Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2010, 1, 279-284 doi:10.4236/msa.2010.15041 Published Online November 2010 (http://www.SciRP.org/journal/msa) Copyright © 2010 SciRes. MSA 279 A Novel Synthesis of Nanostructured ZnO via Thermal Oxidation of Zn Nanowires Obtained by a Green Route Adriana Veloso Maciel, Wagner da Nova Mussel, Vânya Márcia Duarte Pasa 1Department of Chemistry, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil. Email: vmdpasa@terra.com.br Received September 9th, 2010; revised November 1st, 2010; accepted November 5th, 2010. ABSTRACT ZnO nanowires were synthesised in a green and novel, two-step process: (1) the production of Zn nanowires by car- bothermal reduction of a mixture of ZnO/biopitch (Eucalyptus sp. tar pitch) at 900°C for 1 h in a quartz tube placed in an electric furnace in a N2 atmosphere and (2) the oxidation of the as-prepared Zn nanowires in air at 300°C for 3 h and 6 h and at 400°C for 3 h. The structural properties and phase compositions of the oxidised samples were studied by X-ray diffraction (XRD), and the morphologies were investigated by scanning electron microscopy (SEM). The XRD results demonstrated the formation of ZnO phase, as the main product. The oxidised products exhibited good crystallin- ity. Maximal conversion of the Zn nanowires into ZnO nanowires (99% ) resulted from oxidation of the sample for 3 h in air at 300°C. The formation of ZnO was also confirmed by Fourier transform infrared (FTIR) spectroscopy. Keywords: Biopitch, Zn Nanowires, Thermal Oxidation, ZnO Nanowires, XRD, FTIR 1. Introduction Zinc oxide (ZnO) exhibits many interesting properties, such as a wide direct bandgap (3.37 eV), high binding energy of the free excitation (60 meV) at room tempera- ture, high photoconductivity, and important piezoelectric and pyroeletric properties [1-2]. Due to these properties, ZnO has attracted considerable attention for potential applications in various electronic and optoelectronic de- vices. A variety of methods, including thermal evaporation [3], hydrothermal processing [4], chemical vapour depo- sition [5], metal-organic CVD [6], and carbothermal re- duction [7], have been widely used to prepare different ZnO nanostructures. An alternative process for preparing ZnO nanowires involves the synthesis of zinc nanoparti- cles followed by oxidation. The thermal oxidation me- thod has attracted interest due to its simplicity, low cost, lower temperatures, and the lack of a need for a catalyst. Recently, ZnO nanoneedles were selectively grown on the facets of zinc microstructures by thermal oxidation in ambient atmosphere at temperatures from 250 to 400°C for 4 h [8]. ZnO nanowires and nanorods have been pre- pared at an optimal pressure by simple thermal oxidation of wire-like Zn precursors at different temperatures [9]. Wang et al. synthesised ZnO nanoparticles by oxidis- ing Zn nanoparticles prepared through arc discharge in air at 250°C, 300°C and 350°C for different periods of time [10]. ZnO films formed by the oxidation of Zn thin films have been reported [2,11,12]. ZnO nanowires have been formed by annealing Zn nanowires at 500°C for 1 h; the nanowires were synthesised by heating a ZnO and graphite mixture [13]. However, little attention has been given to the preparation of ZnO by thermal oxidation of as-prepared metallic zinc nanoparticles by the carboth- ermal reduction process. This paper describes ZnO nanostructures obtained by the thermal oxidation of Zn nanowires synthesised by a green carbothermal reduction method. A mixture of ZnO/ biopitch was heated, and the gases that evolved during the biopitch pyrolysis and the special carbon generated during the process promoted the reduction of ZnO into metallic Zn. The controlled experimental conditions that allowed the growth of Zn nanowires were recently pre- sented in the literature [14]. The synthesis of nanostruc- tured ZnO using these Zn nanowires as precursors was performed by our research group, and the best results are presented for the first time in this paper. This green method of synthesis is very simple and catalyst-free and  A Novel Synthesis of Nanostructured ZnO via Thermal Oxidation of Zn Nanowires Obtained by a Green Route Copyright © 2010 SciRes. MSA 280 will probably have industrial interest due to its low cost (because biopitch is a remnant of the charcoal production industry) and the fact that the process presents soft ex- perimental conditions. Environmental benefits are also realised because the process uses renewable carbon (bio- pitch) instead of petroleum pitch, graphite or carbon na- notubes. 2. Experimental 2.1. Synthesis of the Zn Nanowires – Step 1 In the present study, zinc nanowires were synthesised by a new carbothermal reduction process by heating a mix- ture of high-purity ZnO (SYNTH, 99.0% minimum pu- rity) powder and biopitch (Eucalyptus sp. tar pitch), us- ing optimal experimental conditions previously obtained by our research group and presented in the literature [14]. The source material, pure ZnO powder mixed with bio- pitch (molar ratio 1:1), was placed in a quartz boat, which was placed at the centre of a quartz tube and in- serted into a horizontal tube furnace. The furnace was heated to 900°C at a heating rate of 3°C/min and kept at 900°C for 1 h under a constant flow of nitrogen, which was used as the carrier gas during the process. After thermal treatment, the furnace was naturally cooled to room temperature. The fluffy, dark, gray products that formed on the inner wall of the quartz tube were col- lected. No catalysts or templates were needed for mate- rial growth through this method. 2.2. Synthesis of the ZnO Nanowires by Thermal Oxidation of Zn Nanowires – Step 2 The as-prepared zinc nanowires were put into a quartz boat and loaded into the centre of a quartz tube with two open ends, which was placed in a horizontal furnace. The Zn nanowires were then heated in air under different conditions to determine the best parameters for promot- ing Zn oxidation. The Zn nanowires were heated to 300°C for 3 h, to 300°C for 6 h, and to 400°C for 3 h in air to promote oxidation into ZnO nanostructures. Sub- sequently, the furnace was cooled to room temperature. The as-synthesised products were then identified as sam- ples A, B and C, as shown in the schematic diagram of the process in Figure 1. 2.3. Characterization of the as-Synthesised Products The morphologies and compositions of the as-synthesis- ed products were characterised by a scanning electron microscope (SEM, Philips XL 30FEG at 30 KV) equip- ed with an energy-dispersive X-ray spectroscope (EDS, Figure 1. Schematic diagram of the synthesis of ZnO nano- wires. JEOL JXA-8900RL at 15 KV and 13 mA). The phases and crystallographies of the products were characterised using X-ray diffraction (XRD), which was carried out on a Siemens D5000 diffractometer equipped with a CuK (=1.54178 Å) radiation tube operating at 40 KV and 30 mA at room temperature with a time con- stant of 0.5 s under a spinning sample holder at 60 cycles per minute, attempting to avoid any preferred orientation. The phases and crystallographies of the products were characterised using X-ray diffraction (XRD), which was carried out on a Siemens D5000 diffractometer equipped with a CuK ( = 1.54178 Å) radiation tube operating at 40 KV and 30 mA at room temperature with a time con- stant of 0.5 s under a spinning sample holder at 60 cycles per minute, attempting to avoid any preferred orientation. The scanning step was 0.05° 2/step. Crystalline phase identification was performed by comparing the sample diffractogram to the PDF2 database from JCDS [15]. The diffraction pattern was fit using the Rietveld algorithm to model the peak profiles, obtain the most reliable lattice parameters and allow quantitative phase analysis. The oxidised products were also characterised by Fou- rier transform infrared FTIR spectroscopy (FT-IR, ABB BOMEM MBSeries) in the range of 4000-250 cm-1. FTIR analyses were performed on powders prepared by the KBr-pellet technique. 3. Results and Discussion 3.1. Characterization of the as-Synthesized Zn Nanowires – Step 1 Figure 2 represents X-rays diffraction (XRD) patterns of the dark, gray product deposited onto the inner wall of the quartz tube after step 1 (Figure 1). The SEM image shown in Figure 3 gives a general view of the morphology of the as-deposited product, ob- served as a large quantity of entangled and curved wire- like structures. In general, the zinc nanowires are several micrometers long and about 70 nm in diameter. 3.2. Characterization of the as-Synthesised ZnO Nanowires – Step 2 The X-ray diffraction patterns of samples A, B and C obtained from the oxidation of Zn nanowires in air are shown in Figure 4. 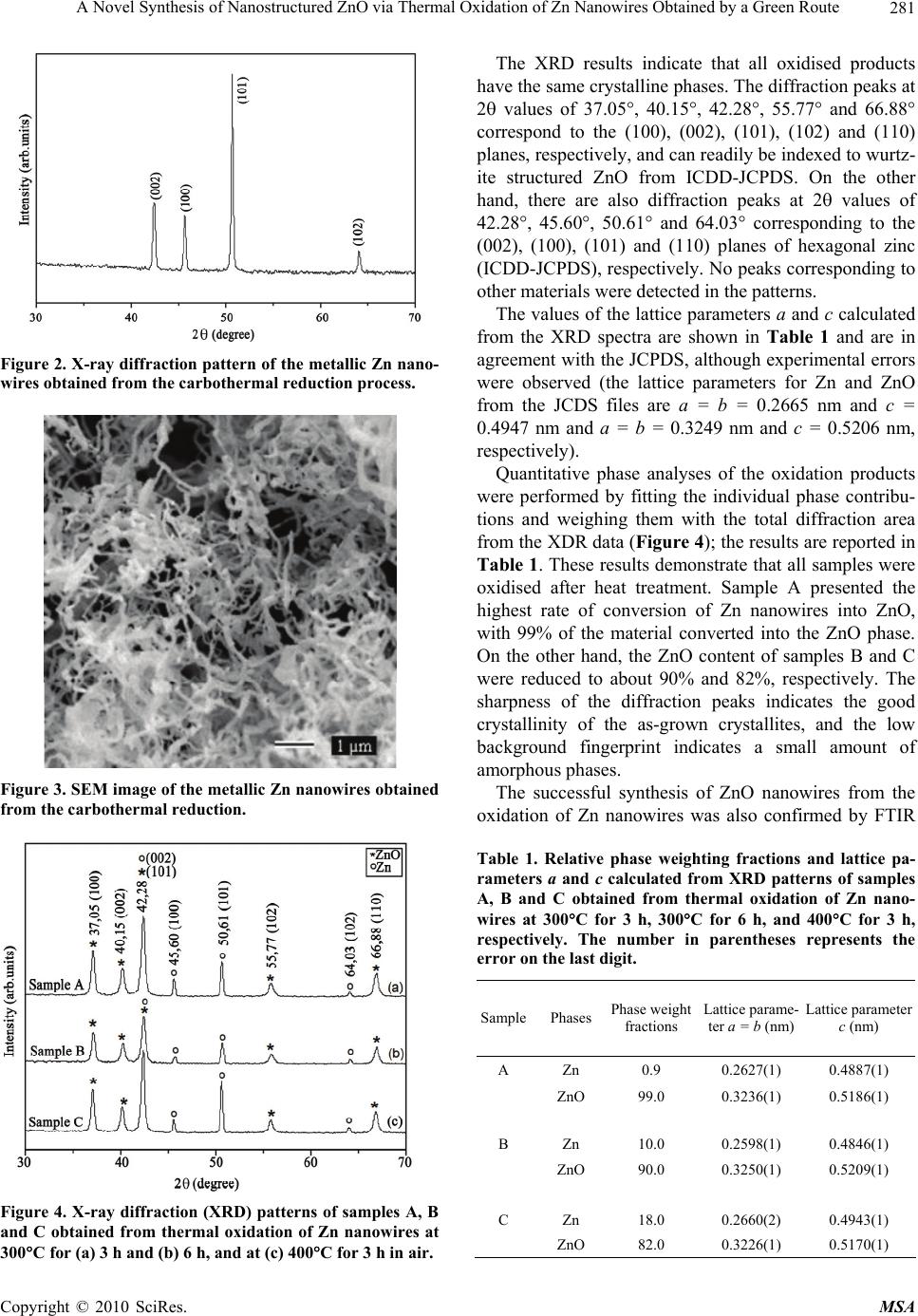 A Novel Synthesis of Nanostructured ZnO via Thermal Oxidation of Zn Nanowires Obtained by a Green Route Copyright © 2010 SciRes. MSA 281 Figure 2. X-ray diffraction pattern of the metallic Zn nano- wires obtained from the carbothermal reduction process. Figure 3. SEM image of the metallic Zn nanowires obtained from the carbothermal reduction. Figure 4. X-ray diffraction (XRD) patterns of samples A, B and C obtained from thermal oxidation of Zn nanowires at 300C for (a) 3 h and (b) 6 h, and at (c) 400C for 3 h in air. The XRD results indicate that all oxidised products have the same crystalline phases. The diffraction peaks at 2 values of 37.05°, 40.15°, 42.28°, 55.77° and 66.88° correspond to the (100), (002), (101), (102) and (110) planes, respectively, and can readily be indexed to wurtz- ite structured ZnO from ICDD-JCPDS. On the other hand, there are also diffraction peaks at 2 values of 42.28°, 45.60°, 50.61° and 64.03° corresponding to the (002), (100), (101) and (110) planes of hexagonal zinc (ICDD-JCPDS), respectively. No peaks corresponding to other materials were detected in the patterns. The values of the lattice parameters a and c calculated from the XRD spectra are shown in Table 1 and are in agreement with the JCPDS, although experimental errors were observed (the lattice parameters for Zn and ZnO from the JCDS files are a = b = 0.2665 nm and c = 0.4947 nm and a = b = 0.3249 nm and c = 0.5206 nm, respectively). Quantitative phase analyses of the oxidation products were performed by fitting the individual phase contribu- tions and weighing them with the total diffraction area from the XDR data (Figure 4); the results are reported in Table 1. These results demonstrate that all samples were oxidised after heat treatment. Sample A presented the highest rate of conversion of Zn nanowires into ZnO, with 99% of the material converted into the ZnO phase. On the other hand, the ZnO content of samples B and C were reduced to about 90% and 82%, respectively. The sharpness of the diffraction peaks indicates the good crystallinity of the as-grown crystallites, and the low background fingerprint indicates a small amount of amorphous phases. The successful synthesis of ZnO nanowires from the oxidation of Zn nanowires was also confirmed by FTIR Table 1. Relative phase weighting fractions and lattice pa- rameters a and c calculated from XRD patterns of samples A, B and C obtained from thermal oxidation of Zn nano- wires at 300C for 3 h, 300C for 6 h, and 400C for 3 h, respectively. The number in parentheses represents the error on the last digit. SamplePhases Phase weight fractions Lattice parame- ter a = b (nm) Lattice parameter c (nm) A Zn 0.9 0.2627(1) 0.4887(1) ZnO 99.0 0.3236(1) 0.5186(1) B Zn 10.0 0.2598(1) 0.4846(1) ZnO 90.0 0.3250(1) 0.5209(1) C Zn 18.0 0.2660(2) 0.4943(1) ZnO 82.0 0.3226(1) 0.5170(1) 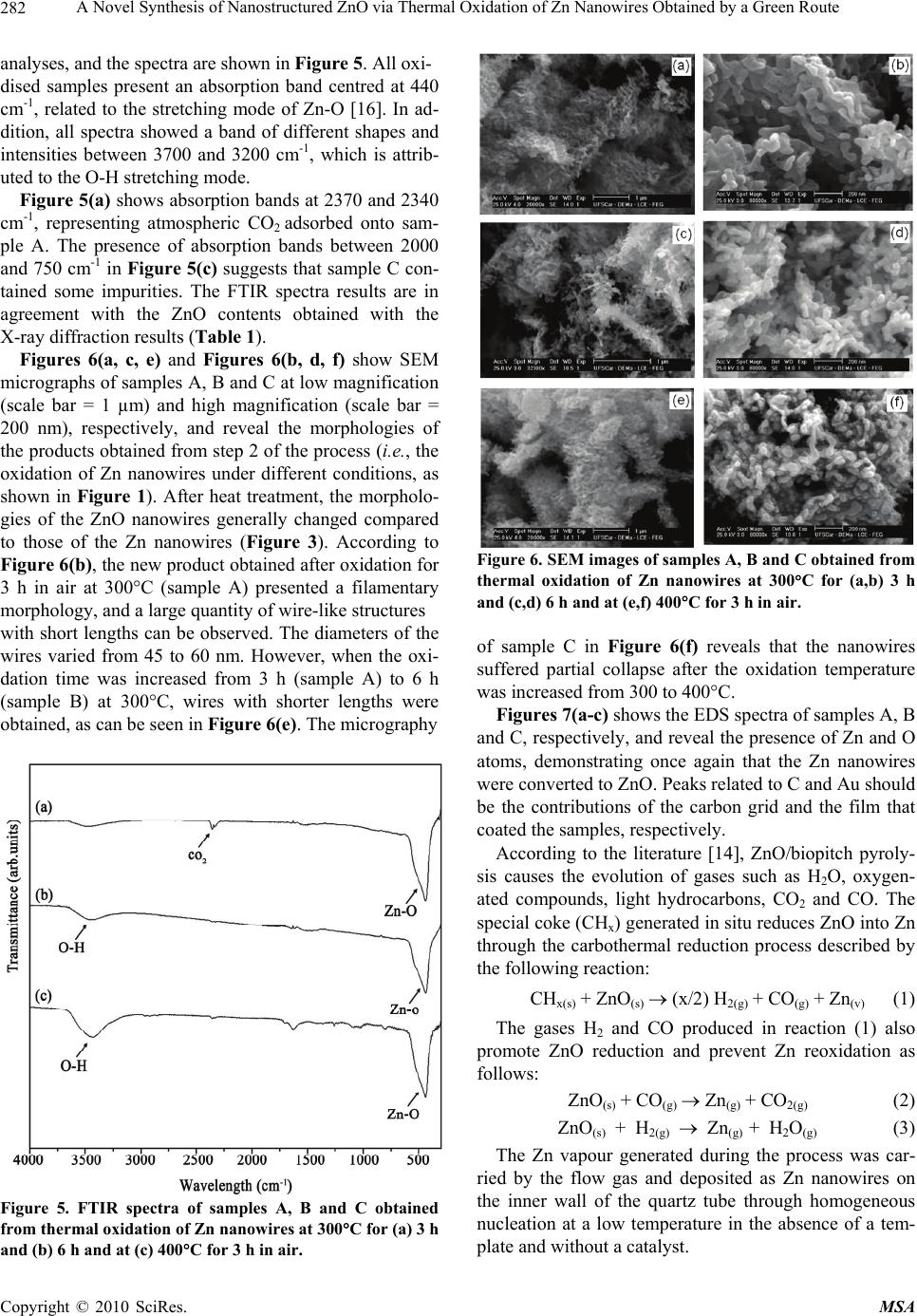 A Novel Synthesis of Nanostructured ZnO via Thermal Oxidation of Zn Nanowires Obtained by a Green Route Copyright © 2010 SciRes. MSA 282 analyses, and the spectra are shown in Figure 5. All oxi- dised samples present an absorption band centred at 440 cm-1, related to the stretching mode of Zn-O [16]. In ad- dition, all spectra showed a band of different shapes and intensities between 3700 and 3200 cm-1, which is attrib- uted to the O-H stretching mode. Figure 5(a) shows absorption bands at 2370 and 2340 cm-1, representing atmospheric CO2 adsorbed onto sam- ple A. The presence of absorption bands between 2000 and 750 cm-1 in Figure 5(c) suggests that sample C con- tained some impurities. The FTIR spectra results are in agreement with the ZnO contents obtained with the X-ray diffraction results (Table 1). Figures 6(a, c, e) and Figures 6(b, d, f) show SEM micrographs of samples A, B and C at low magnification (scale bar = 1 µm) and high magnification (scale bar = 200 nm), respectively, and reveal the morphologies of the products obtained from step 2 of the process (i.e., the oxidation of Zn nanowires under different conditions, as shown in Figure 1). After heat treatment, the morpholo- gies of the ZnO nanowires generally changed compared to those of the Zn nanowires (Figure 3). According to Figure 6(b), the new product obtained after oxidation for 3 h in air at 300°C (sample A) presented a filamentary morphology, and a large quantity of wire-like structures with short lengths can be observed. The diameters of the wires varied from 45 to 60 nm. However, when the oxi- dation time was increased from 3 h (sample A) to 6 h (sample B) at 300°C, wires with shorter lengths were obtained, as can be seen in Figure 6(e). The micrography Figure 5. FTIR spectra of samples A, B and C obtained from thermal oxidation of Zn nanowires at 300C for (a) 3 h and (b) 6 h and at (c) 400C for 3 h in air. Figure 6. SEM images of samples A, B and C obtained from thermal oxidation of Zn nanowires at 300C for (a,b) 3 h and (c,d) 6 h and at (e,f) 400C for 3 h in air. of sample C in Figure 6(f) reveals that the nanowires suffered partial collapse after the oxidation temperature was increased from 300 to 400°C. Figures 7(a-c) shows the EDS spectra of samples A, B and C, respectively, and reveal the presence of Zn and O atoms, demonstrating once again that the Zn nanowires were converted to ZnO. Peaks related to C and Au should be the contributions of the carbon grid and the film that coated the samples, respectively. According to the literature [14], ZnO/biopitch pyroly- sis causes the evolution of gases such as H2O, oxygen- ated compounds, light hydrocarbons, CO2 and CO. The special coke (CHx) generated in situ reduces ZnO into Zn through the carbothermal reduction process described by the following reaction: CHx(s) + ZnO(s) (x/2) H2(g) + CO(g) + Zn(v) (1) The gases H2 and CO produced in reaction (1) also promote ZnO reduction and prevent Zn reoxidation as follows: ZnO(s) + CO(g) Zn(g) + CO2(g) (2) ZnO(s) + H2(g) Zn(g) + H2O(g) (3) The Zn vapour generated during the process was car- ried by the flow gas and deposited as Zn nanowires on the inner wall of the quartz tube through homogeneous nucleation at a low temperature in the absence of a tem- plate and without a catalyst.  A Novel Synthesis of Nanostructured ZnO via Thermal Oxidation of Zn Nanowires Obtained by a Green Route Copyright © 2010 SciRes. MSA 283 Figure 7. EDS spectra of samples A, B and C obtained from thermal oxidation of Zn nanowires at 300C for (a) 3 h and (b) 6 h and at (c) 400C for 3 h in air. Thus, the as-synthesised Zn nanowires were oxidised into nanostructured ZnO at relatively low temperatures (300°C or 400°C) under an ambient air atmosphere: Zn(s) + ½O2 ZnO(s) (4) The mechanism proposed above is consistent with the experimental results. 4. Conclusions ZnO nanowires were obtained with a green, two-step process: 1) the production of Zn nanowires by car- bothermal reduction through heating a mixture of ZnO powder and biopitch (Eucalyptus sp. tar pitch), and 2) the oxidation in air of the as-prepared Zn nanowires into ZnO. Maximal conversion of Zn nanowires into ZnO nanowires (99%) occurred after oxidation for 3 h in air at 300°C. The morphologies of the Zn nanowires changed with oxidation time and temperature. The XRD results showed that all of the synthesised nanowires presented hexagonal structure and good crystallinity. This method is simple and is assisted by Eucalyptus sp tar pitch co-pyrolysis, which generates carbon with spe- cial reactivity and gases (CO and H2) that permitted ZnO reduction and Zn nanowires formation in large scale. The oxidation occurred at low temperature. The entire proc- ess was catalyst-free and performed without a vacuum or a template. This green method will probably generate interest due to its technical and economical feasibility, environmental benefits and the great importance of nanostructured ZnO. 5. Acknowledgements Adriana Veloso Maciel acknowledges a scholarship from CNPq-Conselho Nacional de Desenvolvimento da Pes- quisa (Brazil) and Petrobrás. REFERENCES [1] T.-J. Hsueh and C.-L. Hsu, “Fabrication of Gas Sensing devices with ZnO Nanostructure by the Low-Temperature Oxidation of Zinc Particles,” Sensors and Actuators B: Chemical, Vol. 131, No. 2, 2008, pp. 572-576. [2] G. G. Rusu, M. Girtan and M. Rusu, “Preparation and Characterization of Thin Films Prepared by Thermal Oxidation of Evaporated Zn Thin Films,” Superlattices and Microstructures, Vol. 42, No. 1-6, 2007, pp. 116-122. [3] N. Bouhssira, S. Abed, E. Tomasella, J. Cellier, A. Mosbah, M. S. Aida and M. Jacquet, “Influence of Annealing Temperature on the Properties of ZnO Thin Films Deposited by Thermal Evaporation,” Applied Surface Science, Vol. 252, No. 15, 2006, pp. 5594-5597. [4] L. Z. Pei, H. S. Zhao, W. Tan, H. Y. Yu, Y. W. Chen and Q.-F. Zhang, “Single Crystalline ZnO Nanorods Grown by a Simple Hydrothermal Process,” Materials Chara- cterization, Vol. 60, No. 9, 2009, pp. 1063-1067. [5] G. Z. Wang, Y. Wang, M. Y. Yau, C. Y. To, C. J. Deng and D. H. L. Ng, “Synthesis of ZnO Hexagonal Columnar Pins by Chemical Vapor Deposition,” Materials Letters, Vol. 59, No. 29-30, 2005, pp. 3870-3875. [6] N. Han, P. Hu, A. Zuo, D. Zhang, Y. Tian and Y. Chen, “Photoluminescence Investigation on the Gas Sensing Property of ZnO Nanorods Prepared by Plasmaenhanced CVD Method,” Sensors and Actuators B: Chemical, Vol. 145, No. 1, 2010, pp. 114-119. [7] M. Biswas, E. McGlynn and M. O. Henry, “Carbothermal Reduction Growth of ZnO Nanostructures on Sapphire- Comparisons between Graphite and Activated Charcoal Powders,” Microelectronics Journal, Vol. 40, No. 1, 2009, pp. 259-261. [8] D. Yuvaraj and K. N. Rao, “Selective Growth of ZnO Nanoeedles by Thermal Oxidation of Zn Microstruc- tures,” Materials Science and Engineering B, Vol. 164, No. 3, 2009, pp. 195-199. [9] H.-Q. Liang, L. Z. Pan and Z.-J. Liu, “Synthesis and Photoluminescence Properties of ZnO Nanowires and Nanorods by Thermal Oxidation of Zn Precursors,” Materials Letters, Vol. 62, No. 12-13, 2008, pp. 1797- 1800. [10] Z. H. Wang, D. Y. Geng and H. Z. D. Zhang, “Charac- terization and Optical Properties of ZnO Nanoparticles Obtained by Oxidation of ZnO Nanoparticles,” Materials Letters, Vol. 63, No. 29, 2009, pp. 2533-2535. [11] Z. W. Li, W. Gao and R. J. Reeves, “Zinc Oxide Films by Thermal Oxidation of Zinc Thin Films,” Surface and Coating Technology, Vol. 198, No. 1-3, 2005, pp. 319- 323. [12] W. Gao and Z. W. Li, “Photoluminescence Properties of ZnO Films Grown by Wet Oxidation: Effect of Process- ing,” Journal of Alloys and Compounds, Vol. 449, No. 1-2, 2008, pp. 202-206. [13] A. Khan and M. E. Kordesch, “Large-Scale Fabrication of Metallic Zn Nanowires by Thermal Evaporation,” Physica E, Vol. 33, No. 1, 2006, pp. 88-91. [14] J. V. D. S. Araújo, R. V. Ferreira, M. I. Yoshida and V. M. D. Pasa, “Zinc Nanowires Synthesized on a Large Scale by a Simple Carbothermal Process,” Solid State  A Novel Synthesis of Nanostructured ZnO via Thermal Oxidation of Zn Nanowires Obtained by a Green Route Copyright © 2010 SciRes. MSA 284 Sciences, Vol. 11, No. 9, 2009, pp. 1673-1679. [15] JCPDS-International Center for Diffraction Data. JCPDS- ICDD 2000. [16] Y. J. Kwon, K. H. Kim, C. S. Lim and K. B. Shim, “Characterization of ZnO Nanopowders Synthesized by the Polymerized Complex Method via an Organochemical Route,” Journal of Ceramic Processing Research, Vol. 3, No. 3, 2002, pp. 146-149. |

