium azide 0.002 95 Acetic acid 0.2 93 Honey 0.2 56 the weakest inhibitor. Ascorbic acid and sodium metabi- sulfite have been shown to be strong inhibitors of PPO for Monroe apple peel and Stanley plum [30,40]. The citric, tartaric and oxalic acids did not show any inhibi- tive action against PPO in the sapodilla plum; however, ascorbic acid can be satisfactorily used to control the darkening of this fruit during processing as long as it is in its reduced form. 4. Conclusions This work describes a method for the purification of PPO from sapodilla plum. Polyphenol Oxidase (PPO) was extracted from commercially ripe sapodilla plums that were gathered during the February-May season from an orchard in the Cansahcab town in the state of Yucatan, Mexico. This was the first report on the purification of PPO from sapodilla plum. It can be concluded that the apparent molecular weight of the polyphenoloxidase from sapodilla plum pulp is approximately 60 kD, with a molecular weight (PAGE) of 29 kD; because of we can say that the polypeptide of the sapodilla plum’s PPO is a dimer of identical units. In this study, we achieved a purification factor of ap- proximately 38 times with the Phenylsepharose column, and of 52 with the Sephacryl S-200 column; an optimal reaction pH of 7 and an optimal temperature of approxi- mately 60˚C using pyrocatechol as substrate; the Km value for the sapodilla plum was 12.4 mM, and the H.S. was 69.49 U/min·ml. The PPO inhibitors, ascorbic acid, sodium metabisulfite, sodium azide, acetic acid and honey were particularly effective. Therefore, this study provides useful information about the sapodilla plum. REFERENCES [1] T. Nagai and N. Suzuki, “Polyphenol Oxidase from Bean Sprouts (Glycine max L.),” Journal of Food Science, Vol. 68, No. 1, 2003, pp. 16-20. doi:10.1111/j.1365-2621.2003.tb14107.x [2] M. Rouet, J. Philippon and J. Nicolas, “Enzymatic Brown- ing,” In: Encyclopedia of Food Science. Food Technology and Nutrition, Academic Press, London, 2003, pp. 686- 692. [3] L. Vamos, “Polyphenol Oxidase and Peroxidase in Fruits Copyright © 2013 SciRes. FNS  Purification and Partial Characterization of Polyphenol Oxidase from Sapodilla Plum (Achras sapota) 733 and Vegetables,” Critical Reviews in Food Science and Nutrition, Vol. 15, No. 1, 1981, pp. 49-127. doi:10.1080/10408398109527312 [4] X. Zhang and W. Flurkey, “Phenoloxidases in Portabella Mushrooms,” Journal of Food Science, Vol. 62, No. 1, 1997, pp. 97-100. doi:10.1111/j.1365-2621.1997.tb04376.x [5] S. Fujita, N. B. Saari, M. Maegawa, T. Tetsuka, N. Ha- yashi and T. Tono, “Purification and Properties of Poly- phenol Oxidase from Cabbage (Brassica oleracea L.),” Journal of Agricultural and Food Chemistry, Vol. 43, No. 5, 1995, pp. 1138-1142. doi:10.1021/jf00053a005 [6] J. C. Espín, M. F. Trujano, J. Tudela and F. García, “Monophenolase Activity of Polyphenol Oxidase from Haas Avocado,” Journal of Agricultural and Food Che- mistry, Vol. 45, No. 4, 1997, pp. 1091-1096. doi:10.1021/jf9605815 [7] C.Chang, S. Fujita, M. Asrafuzzaman, N. Nakamura and N. Hayashi, “Purification and Characterization of Poly- phenol Oxidase from Banana (Musa sapiens L.) Pulp,” Journal of Agricultural and Food Chemistry, Vol. 48, No. 7, 2000, pp. 2732-2735. doi:10.1021/jf991037+ [8] F. Gandía, C. García and J. Escribano, “Purification and Characterization of a Latent Polyphenoloxidase from Beet Root (Beta vulgaris L.),” Journal of Agricultural and Food Chemistry, Vol. 52, No. 3, 2004, pp. 609-615. doi:10.1021/jf034381m [9] J. Glenn, C. Kuo, C. Durley and R. Pharis, “Use of In- soluble Polyvinylpyrrolidone for Purification of Plant Ex- tracts and Chromatography of Plant Hormones,” Phyto- chemistry, Vol. 11, No. 1, 1972, pp. 345-351. doi:10.1016/S0031-9422(00)90013-X [10] M. M. Bradford. “A Rapid and Sensitive Method for the Quantification of Gram Quantities of Protein Utilizing the Principle of Protein-Dye Binding,” Analytic Biochemistry, Vol. 72, No. 1-2, 1976, pp. 248-254. doi:10.1016/0003-2697(76)90527-3 [11] D. B. Wetlaufer, “Ultraviolet Spectra of Proteins and Amino Acids,” Advances in Protein Chemistry, Vol. 17, 1963, pp. 303-390. doi:10.1016/S0065-3233(08)60056-X [12] R. K. Scopes, “The Raw Material,” In: Protein Purifica- tion, Principles and Practice, Springer-Verlag, New York, 1994, pp. 85-92. [13] P. Andrews, “The Gel-Filtration Behavior of Proteins Related to Their Molecular Weight over Wide Range,” Biochemical Journal, Vol. 96, No. 3, 1965, pp. 595-605. [14] U. Laemmli, “Cleavage of Structural Proteins during the Assembly of the Head Bacteriophage T4,” Nature, Vol. 227, No. 5244, 1970, pp. 404-427. doi:10.1038/227680a0 [15] I. Oktay, K. Kufrevioglu and H. Sakiroglu, “Polyphen- oloxidase from Amasya Apple,” Journal of Food Science, Vol. 60, No. 3, 1995, pp. 494-496. doi:10.1111/j.1365-2621.1995.tb09810.x [16] O. Arslan, A. Temor and I. Tozlu, “Polyohenol Oxidase from Allium sp.,” Journal of Agricultural and Food Che- mistry, Vol. 45, No. 8, 1997, pp. 2861-2863. doi:10.1021/jf960929w [17] M. Y. Coseteng and C. Y. Lee, “Changes in Apple Poly- phenol Oxidase and Polyphenol Concentrations in Rela- tion to Degree of Browning,” Journal of Food Science, Vol. 52, No. 4, 1987, pp. 985-989. doi:10.1111/j.1365-2621.1987.tb14257.x [18] H. Li, A. Q. Guo and H. Wang, “Mechanisms of Oxida- tive Browning of Wine,” Food Chemistry, Vol. 108, No. 1, 2008, pp. 1-13. doi:10.1016/j.foodchem.2007.10.065 [19] A. Sener and M. Ü. Ünal, “Purification of Polyphenol Oxidase from Akko XIII Loquat (Eriobotrya japonica cv Akko XIII),” Food Biotechnology, Vol. 25, No. 1, 2011, pp. 30-42. doi:10.1080/08905436.2011.547115 [20] J. Zawistowski, C. G. Biliaderis and N. A. M. Eskin, “Polyphenol Oxidase,” In: D. S. Robinson and N. A. M. Eskin, Eds., Oxidative Enzymes in Foods, Elsevier, New York, 1991, pp. 217-273. [21] A. Rocha, M. Cano, M. Galeazzi and A. Morais, “Char- acterization of ‘Starking’ Apple Polyphenoloxidase,” Journal of the Science of Food and Agriculture, Vol. 77, No. 4, 1998, pp. 527-534. doi:10.1002/(SICI)1097-0010(199808)77:4<527::AID-JS FA76>3.0.CO;2-E [22] M. Daniel and J. Stuart, “Protein Methods,” 2nd Edition, Wiley-Liss, Geneva, 1991. [23] R. Jharna, “Purification and Characterization of a Poly- phenol Oxidase from the Kew Cultivar of Indian Pineap- ple Fruit,” Journal of Agricultural and Food Chemistry, Vol. 45, No. 6, 1997, pp. 2031-2035. doi:10.1021/jf9607674 [24] E. Núñez, M. Sojo, F. García and A. Sánchez, “Partial purification of Latent Persimmon Fruit Polyphenol Oxy- dase,” Journal of Agricultural and Food Chemistry, Vol. 51, No. 7, 2003, pp. 2058-2063. doi:10.1021/jf0208583 [25] W. H. Flurkey and J. J. Jen, “Purification of Peach Poly- phenol Oxidase in the Presence of Added Protease In- hibitors,” Journal of Food Biochemistry, Vol. 4, No. 1, 1980, pp. 29-41. doi:10.1111/j.1745-4514.1980.tb00875.x [26] E. P. Wesche and M. W. Montgomery, “Strawberry Poly- phenol Oxidase: Extraction and Partial Characterization,” Journal of Food Science, Vol. 55, No. 5, 1990, pp. 1320- 1325. doi:10.1111/j.1365-2621.1990.tb03925.x [27] J. R. Das, S. G. Bhat andL. R. Gowda, “Purification and Characterization of a Poplyphenol Oxidase from Kew Cultivar of Indian Pineapple Fruit,” Journal of Agricul- tural and Food Chemistry, Vol. 45, No. 6, 1997, pp. 2031-2035. doi:10.1021/jf9607674 [28] A. H. Janovitz-Klapp, F. C. Richard-Forget and J. J. Nicolas, “Polyphenoloxidase from Apple, Partial Purifi- cation and Some Properties,” Phytochemistry, Vol. 28, No 11, 1989, pp. 2903-2907. doi:10.1016/0031-9422(89)80250-X [29] T. Chevalier, D. de Rigal, A. Mbéguié, D. Mbéguié, E. Gaulliard, E. Richard-Forget and B. R. Fils-Lycaon, “Mo- lecular Cloning and Characterization of Apricot Fruit Po- lyphenol Oxidase,” Plant Physiology, Vol. 119, No. 4, 1999, pp. 1261-1269. doi:10.1104/pp.119.4.1261 [30] P. Zhou, N. I. Smith and C. Y. Lee, “Potential Purifica- tion and Some Properties of Monroe Apple Peel Polyphe- nol Oxidase,” Journal of Agricultural and Food Chemis- Copyright © 2013 SciRes. FNS  Purification and Partial Characterization of Polyphenol Oxidase from Sapodilla Plum (Achras sapota) Copyright © 2013 SciRes. FNS 734 try, Vol. 41, No. 4, 1993, pp. 532-536. [31] S. Dogan, Y. Turan, H. Erturk and O. Arslan, “Charac- terization and Purification of Polyphenol Oxidase from Artichoke (Cynarascolymus L.),” Journal of Agricultural and Food Chemistry, Vol. 53, No. 3, 2005, pp. 776-785. doi:10.1021/jf049053g [32] J. J. Nicolas, F. Richard-Forget, P. Goupy, M.-J. Amiot and S. Y. Aubert, “Enzymatic Browning Reactions in Apple and Products,” Critical Reviews in Food Science and Nutrition, Vol. 34, No. 2, 1994, pp. 109-157. doi:10.1080/10408399409527653 [33] C. Queiroz, A. Ribeiro da Silva, M. L. Mendes, E. Fialho, V. Lúcia and V. Mesquita, “Polyphenol Oxidase Activity, Phenolic Acid Composition and Browning in Cashew Apple (Anacardium occidentale, L.) after Processing,” Food Chemistry, Vol. 125, No. 1, 2011, pp. 128-132. doi:10.1016/j.foodchem.2010.08.048 [34] J. Halder, P. Tamuli and A. Bhaduri, “Isolation and Char- acterization of Polyphenol Oxydase from Indian Tea Leaf (Camellia sinesis),” The Journal of Nutritional Biochem- istry, Vol. 9, No. 2, 1998, pp. 75-80. doi:10.1016/S0955-2863(97)00170-8 [35] T. J. Ridgway and G. A. Tucker, “Procedure for the Partial Purification of Apple Leaf Polyphenol Oxydase Suitable for Commercial Application,” Enzyme and Mi- crobial Technology, Vol. 24, No. 3-4, 1999, pp. 225-231. doi:10.1016/S0141-0229(98)00109-4 [36] S. S. Arogba, O. L. Ajiboye, L. A. Ugboko, S. Essienette and P. O. Afolabi, “Properties of Polyphenol Oxydase in Mango (Mangifera inidca) Kernel,” Journal of the Sci- ence of Food and Agriculture, Vol. 77, No. 4, 1998, pp. 459-462. doi:10.1002/(SICI)1097-0010(199808)77:4<459::AID-JS FA61>3.0.CO;2-O [37] E. Núñez, M. Serrano, A. Pérez and J. López, “Polyphe- nol Oxidase from Dominga Table Grape,” Journal of Ag- ricultural and Food Chemistry, Vol. 53, No. 15, 2005, pp. 6087-6093. doi:10.1021/jf050346z [38] C. A.Weemaes, L. R. Ludikhuyze, I. Van den Broeck, M. E. Hendrickx and P. P. Tobback, “Activity, Electropho- retic Characteristics and Heat in Activation of Poly- phenoloxydases from Apples, Avocados, Grapes, Pears and Plums,” LWT—Food Science and Technology, Vol. 31, No. 1, 1998, pp. 44-49. doi:10.1006/fstl.1997.0302 [39] D. Kavrayan and T. Aydemir, “Purification and Charac- terization of Polyphenoloxydase from Peppermint (Men- tha piperita),” Food Chemistry, Vol. 74, No. 2, 2001, pp. 147-154. doi:10.1016/S0308-8146(01)00106-6 [40] M. Siddiq, N. K. Sinha and J. N. Cash, “Characterization of Polyphenoloxydase from Stanley Plums,” Journal of Food Science, Vol. 57, No. 5, 1992, pp. 1177-1179. doi:10.1111/j.1365-2621.1992.tb11292.x
|