J. ESMAILI, M. R. EHSANI
62
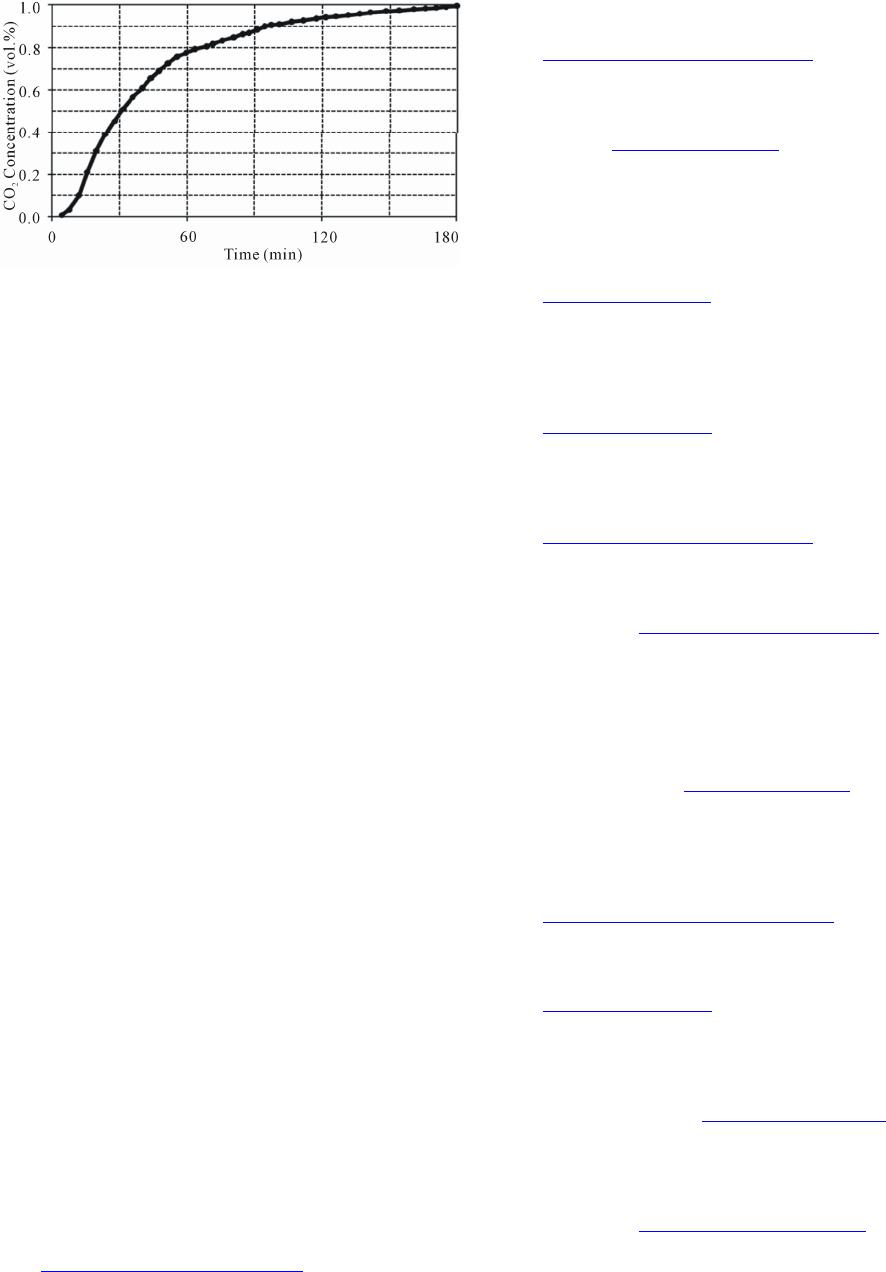
Figure 5. The breakthrough curves of the sorbent prepare
at optimum conditions during CO2 adsorption.
4. Conclusions
The sorbent capacity for carbon dioxide capture from a
gas stream is important for the industrial application of
solid sorbents. The present investigation was carried out
to study combined effects of the initial solution concen-
tration, impregnation time, calcination temperature, and
calcination time on the sorbent capture capacity using a
Box-Behnken design under the Response surface metho-
dology (RSM). The obtained results demonstrate that
sorbent capacity increased with increasing the initial so-
lution concentration and impregnation time. Sorbent ca-
pacity has a maximum point for variation of calcination
temperature and time and further increases of these vari-
ables lead to decrease of sorbent capacity. However,
ANOVA analysis as well as 3D surface plots revealed
that initial solution concentration has the greatest effect
on sorbent capacity. On the basis of the results it can be
concluded that RSM presents an excellent tool which
enables the evaluation of interactions and competitive
effects in multivariable systems and reduces the number
of needed experiments in contrast to the classical method
of changing one variable at a time.
The optimized values obtained for initial solution con-
centration, impregnation time and calcination step tempe-
rature and time were 32.3 wt%, 13.4 hr, 367˚C and 4.1 hr,
respectively, with the predicted maximized response of
sorbent capture capacity (78.66 mg CO2/g sorbent). Stu-
dy on other sorbent characterization such as mechanical
strength require for application of this procedure for pre-
paration of industrial sorbents.
REFERENCES
[1] P. Hagewiesche, S. S. Ashour, H. A. Al-Ghawas and O. C.
Sandall, “Absorption of Carbon Dioxide into Aqueous
Blends of Monoethanolamine and N-Methyldiethanola-
mine,” Chemical Engineering Science, Vol. 50, No. 7,
1995, pp. 1071-1079.
doi:10.1016/0009-2509(94)00489-E
[2] M. Mavroudi, S. P. Kaldis and G. P. Sakellaropoulos,
“Reduction of CO2 Emissions by a Membrane Contacting
Process,” Fuel, Vol. 82, No. 15-17, 2003, pp. 2153-2159.
doi:10.1016/S0016-2361(03)00154-6
[3] R. V. Siriwardane, M. S. Shen, E. P. Fisher and J. A. Po-
ston, “Adsorption of CO2 on Molecular Sieves and Acti-
vated Carbon,” Energy & Fuels, Vol. 15, No. 2, 2001, pp.
279-284. doi:10.1021/ef000241s
[4] H. Hayashi, J. Taniuchi, N. Furuyashiki, S. Sugiyama, S.
Hirano, N. Shigemoto and T. Nonaka, “Efficient Recov-
ery of Carbon Dioxide from Flue Gases of Coal-Fired Po-
wer Plants by Cyclic Fixed-Bed Operations over K2CO3-
on-Carbon,” Industrial and Engineering Chemistry Re-
search, Vol. 37, No. 1, 1998, pp. 185-191.
doi:10.1021/ie9704455
[5] N. Shigemoto, T. Yanagihara, S. Sugiyama and H. Haya-
shi, “Material Balance and Energy Consumption for CO2
Recovery from Moist Flue Gas Employing K2CO3-on-
Activated Carbon and Its Evaluation for Practical Adapta-
tion,” Energy & Fuels, Vol. 20, No. 2, 2006, pp. 721-726.
doi:10.1021/ef058027x
[6] S. C. Lee, B. Y. Choi, S. J. Lee, S. Y. Jung, C. K. Ryu
and J. C. Kim, “CO2 Absorption and Regeneration Using
Na and K Based Sorbents,” Studies in Surface Science
and Catalysis, Vol. 153, No. 1, 2004, pp. 527-530.
doi:10.1016/S0167-2991(04)80307-0
[7] S. C. Lee, B. Y. Choi, C. K. Ryu, Y. S. Ahn and J. C. Kim,
“Absorption and Regeneration of Alkali Metal-Based So-
lid Sorbents,” Catalysis Today, Vol. 111, No. 3-4, 2006,
pp. 385-390. doi:10.1016/j.cattod.2005.10.051
[8] J. S. Hoffman and H. W. Pennline, “Study of Regenerable
Sorbents for CO2 Capture, First National Conference on
Carbon Sequestration, Washington,” 2001.
[9] Y. Liang, D. P. Harrison, R. P. Gupta, D. A. Green and W.
J. McMichael, “Carbon Dioxide Capture Using Dry So-
dium-Based Sorbents,” Energy & Fuels, Vol. 18, No. 2,
2004, pp. 569-575. doi:10.1021/ef030158f
[10] Y. W. Seo, S. H. Jo, C. K. Ryu and C. K. Yi, “Effects of
Water Vapor Pretreatment Time and Reaction Tempera-
ture on CO2 Capture Characteristics of a Sodium-Based
Solid Sorbent in a Bubbling Fluidized-Bed Reactor,”
Chemosphere, Vol. 69, No. 5, 2007, pp. 712-718.
doi:10.1016/j.chemosphere.2007.05.036
[11] C. Zhao, X. Chen and C. Zhao, “CO2 Absorption Using
Dry Potassium-Based Sorbents with Different Supports,”
Energy & Fuels, Vol. 23, No. 9, 2009, pp. 4683-4687.
doi:10.1021/ef900182d
[12] S. Hirano, N. Shigemoto, S. Yamaha and H. Hayashi, Cy-
clic Fixed-Bed Operations over K2CO3-on-Carbon for the
Recovery of Carbon Dioxide under Moist Conditions,”
Bulletin of the Chemical Society of Japan, Vol. 68, No. 3,
1995, pp. 1030-1035. doi:10.1246/bcsj.68.1030
[13] A. G. Okunev, V. E. haronov, Y. I. Aistov and V. N. Parmon,
“Sorption of Carbon Dioxide from Wet Gases by K2CO3-
in-Porous Matrix: Influence of the Matrix Nature,” Reac-
tion Kinetics and Catalysis Letters, Vol. 71, No. 2, 2000,
pp. 355-362. doi:10.1023/A:1010395630719
[14] A. G. Okunev, V. E. Sharonov, A. V. Gubar, I. G. Danilo-
va, E. A. Paukshtis, E. M. Moroz, T. A. Kriger, V. V. Ma-
lakhov and Y. I. Aistov, “Sorption of Carbon Dioxide by
Copyright © 2013 SciRes. JEAS