Paper Menu >>
Journal Menu >>
 Journal of Environmental Protection, 2010, 1, 337-345 doi:10.4236/jep.2010.14040 Published Online December 2010 (http://www.SciRP.org/journal/jep) Copyright © 2010 SciRes. JEP 337 Biological Activity of Bacillus thuringiensis (Berliner) Strains on Larvae and Adults of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) Houda Aboussaid1,2, Loubna El-Aouame1,2, Said El-Messoussi2, Khalid Oufdou1 1Laboratory of Biology and Biotechnology of Microorganisms, Cadi Ayyad University, Marrakech, Morocco; 2Laboratory of Molecular Modeling and Ecophysiology, Faculty of Sciences-Semlalia, Cadi Ayyad University, Marrakech, Morocco. Email: oufdou@ucam.ac.ma Received August 9th, 2010; revised August 29th, 2010; accepted September 9th, 2010. ABSTRACT The objective of this study was to evaluate the efficiency of Moroccan Bt strains against neonate larvae, third instar larvae and emerged adults of Ceratitis capitata. This Mediterranean fruit fly causes serious damages to Argan forest and other agricultural plants. There is no successful control program of this pest fly in the endemic Argan forest in Morocco. A single-dose test was performed on neonate larvae (25 µL/g) and adult (333.33 µL/g), when three doses of Bt toxins (50 µL/g, 100 µL/g and 150 µL/g) were tested against third instar of C. capitata. Among the twenty-six Bt strains examined, local Bt 13.4 and Bt A7 strains showed highest toxicity levels against larvae and adults, when com- pared to the reference strain, Bacillus thuringiensis subsp. israelensis HD567 “code 4Q1”, and commercial product “Skeetal”. One hundred percent mortality was observed against neonate larvae after 7 days of application by Bt 13.4 toxin. Third instar larvae were very susceptible to Bt A7 and Bt M-Ag 21.6 strains with 68% mortality (Lethal Concen- tration: LC50 = 1.115) at a dose of 150 µL/g. The Bt A7 strain was also highly toxic to adults with 81.66% of mortality after 7 days of application. This study demonstrated that some of our collection Bt strains can contribute to integrated C. capitata management system with strong biological control components. Keywords: Argan Forest, Bacillus thuringiensis, Biological Control, Ceratitis capitata, Diptera 1. Introduction Control of the Mediterranean fruit fly or medfly Ceratitis capitata (Wiedemann) (Diptera: Tephritidae); one of the world’s most destructive insect, continues to rely on the use of broad-spectrum chemical insecticides in bait sprays [1]. While these chemical products are generally effective, they are controversial because of concerns for adverse effects on human health and non-target organ- isms, environmental pollution and development of insec- ticide resistance [2]. Therefore there is a need for alterna- tive strategies such as bacterial entomopathogenic toxins: Bacillus thuringiensis. Commercial preparations of Bt have been shown to be successful biological control products worldwide [3,4]. Bt insecticidal activity primarily lies in its parasporal crystals. They are predominantly composed of one or more types of proteins, known as δ-endotoxins: the Cry proteins and the Cyt (cytolitic) proteins [5]. Nevertheless, other factors may contribute to Bt toxicity, such as the spore itself and extracellular soluble factors like β-exo- toxins, VIP (vegetative insecticidal proteins) toxins, phospholipases, proteases and chitinases [6]. Cry toxins are highly specific to their target insects, are harmless to humans, vertebrates and plants, and are easily biodegradable [7]. To date, several invertebrates have been described to be susceptible to Bt strains, mostly insects from Lepidoptera, Diptera and Coleoptera, but also from other orders (Hymenoptera, Homoptera, Orthoptera and Mallophaga) and also species from nematodes, mites and protozoa. However, for many crystal-bearing Bt strains, no toxic activity has been de- tected yet [8]. Thus, in recent decades, there has been a great interest in the screening of Bt collections to find isolates useful for pest control [9-12]. Many dipteran species have been found to be susceptible to Bt; these  Biological activity of Bacillus thuringiensis (Berliner) strains on larvae and adults of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) Copyright © 2010 SciRes. JEP 338 include mosquitoes, blackflies, chironomids, tipulids, muscids, sciarids, drosophilids [13] and tephritidis; such as the olive fly Bactrocera oleae [14,15], the Mexican fruit fly Anastrepha ludens [16,17] and the Mediterra- nean fruit fly C. capitata [18-20]. According to our knowledge, there is no available data on the effect of Moroccan Bt strains isolated especially from the endemic Argan forest which is strongly infested by C. capitata. In the present study, we evaluate the biological ento- mopathogenic activity of Bt strains isolated from Marra- kech region and Argan region in Morocco. We test the insecticidal effects of Bt strains against neonate larvae, third instar larvae and emerged adults of C. capitata. 2. Materials and Methods 2.1. Insect Rearing The insects used in the bioassays came from the labora- tory colony of C. capitata, maintained at the Faculty of Sciences Semlalia, Cadi Ayyad University, Marrakech (Morocco). The laboratory conditions used for rearing and bioas- says were 25 ± 1℃, 60 ± 10% relative humidity (RH) and 12 h of light and 12 h of darkness. Eggs laid through a net placed on one side of an insect-rearing cage were collected in a plastic container that was filled with dis- tilled water. Larvae from 0.5 ml of eggs were raised on an artificial diet containing 250 g wheat bran, 70 g sucrose, 36.3 g brewer’s yeast, 2.8 g (methyl 4-hydroxybenzoate; Sigma), 2.8 g Nipasol (propyl 4-hydroxybenzoate; Sigma) nipagin, 2.5 g Benzoic acid and 600 mL distilled water. Third-instar larvae were transferred into a pupation chamber with sand and were maintained there for at least 1 week before they were collected using a sieve. Adult flies were fed an artificial diet comprised of 20% (wt/wt) yeast autolysate and 80% (wt/wt) sucrose. Water was provided to the flies via a damp yellow sponge. 2.2. B. thuringiensis Strains Samples were collected from different areas of Mar- rakesh region and endemic argan forests (southwest of Morocco) (Table 1). Sampling was performed from het- erogeneous sources: soils underground surface (to avoid spore inactivation caused by UV radiation), Citrus Phyll- plane, Farm animal excrement, wastewater and sludge of wastewater treatment system. Bt isolation was carried out as described previously by Bel et al. [21]. Colonies with characteristic Bt morphol- ogy and presence of parasporal inclusions in the sporan- gium were selected and plated again for single colony isolation. After a second microscopic observation to ver- Table 1. Local Bt strains isolated from different habitats in Morocco. Localisation Habitat No. of samples No. of Bt isolates Agadir Argan soils 8 13 Essaouira Argan soils 2 4 Olive-cultivated soil 1 3 Bean-cultivated soil 1 1 Farm animal excrement 1 1 Citrus phylloplane 1 1 Wastewater 1 1 Marrakech Sludge of wastewater treatment system 2 2 Total 17 26 ify presence of protein inclusions, strains were cultured in CCY (Casein Casein Yeast) liquid medium [22], for 2 days until sporulation. Vegetative cells were eliminated by heating the sample at 70℃ for 20 min. Aliquots were taken and stored in 25% glycerol at −20℃. The standard strains, other than those isolated in this study were Bacillus thuringiensis subsp. israelensis HD 567 (code 4Q1, Bacillus Genetic Stock Center, B.G.S.C., Ohio State University, Columbus, OH, U.S.A.) and Skeetal, a commercial product based on B. thuringiensis subsp. israelensis (Valent Biosciences Corporation, Lib- ertyville, U.S.A.). 2.3. Culture Conditions and Production of Spore – Crystal Biomass A loopful of cells from a single colony of each strain grown in CCY agar medium were inoculated in 4.5 ml of liquid CCY medium (pre-culture) and grown for 48 h at 28℃ and agitated at 200 rpm. An aliquot was taken to verify spore and crystal formation (over 90% sporulation is optimum), and the pre-culture was incubated for 20 min at 70℃ to eliminate vegetative cells (synchroniza- tion). The main culture (40 ml) was inoculated with 1/1,000 volumes of synchronized pre-culture and grown as men- tioned above. Optimal crystal formation was checked by phase-contrast microscopy (DM2500, Leica Microsys- tems, Germany). The total number of cells in a culture was determined by plating 10-fold serial dilutions on CCY plates. Then the whole culture was centrifuged at 9,000 ×g for 10 min. The pellet was washed once with ice-cold 1 mol/l NaCl, 10 mmol/l EDTA solutions. Finally, the pellet was suspended in 1 ml of 10 mmol/l KCl. Optical Density  Biological activity of Bacillus thuringiensis (Berliner) strains on larvae and adults of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) Copyright © 2010 SciRes. JEP 339 (OD) was measured at 600 nm and the suspensions were stored at −20℃ until bioassay. All steps after centrifuga- tion were done on ice to limit proteolysis. 2.4. Bioassays In order to determine and optimize the toxicity of 26 Bt strains, three kinds of bioassays were carried out: 2.4.1. Activity against First Instar Larvae A single-dose test was performed; about 50 µL of the culture of each strain corresponding to 1.9·108 UFC/mL added onto 2 g of the artificial diet surface contained in 24-well polystyrene plates. One-first instar larvae of C. capitata was added per well, and the mortality was re- corded after 7 days of incubation at 25℃ in dark condi- tions. Two replicates of 24 larvae were conducted for each Bt strain. B. thuringiensis subsp. israelensis HD567 strain (Bti) (B.G.S.C. code 4Q1) and Skeetal, a commercial product based on Bti and reported to be active against dipterans, were used as standard controls. Sterile distilled water was used as a negative control. 2.4.2. Activity against Third Instar Larvae Five third instar larvae were transferred to plastic recipi- ents (30 mm diam × 70 mm depth) containing 10 g of diet (see insect rearing) mixed with the toxin, except for the control group, which had just received water with the diet. Three doses of Bt spore-crystals (50 µL/g, 100 µL/g and 150 µL/g) were used in five repetitions for each strain to confirm the results. The recipients were covered with nylon mesh, and fixed around the edges with a rub- berband. After 48 h of exposure, the number of dead larvae was counted and the pupae were separated from diet and then transferred to Petri dish (9 cm), until adult emergence. Bioassay tests were carried out to determine LC50 values of the Bt strains against C. capitata larvae. 2.4.3. Activity against C. capitata Adults 20 adults newly emerged (1-2 days old) were kept in the cage (25 cm by 25 cm) containing artificial diet (see in- sect rearing) mixed with 333.33 µL/g of spores and crys- tals mixture. The sweet substances (hydrolyzed protein and sugar) was used in the bioassay to attract the adults recently emerged to feed Bt spore-crystal. Water was provided to the flies via a damp yellow sponge. For each toxin, a negative control was prepared with distilled wa- ter and fly feeding. Three replicates per assay were car- ried out. Fly mortality was recorded daily for 7 days. 2.5. Statistical Analysis The mortality assay was calculated according to the Ab- bot’s formula: Mortality corrected % = (1 − n in T after treatment/n in Co after treatment) * 100 Where: n = Insect population, T = treated, Co = con- trol. The LC50 regression equation and the 95% confidence limit were calculated by using Probit analysis. Percent- ages of mortality obtained from bioassay tests were ana- lyzed using one-way analysis of variance (ANOVA, p < 0.05). Tukey’s test (p < 0.05) was used to analyze f sig- nificant differences among the Bt strains tested against C. capitata. 3. Results 3.1. Activity against First Instar Larvae Results of the larvicidal effectiveness of 26 Bt strains are shown in Table 2. The spore-crystal mixture of selected strains showed significantly larvicidal activity (df: 27, F: 10.42, p < 0.005 at 95%). The mortality rate was ranging from 8.33 to 100%, obtained after 7 days of treatment with single dose 25 µL/g. The toxin produced by Bt 13.4 strain showed the highest effect, with 100% of corrected mor- tality. The effect of Bt 2.1 strain was the lowest, with 8.33% as percentage of larval mortality. The reference strain 4Q1 (45.83%) and Skeetal (60.83%) caused mor- tality to the same degree with some of our strains or low like Bt M-Ag 2.7 (47.92%). 3.2. Activity against Third Instar Larvae Screening for toxicity against C. capitata adults was performed using spore-crystal mixture. In Table 3, the results of bioassays on the biological activity of Bt strains tested against C. capitata third instar larvae are presented. The susceptibility of third instar of C. capitata, evalu- ated as percentage of larvae that did not survive to adulthood, varied significantly between 0-8%, 8-48% 20-68% at dosages of 50 µL/g, 100 µL/g and 150 µL/g respectively (Table 3). At 150 µL/g, Bt A7 and Bt M-Ag 21.6 strains proved most effective activity (68%) whereas Bt A10 strain showed the lowest corrected mor- tality (20%). The LC50s determined after 48 h of application were ranging between 1.115 µL/g for Bt A7 and 3.199 µL/g for Bt A14. Significant differences were noted with tested doses (df: 2, F: 49.97, p < 0.005 at 95%). 3.3. Activity against C. capitata Adults Table 4 shows biological activity of the spore-crystal mixture of Bt strains in newly emerged adults of C. 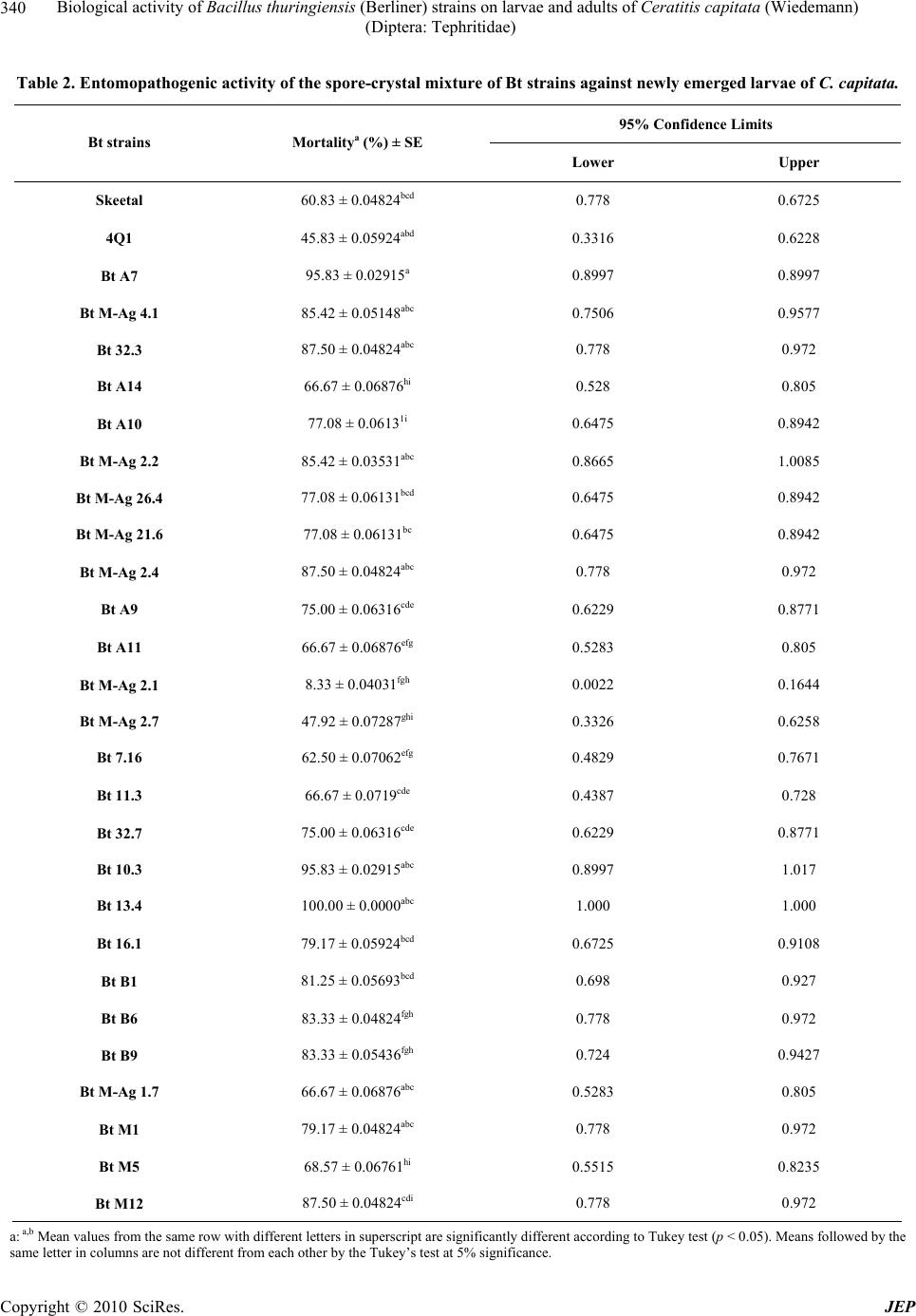 Biological activity of Bacillus thuringiensis (Berliner) strains on larvae and adults of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) Copyright © 2010 SciRes. JEP 340 Table 2. Entomopathogenic activity of the spore-crystal mixture of Bt strains against newly emerged larvae of C. capitata. 95% Confidence Limits Bt strains Mortalitya (%) ± SE Lower Upper Skeetal 60.83 ± 0.04824bcd 0.778 0.6725 4Q1 45.83 ± 0.05924abd 0.3316 0.6228 Bt A7 95.83 ± 0.02915a 0.8997 0.8997 Bt M-Ag 4.1 85.42 ± 0.05148abc 0.7506 0.9577 Bt 32.3 87.50 ± 0.04824abc 0.778 0.972 Bt A14 66.67 ± 0.06876hi 0.528 0.805 Bt A10 77.08 ± 0.06131i 0.6475 0.8942 Bt M-Ag 2.2 85.42 ± 0.03531abc 0.8665 1.0085 Bt M-Ag 26.4 77.08 ± 0.06131bcd 0.6475 0.8942 Bt M-Ag 21.6 77.08 ± 0.06131bc 0.6475 0.8942 Bt M-Ag 2.4 87.50 ± 0.04824abc 0.778 0.972 Bt A9 75.00 ± 0.06316cde 0.6229 0.8771 Bt A11 66.67 ± 0.06876efg 0.5283 0.805 Bt M-Ag 2.1 8.33 ± 0.04031fgh 0.0022 0.1644 Bt M-Ag 2.7 47.92 ± 0.07287ghi 0.3326 0.6258 Bt 7.16 62.50 ± 0.07062efg 0.4829 0.7671 Bt 11.3 66.67 ± 0.0719cde 0.4387 0.728 Bt 32.7 75.00 ± 0.06316cde 0.6229 0.8771 Bt 10.3 95.83 ± 0.02915abc 0.8997 1.017 Bt 13.4 100.00 ± 0.0000abc 1.000 1.000 Bt 16.1 79.17 ± 0.05924bcd 0.6725 0.9108 Bt B1 81.25 ± 0.05693bcd 0.698 0.927 Bt B6 83.33 ± 0.04824fgh 0.778 0.972 Bt B9 83.33 ± 0.05436fgh 0.724 0.9427 Bt M-Ag 1.7 66.67 ± 0.06876abc 0.5283 0.805 Bt M1 79.17 ± 0.04824abc 0.778 0.972 Bt M5 68.57 ± 0.06761hi 0.5515 0.8235 Bt M12 87.50 ± 0.04824cdi 0.778 0.972 a: a,b Mean values from the same row with different letters in superscript are significantly different according to Tukey test (p < 0.05). Means followed by the same letter in columns are not different from each other by the Tukey’s test at 5% significance.  Biological activity of Bacillus thuringiensis (Berliner) strains on larvae and adults of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) Copyright © 2010 SciRes. JEP 341 Table 3. Entomopathogenic activity of the spore-crystal mixture of Bt strains against last instar larvae of C. capitata. Mortality (%) ± SE Strains 50 µL/g 100 µL/g 150 µL/g LC50 95% Confidence Limits Skeetal 0 ± 0.0000 36 ± 1.30384 52 ± 1.14018 1.328 1.143 ± 1.650 4Q1 0 ± 0.0000 28 ± 0.89443 40 ± 0.0000 1.351 1.144 ± 1.833 Bt A7 8 ± 54772 48 ± 54772 68 ± 54772 1.115 0.938 ± 1.370 Bt M-Ag 4.1 1 ± 0.0000 40 ± 0.0000 60 ± 0.0000 1.250 1.067 ± 1.554 Bt 32.3 4 ± 0.44721 36 ± 0.44721 60 ± 0.0000 1.268 1.028 ± 1.833 Bt A14 4 ± 0.44721 12 ± 0.54772 24 ± 0.83666 3.199 1.738 ± % 100000002.000E + 12* Bt A10 0 ± 0.0000 8 ± 0.54772 20 ± 0.70711 2.394 1.656 ± 4815083.000 Bt M-Ag 2.2 4 ± 0.44721 44 ± 0.44721 60 ± 0.0000 1.212 1.007 ± 1.592 Bt M-Ag 26.4 8 ± 0.54772 28 ± 0.54772 40 ± 0.0000 1.852 1.298 ± 11.893 Bt M-Ag 21.6 0 ± 0.0000 44 ± 0.44721 68 ± 0.54772 1.164 1.001 ± 1.381 Bt M-Ag 2.4 8 ± 0.54772 36 ± 0.44721 60 ± 0.70711 1.268 1.028 ± 1.833 Bt A9 0 ± 0.0000 28 ± 0.54772 40 ± 0.70711 1.604 1.297 ± 2.886 Bt A11 0 ± 0.0000 24 ± 0.44721 32 ± 0.54772 1.847 1.413 ± 5.420 Bt M-Ag 2.1 0 ± 0.0000 28 ± 0.54772 32 ± 0.54772 1.164 1.001 ± 1.381 Bt M-Ag 2.7 0 ± 0.0000 16 ± 0.44721 32 ± 0.54772 1.872 1.458 ± 6.085 Bt 7.16 0 ± 0.0000 20 ± 0.70711 36 ± 0.44721 1.743 1.390 ± 3.959 Bt 11.3 0 ± 0.0000 16 ± 0.44721 52 ± 0.54772 1.464 1.273 ± 2.005 Bt 32.7 0 ± 0.0000 16 ± 0.44721 53 ± 0.54772 1.464 1.273 ± 2.005 Bt 10.3 4 ± 0.44721 24 ± 0.83666 60 ± 0.70711 1.360 1.137 ± 1.877 Bt 13.4 0 ± 0.0000 32 ± 0.54772 64 ± 0.44721 1.264 1.094 ± 1.532 Bt 16.1 4 ± 0.44721 16 ± 0.44721 52 ± 0.54772 1.548 1.260 ± 2.603 Bt B1 4 ± 0.44721 20 ± 0.70711 52 ± 0.54772 1.515 1.231 ± 2.490 Bt B6 0 ± 0.0000 8 ± 0.54772 44 ± 0.44721 1.574 1.376 ± 2.400 Bt B9 0 ± 0.0000 12 ± 0.54772 40 ± 0.70711 1.654 1.391 ± 3.203 Bt M-Ag 1.7 0 ± 0.0000 36 ± 0.83666 56 ± 0.44721 1.313 1.117 ± 1.695 Bt M1 0 ± 0.0000 28 ± 0.54772 56 ± 0.44721 1.362 1.169 ± 1.768 Bt M5 0 ± 0.0000 8 ± 0.54772 28 ± 0.54772 1.947 1.529 ± 18.077 Bt M12 4 ± 0.44721 12 ± 0.54772 52 ± 0.54772 1.580 1.288 ± 2.723 a: a,b Mean values from the same row with different letters in superscript are significantly different according to Tukey test (p < 0.05). Means followed by the same letter in columns are not different from each other by the Tukey’s test at 5% significance. *: Upper limits greater than or equal to 1.E20 are really infinite.  Biological activity of Bacillus thuringiensis (Berliner) strains on larvae and adults of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) Copyright © 2010 SciRes. JEP 342 Table 4. Entomopathogenic activity of the spore-crystal mixture of Bt strains against emerged adults of C. capitata. 95% Confidence Limits Strains Mortality (%) ± SE Lower Upper Skeetal 48.33 ± 0.2801bc 0.7967 1.9652 4Q1 33.33 ± 0.25332bc 0.424 1.4808 Bt A7 81.66 ± 0.52834a 1.4217 3.6259 Bt M-Ag 4.1 80 ± 0.43799a 1.2292 3.0565 Bt 32.3 70 ± 0.371c 1.3213 2.8691 Bt A14 68 ± 0.55838c 1.2162 3.5457 Bt A10 60 ± 0.347ca 1.1333 2.581 Bt M-Ag 2.2 80 ± 0.53282a 1.3647 3.5876 Bt M-Ag 26.4 70 ± 0.4555c 1.4784 3.3787 Bt M-Ag 21.6 80 ± 0.4555a 1.4784 3.3787 Bt M-Ag 2.4 80 ± 0.59074a 0.9106 3.3751 Bt A9 60 ± 0.4555ca 1.4784 3.3787 Bt A11 56.66 ± 0.347d 1.1333 2.581 Bt M-Ag 2.1 68.66 ± 0.32819ca 1.1249 2.4941 Bt M-Ag 2.7 78.66 ± 0.371a 1.3213 2.8691 Bt 7.16 75 ± 0.56625a 1.1522 3.5145 Bt 11.3 70 ± 0.45848c 1.3293 3.2421 Bt 32.7 75 ± 0.59074a 0.9106 3.3751 Bt 10.3 81.66 ± 0.30971a 1.6397 2.9318 Bt 13.4 80 ± 0.53282a 1.3647 3.5876 Bt 16.1 80 ± 0.4555a 1.4784 3.3787 Bt B1 80 ± 0.4555a 1.4784 3.3787 Bt B6 75 ± 0.40908a 1.4324 3.139 Bt B9 80 ± 0.26342a 1.8791 2.978 Bt M-Ag 1.7 81.66 ± 0.4555a 1.4784 3.3787 Bt M1 81.66 ± 0.53282a 1.3647 3.5876 Bt M5 60 ± 0.347ca 1.1333 2.581 Bt M12 80 ± 0.33503a 1.7297 3.1274 a: a,b Mean values from the same row with different letters in superscript are significantly different according to Tukey test (p < 0.05). Means followed by the same letter in columns are not different from each other by the Tukey’s test at 5% significance.  Biological activity of Bacillus thuringiensis (Berliner) strains on larvae and adults of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) Copyright © 2010 SciRes. JEP 343 capitata. The difference of fly’s mortality was significantly higher between those exposed to the spore-crystal mix- tures and the ones exposed to the water control. The majority of Bt strains killed more than 50% of C. capitata adults. The mortality values varied significantly between strains. They produced significant mortalities from 56.66 to 81.66% (df: 27, F: 0.68, p < 0.005 at 95%); when using both spore-crystal suspensions of Bt toxin after 7 days exposure time. The more toxic Bt strains caused adult mortality starting from the 2nd day or the 3rd day of ingestion. The commercial product based on Bti (Skeetal) and the reference Bti strain (code 4Q1) were also tested against C. capitata adults. They were in general less effi- cient, showing corrected mortality about 48.33% and 33.33% respectively (Table 4). 4. Discussion During sporulation, the Gram-positive bacterium, Bt forms crystalline protein inclusions, which possess insec- ticidal activity. These parasporal inclusions differentiate this species from other related species such as Bacillus cereus and Bacillus mycoides [23]. The crystals are gen- erally smaller than the spores and can represent ±30% of the dry weight of the cell [24]. A total of 26 Bt strains were tested on both larvae and adults; and it was found that 100% of strains were toxic against adults of C. capitata, 92% on neonate larvae on adults, and 60% on third instar larvae. Neonate larvae and adults were more susceptible than third instar larvae of C. capitata; in fact, several strains produced high mortality only after 3 days of exposure. The toxicity of spore-crystal mixtures against third larvae was seldom high but some strains yielded 68% mortality after 48 hours of application at the doses used in these experi- ments. The biological activity of Bt strains has been studied by many authors, who determined the insecticide poten- tial of obtained toxins using both spore and crystal. Our results are similar to that reported by Gingrich [18]. This author have tested 94 strains of Bt and found that 15 of them killed at least 80% of C. capitata adults that fed pellets for 9 days. Hassani and Gaouar Benyelles [19] have tested the ef- fect of the preparation of Bti on wild third instar larvae and adults of C. capitata isolated from Citrus fruit or- chards in Algeria, and observed toxicity in high doses (100 mg/g) with a reduction in average emergence (84.62%), concluding that the stage L3 and adults of the pest are very susceptible at this dose of Bti product. Molina et al. [25] have tested sporulated cultures of 115 bacterial strains of Bacillus pumilus (4.65 × 108 to 1.45 × 107 CFU/mL) against adults and neonate larvae of C. capitata. None of these strains were caused significant mortality of C. capitata adults compared with the nega- tive controls. The mortality rates with the 115 bacterial strains at the end of the experiment ranged from 0% to 40%, while the average mortality rates with the negative controls ranged from 5% to 30%. Similar results were obtained in bioassays with larvae, the maximal corrected mortality rates with the 115 bacterial isolates ranged from 0% to 36%, while the average mortality rates with the negative controls ranged from 1% to 12% after 15 days, at the end of the experiment. After this toxicity screening, they obtained a novel Bacillus pumilus strain, which is highly toxic to C. capitata larvae. The mortality rate for C. capitata larvae ranged from 68 to 94% de- pending on the conditions under which the culture was kept before the bioassay. Karamanlidou et al. [14] and Yamvrias and Anagnou [26] have reported a mortality > 80% when they used various Bt strains against old larvae of the dipteran olive fruit fly, B. oleae (Gmelin). Robacker et al. [16] evalu- ated the action of 55 Bt strains on larvae of Mexican fruit fly, Anastrepha ludens (Loew). Only 7 strains were found toxic on adults; the centrifuge pellets or precipi- tates of Bt strains have killed > 50% of larvae. The mor- tality rates varied between 4 to 62% after application of the pellets of these strains against adult flies. Only 5 of them killed 65-80% of adults in 10 days compared with 2.7% mortality in controls. The other killed 40% of adults in the same experiment. Alberola et al. [15] studied the Bt activity against sec- ond instar of B. oleae larvae and new emerged adults. These authors have reported that both spore-crystal mix- tures (109/mL) caused 70% mortality for larvae in 72 hours and 80% of mortality for adult flies in 6 to 10 days of application. The obtained results during our study showed that some Bt strains were most toxic against adults than on larvae and vice versa. For example, the insecticidal activ- ity of Bt M-Ag 2.1 against larvae of C. capitata is 8.33, whereas the activity of this strain towards C. capitata adults is 68.66 (Tables 2 and 4). The genes which coding for insecticidal activity against the two stages of the medfly, could be different [15,16]. Alberola et al. [15] have also reported that there are some different chemical conditions in the gut of the two stages of the insect that can control the activation or activity of the protoxin. On the other hand, some Bt strains expressed high ac- tivities against the both stages of C. capitata such as Bt  Biological activity of Bacillus thuringiensis (Berliner) strains on larvae and adults of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) Copyright © 2010 SciRes. JEP 344 A7, Bt 13.4, Bt M-Ag 4.1, Bt M-Ag 2.2, Bt M-Ag 21.6 (Tables 2 and 4). 5. Conclusions Sustainable agriculture will rely increasingly to biologi- cal control of pests such as C. capitata that is considered as a quarantine pest over the world and particularly in the Argan area. No control program has been undertaken until now against C. capitata in the Argan forest. The use of biopesticides is environmentally friendly and reduces the contact of human to chemical pesticides. The present study provides evidence for the insecticidal activity of Moroccan Bt strains against C. capitata. Some Bt strains showed a great activity against neonate larvae, third in- star larvae and especially towards adults of C. capitata. A number of our collection Bt strains showed high insec- ticidal activity against C. capitata in comparison to that noted for the Bti HD567 strain and the commercial product Skeetal previously used in the control of harmful fruit flies. Some of our Bt strains can be used in the bio- logical control system to fight against C. capitata and may contribute to reduce the use of chemical insecticides harmful to the consumers and the environment. REFERENCES [1] J. P. Ros, E. Wong, J. Olivero and E. Castillo, “Mejora de los Mosqueros, Atrayentes y Sistemas de Retención Contra la Mosca Mediterránea de la Fruta Ceratitis capitata Wied. Como Hacer de la Técnica del Trampeo Masivo una Buena Herramienta Para Controlar esta Plaga,” Boletín Sanidad Vegetal Plagas, Vol. 28, No. 4, 2002, pp. 591-597. [2] C. Magaña, P. Hernández-Crespo, A. Brun-Barale, F. Couso-Ferrer, J. M. Bride, P. Castañera, R. Feyereisen and F. Ortego, “Mechanisms of Resistance to Malathion in the Medfly Ceratitis capitata,” Insect Biochemisry and Molecular Biology, Vol. 38, No. 8, 2008, pp. 756-762. [3] V. Sanchis, and D. Bourguet, “Bacillus thuringiensis: Applications in Agriculture and Insect Resistance Mana- gement,” Agronomy for Sustainable Development, Vol. 28, No. 1, 2008, pp. 11-20. [4] K. van Frankenhuyzen, “Insecticidal Activity of Bacillus thuringiensis Crystal Proteins,” Journal of Invertebrate Pathology, Vol. 101, No. 1, 2009, pp. 1-16. [5] N. Crickmore, D. R. Zeigler, J. Feitelson and E. Schnepf, “Revision of the Nomenclature for the Bacillus thurin- giensis Pesticidal Crystal Proteins,” Microbiology and Molecular Biology Reviews, Vol. 62, No. 3, 1998, pp. 807-813. [6] M. Porcar and V. M. Juárez-Pérez, “PCR-Based Iden- tification of Bacillus thuringiensis Pesticidal Crystal Genes,” FEMS Microbiology Reviews, Vol. 26, No. 5, 2003, pp. 419-432. [7] IPSC-WHO, “Bacillus thuringiensis. Environmental Health Criteria of the International Program on Chemical Safety,” IPCS WHO International Program on Chemical Safety, No. 217. 1999. [8] E. Schnepf, N. Crickmore, J. Van Rie and D. Lereclus, “Bacillus thuringiensis and Its Pesticidal Crystal Pro- teins,” Microbiology and Molecular Biology Reviews, Vol. 62, No. 3, 1998, pp. 775-806. [9] K. F. Chak, D. C. Chao, M. Y. Tseng and S. S. Kao, “Determination and Distribution of Cry-Type Genes of Bacillus thuringiensis Isolates from Taiwan,” Applied and Environmental Microbiology, Vol. 60, No. 7, 1994, pp. 2415-2420. [10] A. Bravo, S. Sarabia, L. López and H. Ontiveros, “Char- acterization of Cry Genes in a Mexican Bacillus thur- ingiensis Strain Collection,” Applied and Environmental Microbiology, Vol. 64, No. 12, 1998, pp. 4965-4972. [11] J. E. Ibarra, M. C. Del Rincon, S. Orduz and D. Noriega, “Diversity of Bacillus thuringiensis Strains from Latin America with Insecticidal Activity against Different Mosquito Species,” Applied and Environmental Micro- biology, Vol. 69, No. 9, 2003, pp. 5269-5274. [12] E. Quesada-Moraga, E. García-Tovar, P. Valverde-García and C. Santiago-Álvarez, “Isolation, Geographical Diver- sity and Insecticidal Activity of Bacillus thuringiensis from Soils in Spain,” Microbiology Research, Vol. 159, No. 1, 2004, pp. 59-71. [13] C. Itoua-Apoyolo, L. Drif, J. M. Vassal, H. DeBarjac, J. P. Bossy, F. Leclant and R. Frutos, “Isolation of Multiple Subspecies of Bacillus thuringiensis from a Population of the European Sunflower Moth, Homoeosoma nebulella,” Applied and Environmental Microbiology, Vol. 61, No. 12, 1995, pp. 4343-4347. [14] G. Karamanlidou, A. F. Lambropoulos, S. I. Koliais, T. Manousis, D. Ellar and C. Kastritsis, “Toxicity of Bacil- lus thuringiensis to Laboratory Populations of the Olive Fruit Fly (Dacus oleae),” Applied and Environmental Microbiology, Vol. 57, No. 8, 1991, pp. 2277-2282. [15] T. M. Alberola, S. Aptosoglou, M. Arsenakis, Y. Bel, G. Delrio, D. J. Ellar, J. Ferre, S. P. Gash, F. Granero, S. Ko- liais, M. J. Martinez-Sebastian, R. Prota, S. Rubino, A. Satta, G. Scarpellini, A. Sivropoulou and E. Vasara, “In- secticidal Activity of Strains of Bacillus thuringiensis on Larvae and Adults of Bactrocera oleae Gmelin (Dipt. Tephritidae),” Journal of Invertebrate Pathology, Vol. 74, No. 2, 1999, pp. 127-136. [16] D. C. Robacker, A. J. Martínez, J. A. García, M. Díaz and C. Romero, “Toxicity of Bacillus thuringiensis to Mexi- can Fruit Fly (Diptera: Tephritidae),” Journal of Eco- nomic Entomology, Vol. 89, No. 1, 1996, pp. 104-110. [17] J. Toledo, P. Liedo, T. Williams and J. Ibarra, “Toxicity of Bacillus thuringiensis β-Exotoxin to Three Species of Fruit Flies (Diptera: Tephritidae)”, Journal of Economic Entomology, Vol. 92, No. 5, 1999, pp. 1052-1056. [18] R. E. Gingrich, “Demonstration of Bacillus thuringiensis as a Potential Control Agent for the Adult Mediterranean  Biological activity of Bacillus thuringiensis (Berliner) strains on larvae and adults of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) Copyright © 2010 SciRes. JEP 345 Fruit Fly, Ceratitis capitata (Wied.),” Journal of Applied Entomology, Vol. 104, No. 1-5, 1987, pp. 378-385. [19] F. Hassani and N. Gaouar Benyelles, “Application of Bacillus thuringiensis (Bti) Struggling Microbiological Control of the Fruit Fly Ceratitis capitata (wied) (Diptera: Tephritidae),” IBSCientific Journal of Science, Vol. 3, No. 1, 2008, pp. 10-13. [20] J. C. Vidal-Quist, P. Castañera and J. González-Cabrera, “Diversity of Bacillus thuringiensis Strains Isolated from Citrus Orchards in Spain and Evaluation of Their Insecti- cidal Activity against Ceratitis capitata,” Journal of Mi- crobiology and Biotechnology, Vol. 19, No. 8, 2009, pp. 749-759. [21] Y. Bel, F. Granero, T. M. Alberola, M. J. Martínez- Sebastian and J. Ferré, “Distribution, Frequency and Di- versity of Bacillus thuringiensis in Olive Tree Environ- ments in Spain,” Systematic and Applied Microbiology, Vol. 20, No. 4, 1997, pp. 652-658. [22] G. S. A. B. Stewart, K. Johnstone, E. Hagelberg and D. J. Ellar, “Commitment of Bacterial Spores to Germinate,” Biochemistry Journal, Vol. 198, No. 1, 1981, pp. 101-106. [23] E. Helgason, O. A. Okstad, D. A. Caugant, H. A. Johansen, A. Fouet, M. Mock, I. Hegna and A. B. Kolsto, “Bacillus Anthracis, Bacillus cereus, and Bacillus thur- ingiensis - One Species on the Basis of Genetic Evi- dence,” Applied and Environmental Microbiology, Vol. 66, No. 6, 2000, pp. 2627-2630. [24] J. A. Baum and T. Malvar, “Regulation of insecticidal crystal protein production in Bacillus thuringiensis,” Mo- lecular Microbiology, Vol. 18, No. 1, 1995, pp. 1-12. [25] C. A. Molina, J. F. Cana-Roca, A. Osuna and S. Vilchez, “Selection of a Bacillus pumilus Strain Highly Active against Ceratitis capitata (Wiedemann) Larvae,” Applied and Environmental Microbiology, Vol. 76, No. 5, 2010, pp. 1320-1327. [26] C. Yamvrias and M. Anagnou, “Preliminary Tests on the Sensitivity of the Larvae of Dacus oleae to Bacillus thur- ingiensis var. israelensis, in Fruit Flies of Economic Im- portance,” R. Cavalloro, Ed., Balkema, Rotterdam, Vol. 87, 1989, pp. 345-348. |

