Paper Menu >>
Journal Menu >>
 J. Mod. Phys., 2010, 1, 201-205 doi:10.4236/jmp.2010.14030 Published Online October 2010 (http://www.SciRP.org/journal/jmp) Copyright © 2010 SciRes. JMP Physical Properties of Lithium Containing Cadmium Phosphate Glasses Muhammad Altaf 1, M. Ashraf Chaudhry2 1Department of Physics, Govt. College of Sc ience, Multan, Pakistan 2Department of Physics, Bahauddin Zakariya University, Multan, Pakistan E-mail: altaf_sal@yahoo.com Received May 1, 2010; revised August 15, 2010; accepted May 14, 2010 Abstract Lithium cadmium phosphate glasses were prepared by melt quench technique. These glasses contain a mole % composition of x% Li2O-(50-x)% CdO-50%P2O5. The quantity x varies from 0-40 mole%. The physical properties reported in this paper are mass density ρ, modulus of rigidity η, coefficient of linear expansion α, transition temperature Tg, Softening temperature Ts, Oxygen packing density, Molar volume and lithium ion concentration. The mass density, oxygen packing density, modulus rigidity, transition temperature and sof- tening temperature show decreasing trend with increasing concentration of lithium ions in these glasses, where molar volume and coefficient of linear expansion increases with increasing concentration of Li2O. Keywords: Phosphate Glasse, Mass Density, Modulus of Rigidity, Coefficient of Linear Expansion, Transition Temperature, Molar Volume 1. Introduction The competition of speed, cost and reliability of elec- tronic devices in various applications has led to the re- search for new materials which can met the specific re- quirement. The phosphate glasses are among those mate- rials which have a number of technical and biological applications [1-3]. Phosphate glasses have a variety of technological application due to several unique proper- ties [4]. To investigate the nature of these glasses for their appropriate applications, study of optical, electrical and physical properties are necessary. Most of the re- searchers studied optical and electrical properties to characterize the phosphate glasses [5-8], whereas the knowledge of physical properties is equally very helpful to study the nature of materials. A general observation of the thermal expansion curve tells whether the materials under investigation are amor- phous are crystalline in nature. Experimental results on physical properties in glassy material have been reported in literature [9-11]. In the present work physical properties such as mass density, oxygen packing density, molar volume, modulus of rigidity, transition temperature, softening temperature and coefficient of linear expansion were studied to ex- amine the effect of alkali metal oxide i.e. Li2O on the cadmium phosphate glasses. According to our survey, no data has been reported on the physical properties of cad- mium phosphate glass system containing lithium oxide. 2. Experimental Chemicals Li2CO3, CdO and P2O5 of purity (99.99%) were used to prepare Lithium-cadmium-phosphate glasses. The glass samples were prepared by using 15 g ingredients mixture of composition x%Li2O-(50-x) %CdO-50%P2O5 in a platinum crucible. The details of preparation of sam- ples and method of density measurements and modulus of rigidity have already been described else where [12,13]. Oxygen packing density, molar volume and lithium ion concentration were estimated by using fol- lowing equations respectively, Oxygen packing density = {1000 × ρ× (O)}/M (1) Molar volume = M/ρ (2) Ion concentration = {ρ Navo P}/M (3) where ρ = mass density M = Molecular weight of glass composition O = number of oxygen atoms in the composition Navo = Avogadro number and P = nx where ‘x’ is the mole fraction in glass composition and  M. ALTAF ET AL. Copyright © 2010 SciRes. JMP 202 ‘n’ is the number of atoms of element ions in a given oxide, i.e. n = 1 for oxides like CdO, ZnO etc and n = 2 for oxides like Li2O, Na2O, etc. Thermal expansion and coefficient of linear expansion of fibers of lithium phosphate glasses have been studied up to 600˚C. These parameters were measured by using a horizontal tube heating system as shown in Figure 1. The heating system consists of two equal bore Pyrex tubes each of 10 cm length. The tubes are fitted together in a single heating unit so that both the tubes can have the same temperature. A Cromel-Alumel thermocouple connected to a Fenwal controller is fitted in one tube and the glass fiber ‘F’ of length Lo = 10.5 cm is placed in the other tube. The temperature of the system is controlled by a temperature controller while the heating rate is con- trolled through a voltage regulator. Each temperature was maintained for five minutes to achieve the equilib- rium. The increase in the length of the fiber for every 50˚C rise in temperature (with constant rate of heating) was measured with a traveling microscope whose least count is 10 μm. The linear expansion was plotted against tem- perature as shown in Figure 2. This curve was used to estimate transition temperature Tg and softening tem- perature Ts. The coefficient of linear expansion ‘α’ is then calculated using equation α= (L)/(Lo t) (˚C)-1 (4) 3. Results and Discussion The measured results and estimated values of mass den- sity are listed in Table 1. These results indicate a de- creasing trend in the density of. x% Li2O-(50-x)% CdO-50%P2O5 glass system with increasing concentra- tion of Li2O. Theoretical values of the density were es- timated by using the relation ρ = ∑ρi xi, where ρi and xi are the density and fraction of the free oxides respec- tively. The estimated and the measured values of density of these glasses are depicted in Figure 3 as a function of Li2O concentration. It can be seen that estimated values are higher than measured values. This difference in val- ues of density may be due to the variation in atomic ar- rangement between the structure of glass and molecules of the free oxides. The decrease in the measured density with the increasing concentration of Li2O agrees qualita- tively with that predicted by the composition relation, and it may be due to the replacement of high density CdO (8.15 g/cm3) as compared with Li2O (2.02 g/cm3). The estimated oxygen packing density and molar volume and lithium ion concentration are listed in Table 1 and are shown in Figures 4, 5, and 6 respectively. These re- sults show that oxygen packing density decreases where as molar volume and lithium ion concentration increase Figure 1. Tube furnace along with temperature controller for heating the fiber to measure its linear expansion with the variation temperature. Figure 2. A plot of linear expansion l versus temperature oC. Figure 3. Var iation of mass dens ity relative to lithium oxide concentration (mole%). 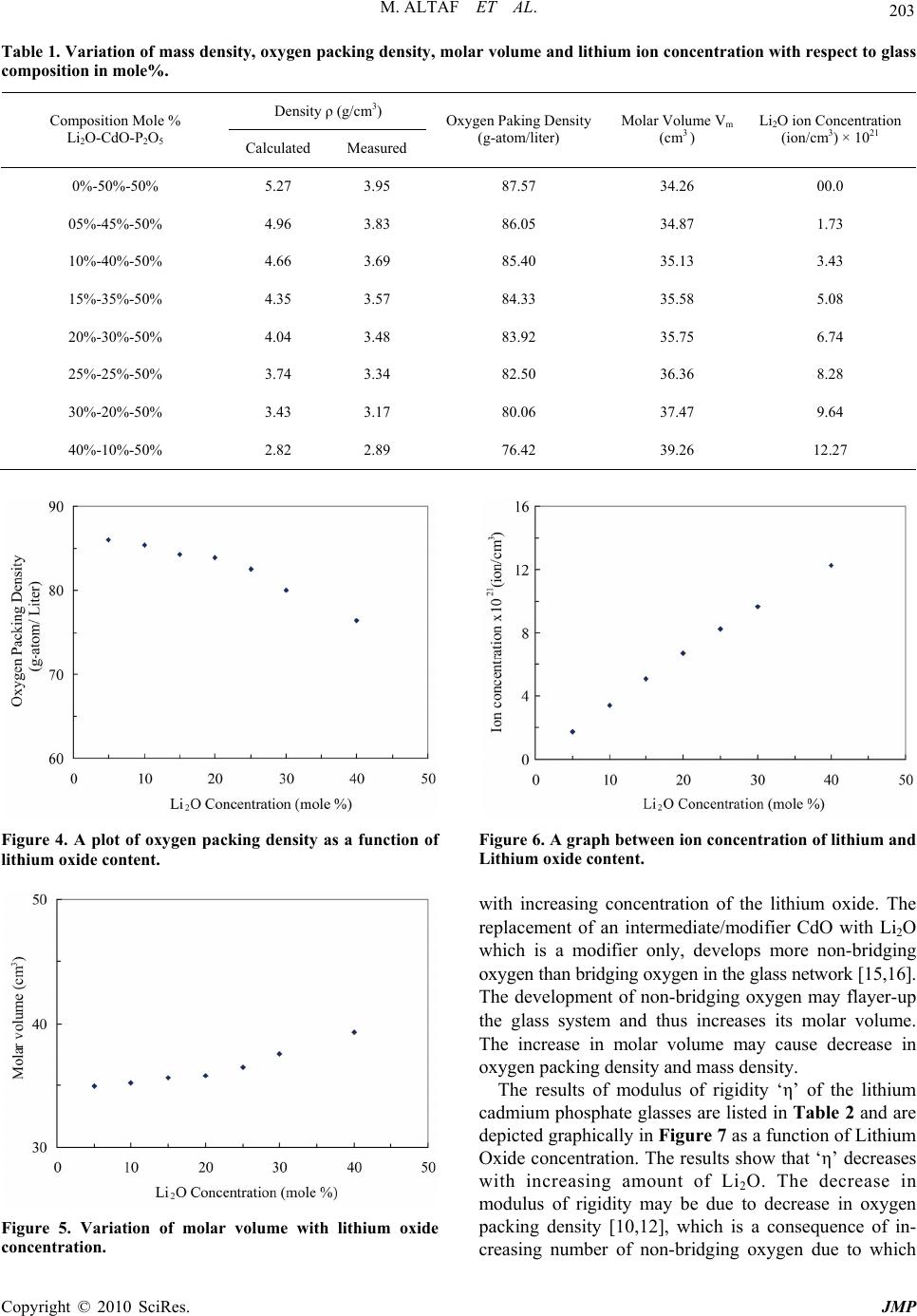 M. ALTAF ET AL. Copyright © 2010 SciRes. JMP 203 Table 1. Variation of mass density, oxy gen packing density, molar volume and lithium ion concentration with respect to glass composition in mole%. Density ρ (g/cm3) Composition Mole % Li2O-CdO-P2O5 Calculated Measured Oxygen Paking Density (g-atom/liter) Molar Volume Vm (cm3 ) Li2O ion Concentration (ion/cm3) × 1021 0%-50%-50% 5.27 3.95 87.57 34.26 00.0 05%-45%-50% 4.96 3.83 86.05 34.87 1.73 10%-40%-50% 4.66 3.69 85.40 35.13 3.43 15%-35%-50% 4.35 3.57 84.33 35.58 5.08 20%-30%-50% 4.04 3.48 83.92 35.75 6.74 25%-25%-50% 3.74 3.34 82.50 36.36 8.28 30%-20%-50% 3.43 3.17 80.06 37.47 9.64 40%-10%-50% 2.82 2.89 76.42 39.26 12.27 Figure 4. A plot of oxygen packing density as a function of lithium oxide content. Figure 5. Variation of molar volume with lithium oxide concentration. Figure 6. A graph be tween ion c oncentration of lithium and Lithium oxide content. with increasing concentration of the lithium oxide. The replacement of an intermediate/modifier CdO with Li2O which is a modifier only, develops more non-bridging oxygen than bridging oxygen in the glass network [15,16]. The development of non-bridging oxygen may flayer-up the glass system and thus increases its molar volume. The increase in molar volume may cause decrease in oxygen packing density and mass density. The results of modulus of rigidity ‘η’ of the lithium cadmium phosphate glasses are listed in Table 2 and are depicted graphically in Figure 7 as a function of Lithium Oxide concentration. The results show that ‘η’ decreases with increasing amount of Li2O. The decrease in modulus of rigidity may be due to decrease in oxygen packing density [10,12], which is a consequence of in- creasing number of non-bridging oxygen due to which 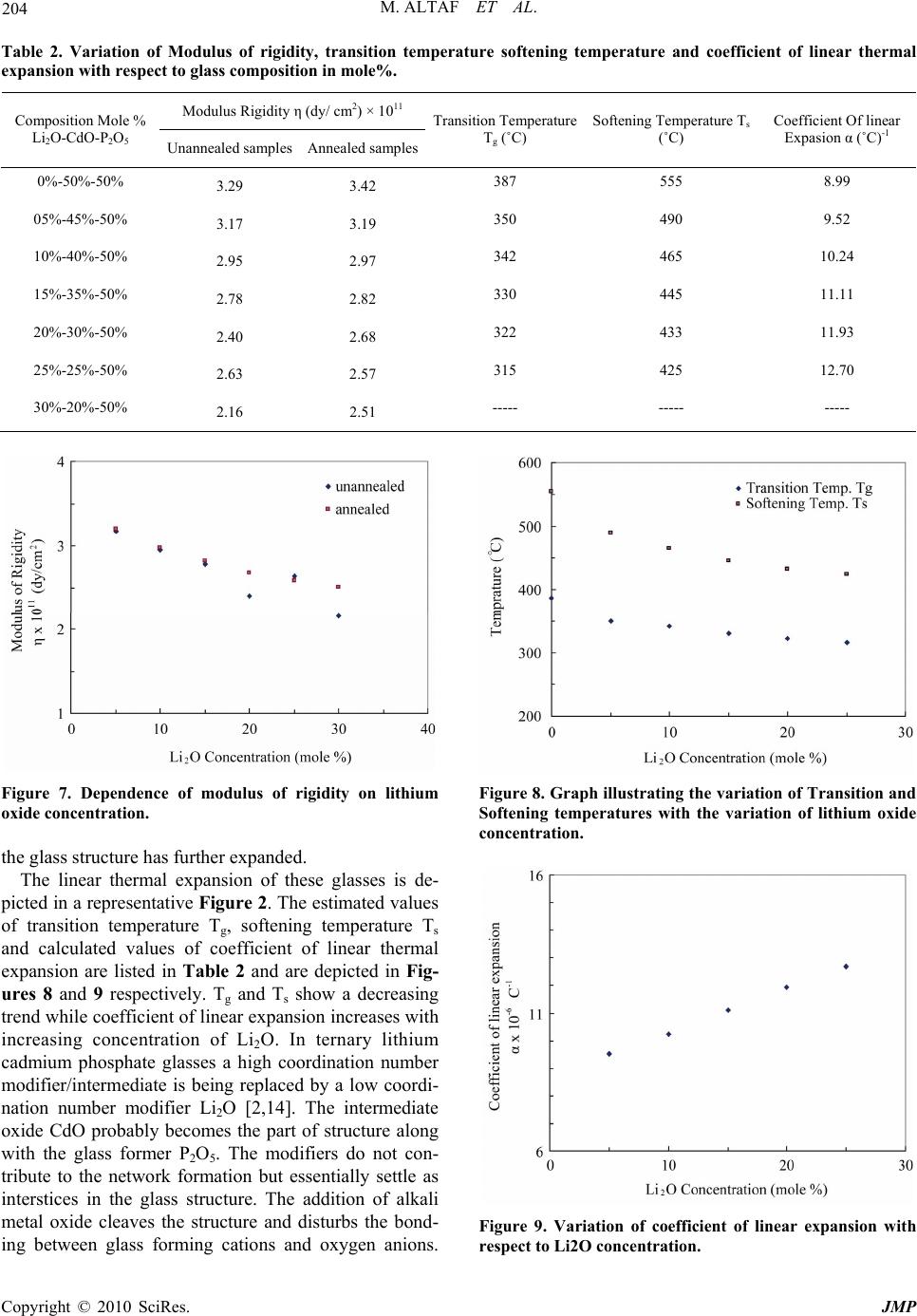 M. ALTAF ET AL. Copyright © 2010 SciRes. JMP 204 Table 2. Variation of Modulus of rigidity, transition temperature softening temperature and coefficient of linear thermal expansion with respect to glass comp os ition in mole%. Modulus Rigidity η (dy/ cm2) × 1011 Composition Mole % Li2O-CdO-P2O5 Unannealed samples Annealed samples Transition Temperature Tg (˚C) Softening Temperature Ts (˚C) Coefficient Of linear Expasion α (˚C)-1 0%-50%-50% 3.29 3.42 387 555 8.99 05%-45%-50% 3.17 3.19 350 490 9.52 10%-40%-50% 2.95 2.97 342 465 10.24 15%-35%-50% 2.78 2.82 330 445 11.11 20%-30%-50% 2.40 2.68 322 433 11.93 25%-25%-50% 2.63 2.57 315 425 12.70 30%-20%-50% 2.16 2.51 ----- ----- ----- Figure 7. Dependence of modulus of rigidity on lithium oxide concentration. the glass structure has further expanded. The linear thermal expansion of these glasses is de- picted in a representative Figure 2. The estimated values of transition temperature Tg, softening temperature Ts and calculated values of coefficient of linear thermal expansion are listed in Table 2 and are depicted in Fig- ures 8 and 9 respectively. Tg and Ts show a decreasing trend while coefficient of linear expansion increases with increasing concentration of Li2O. In ternary lithium cadmium phosphate glasses a high coordination number modifier/intermediate is being replaced by a low coordi- nation number modifier Li2O [2,14]. The intermediate oxide CdO probably becomes the part of structure along with the glass former P2O5. The modifiers do not con- tribute to the network formation but essentially settle as interstices in the glass structure. The addition of alkali metal oxide cleaves the structure and disturbs the bond- ing between glass forming cations and oxygen anions. Figure 8. Graph illustrating the variation of Transition and Softening temperatures with the variation of lithium oxide concentration. Figure 9. Variation of coefficient of linear expansion with respect to Li2O concentration.  M. ALTAF ET AL. Copyright © 2010 SciRes. JMP 205 This increase, the number of non-bridging oxygens and thus develop a more open structure. Consequently the structure expands the molar volume which causes a decrease in the oxygen packing density and hence a de- crease in the density of the glass sample. The decrease in oxygen packing density along with the decrease of den- sity and increase of molar volume of these glasses make them less mechanical resistive which may have caused an increase in the coefficient of linear expansion and decrease in the transition temperature and the softening temperature. The results reported here are similar to those reported by other research workers [13,15-17]. 4. References [1] M. Altaf, M. A. Chaudhry and S. A. Siddiqi, “Effect of Li2O on the Refractive Index and Optical Band Gap of Cadmium Phosphate Glasses,” Journal of Materials Sci- ence and Technology, Vol. 12, No. 2, 2004, p. 150. [2] S. A. Siddiqi, M. Masih and A. Mateen, “Optical Band Gap in Cd-Mg Phosphate Glasses,” Materials Chemistry and Physics, Vol. 40, No. 1, 1995, pp. 69-72. [3] B. Kumar, “Phosphate Glasses and Glass Ceramics Bio Materials,” Indian Ceramic Society, Vol. 44, No. 6, 1985, p. 123. [4] J. E. Pemberton, L. Latif Zadeh, J. P. Fletcher and S. H. Risbub, “Non-linear optical properties,” Chemistry of Materials, Vol. 3, No. 1, 1991, p. 195. [5] A. Mogus-Milankovic, A. Santic, A. Gajovic and D. E. Day, “Electrical Properties of Sodium Phosphate Glasses Con- taining Al2O3 and/or Fe2O3,” Journal of Non-Crystalline Solids, Vol. 296, No. 1, 2001, pp. 57-64. [6] M. Ahmad, F. Salman, M. Morsi, K. El-Badry and F. Metwall, “Electrical Proties of Some Copper-Containing Phosphate Glasses,” Journal of Materials Science, Vol. 41, No. 5, 2006, pp. 1667-1669. [7] A. Pan and A. Ghosh, “Structural and Optical Properties of Lithium Bismuthate Glasses,” Materials Research So- ciety, Vol. 17, No. 8, 2002, p. 1941. [8] G. Y. Guo and Y. L. Chen, “Optical Properties of a Chemically Durable Phosphate Glass,” Journal of Mate- rials Science Letters, Vol. 12, No. 5, 1993, pp. 265-267. [9] G. W. Morey, “The Properties of Glasses,” 2nd Edition, Reinhold Publishing Corporation, New York, 1960. [10] D. G. Holloway, “The Physical Properties of Glass,” Wyke- ham Publications Ltd., London, 1973. [11] W. D. Kingery, “Introduction to Ceramics,” John Willy and Sons, Inc., NewYork, 1960. [12] M. A. Chaudhry and M. Altaf, “Electrical Properties of Na2o-CdO-P2O5 Glasses,” Modern Phy sics Letters B, Vol. 14, No. 9, 2000, pp. 319-326. [13] M. Altaf and M. A. Chaudhry, “Study of Some Physical Properties of Cadmium Lanthanum Phosphate Glasses,” Journal of Materials Science and Technology, Vol. 13, No. 1, 2005, p. 33. [14] K. Othmer, “Encyclopedia of Chemical Technology,” Clan- rendon Press, Oxford, Vol. 11, 1963. [15] D. S. Rao and P. P. Karat, “Elastic Constants of Glasses in the System P2O5-Na2O-ZnO,” Physics & Chemistry of Glasses, Vol. 35, No. 3, 1994, p. 124. [16] A. P. N. de Oliveira, L. B., C. Leonelli, T. Manfredini, and G. C. Pellacani, “Physical Properties of Quenched Glasses in the Li2O-ZrO-SiO2 System,” Journal of Ameri- can Ceramic Society, Vol. 79, No. 4, 1996, pp. 1092- 1097. [17] M. Farley and G. A. Saunders, “The Application of Small Polaron Theory to Transition Metaloxide Theory,” Physica Status Solidi (a), Vol. 28, 1975, p. 199. |

