 Advances in Bioscience and Biotechnology, 2013, 4, 654-664 ABB http://dx.doi.org/10.4236/abb.2013.45086 Published Online May 2013 (http://www.scirp.org/journal/abb/) Construction, expression and binding specificity of bispecific CD3 × VEGFR-2 and CD3 × NCAM antibodies in the single chain and diabody format Anke Kopacek1, Thomas Böldicke1, Sarah Lergenmüller1, Frank Berthold2, Markus Jensen2, Peter P. Müller1, Ludger Grosse-Hovest3 1Helmholtz Center for Infection Research, Department of Molecular Biotechnology, Braunschweig, Germany 2Department of Pediatric Oncology and Hematology, University of Cologne, University Hospital Cologne, Cologne, Germany 3Institute for Cell Biology, Department of Immunology, Eberhard Karls University, Tübingen, Germany Email: anke.kopacek@googlemail.com, thomas.boeldicke@helmholtz-hzi.de, sarah.lergenmueller@gmx.de, frank.berthold@uk-koeln.de, jensen@uni-koeln.de, pmu@helmholtz-hzi.de, ludger.grosse-hovest@uni-tuebingen.de Received 8 March 2013; revised 19 April 2013; accepted 13 May 2013 Copyright © 2013 Anke Kopacek et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Bispecific antibodies are recombinant proteins with novel immunological properties and therapeutic po- tential. Recombinant protein quality and activity of several bispecific antibodies comprising different va- riable domain combinations with respect t o the paren- tal monospecific single chain fragments (scFv) were evaluated after expression in bacteria or mammalian cells. The parental scFv proteins humanized anti- NCAM scFv, murine anti-VEGFR-2 scFv, murine and humanized anti-CD3 scFv, respectively, could success- fully be expressed in E. coli, whereas the murine anti- NCAM scFv version could not be reliably detected. Bispecific CD3 × VEGFR-2 and CD3 × NCAM anti- bodies were expressed in the bispecific single chain and the single chain diabody format. However, the di- abody derived from the murine anti-NCAM scFv could not efficiently be expressed in E. coli or in mam- malian cells. Significant binding of the CD3 × NCAM single chain diabody comprising the humanized ver- sion of anti-CD3 and humanized version of anti- NCAM was efficient to both antigens. Nevertheless, binding of the bispecific single chain version to the NCAM antigen was inefficient in comparison to CD3 binding. In conclusion, the data could indicate that the result of scFv expression in bacteria may be pre- dictive for the chances of success for functional ex- pression of more complex bispecific derivatives. Keywords: Recombinant Antibody; Single Chain Diabody; Bispecific An tibody; Protein Expression 1. INTRODUCTION Conventional monoclonal antibodies specifically recog- nize a single antigen with their N-terminal variable do- mains. In addition, via their constant C-terminal Fc do- main, they are able to activate the complement system and various immune cells such as neutrophils, monocytes, macrophages or dendritic cells. This can lead to inflam- matory cytokine expression and even to tissue toxicity. However, for envisioned clinical applications of recom- binantly produced an tibodies such as immun e therap ies it is of interest to avoid potential excess inflammatory re- actions or cytotoxicity. The C-terminal antibody domain and its potential to arouse side effects could be omitted by novel antibody constructs that combine two binding specificities in this way that they can directly activate and link immune effector cells to target cells of interest [1]. Recombinant bispecific antibodies lack the Fc part and are composed of the variable antigen binding do- mains only [2 ,3]. One future application of su ch bispeci- fic antibodies (BsAbs) could be the treatment of cancer by activating and crosslinking cytotoxic T cells to tumor cells and thereby induce tumor cell death [4]. Due to their reduced size, engineered antibodies that lack the Fc domain are expected to assemble more easily, to be less immunogenic and to be able to penetrate solid tumor tis- sue more easily than conventional antibodies [5]. The prerequisite for the construction of recombinant bispecific antibodies is the availability of two parental single chain antibodies. DNA encoding antigen-binding domains can be derived from hybridoma cells or from universal phage display libraries [6-8]. BsAbs have been raised against various cancer antigens and successfully OPEN ACCESS  A. Kopacek et al. / Advances in Bioscience and Biotechnology 4 (2013) 654-664 655 demonstrated anti-tumor activity in vitro and in vivo [9- 18]. Various formats for bispecific antibodies have been proposed, such as bicistronic diabody expression constructs that express two different peptide chains from a single mRNA and the single chain diabody or bispecific single chain formats. An advantage of the single chain format is that no assembly is required and folding is thought to be more robust since both binding specificities are present in a single polypeptide chain. Even though BsAbs are already being tested in the clinic, further research is necessary to improve charac- teristics like the reduction of immunogenicity by hu- manizing antibodies isolated from other species, by im- proving the target specificity of novel antibodies that differentiate between protein modifications present only on tumor cells but not on differentiated cells that express the same protein, by cost-effective production, antibody stability in the human circulation and by improving the efficacy in vivo [19]. With respect to full-length antibod- ies, for in vitro investigations recombinant antigen bind- ing fragments can be expressed with a good success rate —comparatively quick ly and with limited efforts even in bacteria [20]. However, for clinical use, due to the ab- sence of inflammatory side products and the human- compatible posttranslational modifications the preferred expression system for therapeutical proteins is mammal- ian cell culture s. Suitable production cell lin es must fulfill multiple requirements su ch as the absenc e of endogeno us virus production and robust cell growth in suspension cultures in the absence of serum. Few cell lines meet these conditions. Preferred producer cells are human kidney cell lines, hamster cells or murine myeloma cells [21]. Subsequent purification of the recombinant antibodies can efficiently be performed in a single step using affinity chromatography in combination with peptide-tagged an- tibody [22]. The specific binding capabilities of antibody variable domains can be influenced by the domain positions rela- tive to each other, by amino acid sequences outside of the complementarity determining regions and by the produc- tion system. Therefore, the strategy of this study was to generate, to characterize and to compare the functionality of various antibody formats and production systems. The construction, production, purification and antigen bind- ing capabilities of recombinant bispecific antibodies that bind T cells and either of two tumor-associated antigens were investigated. The tumor antigen neural cell adhe- sion molecule (NCAM) is expressed on several tumor cells, such as neuroblastoma cells, the second most co m- mon malignancy among childhood cancers [23]. The se- cond antigen, vascular endothelial growth factor receptor 2 (VEGFR-2) is expressed on cancer cells and non-tu- morigenic cells such as vascular endo thelial cells. VEGF directly stimulates cancer cell mitosis and additionally, indirectly supports tumor growth by stimulating blood vessel sprouting to enhance tumor oxygenation and nu- trient supply [24-26]. A full length CD3 × NCAM anti- body has previously been shown to induce cytotoxicity of T cells to NCAM expressing tumor cells [16]. CD3 × NCAM and CD3 × VEGFR-2 BsAbs were both con- structed as single chain antibodies and as single chain diabodies. With the exception of constructs derived from one specific antibody, the BsAb proteins investigated in this study could be expressed in bacteria as well as in mammalian cells, and in addition, they could bind to both cognate antigens expressed on the surface of cells. 2. MATERIALS AND METHODS 2.1. Construction of CD3 × VEGFR-2 Single Chain Antibody bs2,5 and CD3 × NCAM Single Chain Antibodies bs1,3 and bs2,4 The cDNA of the mouse anti-VEGFR-2 scFv A7 was PCR amplified from the pCANTAB5EscFvA7 construct [27]. The fragment was cloned into an expression vector pKozak-Splice that already contained the humanized anti-CD3 scFv UCHT1 as previously described [28]. A C-terminal c-myc epitope and mRNA processing signals were introduced into the cDNA of scFv A7 by three am- plification step s usi ng one f orwar d pri mer V-BspEI (atccggacaggtgaaactgcaggagtctggacctgagctggtg) and four reverse p ri mers V-rev (ggggggaagatgaacacactgggggcagccactgtccgtttcagctccagct tggtcccccctccg), universal-rev (gatcagcttctgctcttcagcagaaggggggaagatgaacacactggggg- cagccactgt), universal-rev2 (gagttcaggtcctcttcagagatcagcttctgctctt cagcagaaggggggaag) and universal-rev3 (tactagttatcagtccacagcagagttcaggtcctcttcagagatcagcttctg- ctc). The final amplicon was cloned via BspEI and SpeI in the expression vector resulting in the following 5’-3’ orientation: (VL-VH) UCHT1-18aa linker-(VH-VL)A7 yielding bs2,5. The 18 amino acid linker between the scFv fragments was derived from the human IgG elbow region of the CH1 domain [29]. The same strategy was used to construct bs1,3 and bs2,4. 2.2. Assembly of the CD3 × VEGFR-2 Single Chain Diabody db2,5 and of the CD3 × NCAM Single Chain Diabodies db1,3 and db2,4 The coding sequences of VH and VL from OKT3 and UCHT1 were amplified from the cDNA of the hybrido- ma clone OKT3 and from pCANTAB5EscFv UCHT1, re- spectively; the cds of VH and VL from scFv A7, ERIC1 and D29 was amplified from pCANTAB5E scFvA7, the cDNA of hybridoma clone ERIC1 and from the pCAN- TAB5E scFv D29 clone. scFv Fragments in the configu- Copyright © 2013 SciRes. OPEN ACCESS  A. Kopacek et al. / Advances in Bioscience and Biotechnology 4 (2013) 654-664 656 ration VH (CD3)-Gly4Ser-VL(NCAM) and VH(NCAM)-Gly4Ser- VL(CD3) and VH(CD3)-Gly4Ser-VL(VEGFR-2) and -VH(VEGFR-2)- Gly4Ser-VL(CD3) were generated by assembly extension PCR as described previously [30]. In a second step the inverse scFv fragments were assembled introducing a (Gly4Ser)3-linker between both partners resulting in single chain diabodies with the following configuration: VH (CD3)-Gly4Ser-VL(NCAM)-(Gly 4Ser)3-VH(NCAM)-Gly4Ser-VL(CD 3) and VH(CD3)-Gly4Ser-VL(VEGFR-2)-(Gly4Ser)3-VH(VEGFR-2)- Gly4Ser-VL(CD3). All assembled PCR products contained a 5’ SfiI and a 3’ NotI recognition site. Then the PCR products were cloned via SfiI and NotI into the mammal- ian cell expression vector pSecTag/HygroA (Invitrogen). Using this assembly procedure db1,3; db2,4 and db2,5 were obtained. 2.3. Recombinant Antibody Expression in Bacteria For recombinant protein expression in bacteria, the diabo- dies UCHT1 × A7, OKT3 × ERIC1, UCHT1 × D29, OKT-3 × ERIC1 single chain antibody and the scFv co- ding sequences of OKT3, UCHT1, ERIC1 and D29 were cloned in to the E. coli expression vector pCANTAB5E (GE Healthcare) using SfiI and NotI sites. After transfor- mation of diabodies, single chain antibody and scFv ex- pression plasmids in E. coli HB2151 recombinant anti- body expression was induced at OD600 1.0 with 0.2 mM IPTG and the cells were further cultured for 16 h at 25˚C in 100 ml in 2 × YTS medium (16 g/l trypton, 10 g/l yeast extract, 5 g/l NaCl, 0.4 M sucrose). The periplas- matic fraction and the culture supernatant were collected and the proteins separated by SDS-PAGE followed by either silver staining or Western blot analysis [31]. 2.4. Mammalian Cell Culture Procedures VEGF-receptor 2 (VEGFR-2) expressing porcine aortic endothelial cell line PAE-KDR or PAE-FUA [32] were cultured in DMEM, 10% FCS (heat-inactivated; Bio- chrom AG, Berlin, Germany), 2 mM glutamine (Serva, Heidelberg, Germany), 0.4 mg/ml G418 (Gibco, Serva, Heidelberg, Germany) under standard conditions in a humidified atmosphere with 5% CO2 at 37˚C. Jurkat cells (ACC-282, DSMZ, Braunschweig, Germany), TE671 and LS cells were grown in RPMI 1640 supplemented with 10% FCS and 2 mM glutamine. For purification of re- combinant db2,5 stable transfected BHK21 cells (ATCC® CCL-10) were grown in serum-free Pro-CHO5 medium (Lonza, Basel, Switzerland) supplemented with 7.5 mM glutamine and 0.8 mg/ml hygromycin B. For purification of recombinant bs2,5 stable transfected Sp2/0-Ag14 mu- rine myeloma cells (CRL-1581, ATCC) were cultured in serum-free CD Hybridoma Medium (Gibco) with 7.5 mM glutamine and 1.0 mg/ml G418 an d cholesterol (250 × lipid concentrate, Gibco). 2.5. Expression of CD3 × VEGFR-2 Single Chain Antibody bs2,5 and CD3 × NCAM Single Chain Antibodies bs1,3 and bs2,4 To enhance vector integration the bs2,5 DNA construct was linearized with AhdI (New England Biolabs, Frank- furt/Main, Germany) and stably transfected into Sp2/0- Ag14 murine myeloma cells by electroporation at 230 V and a capacity of 975 µF (Gene Pulser, Biorad). Cells were cultured under standard conditions in the presence of G418 (1 mg/ml final concentration) to select stable bs2,5 antibody producing cell clones. The same proce- dure was used to assemble and express the construct (VL-VH)OKT3-18aa linker-(VH-VL)mu rin e NCAM resulting in bs1,3 and (VL-VH)UCHT1-18aa linker-(VH-VL)humanized NCAM resulting in bs2,4. 2.6. Generation of Cells Expressing the CD3 × VEGFR-2 Single Chain Diabody db2,5 or the CD3 × NCAM Single Chain Diabodies db1,3 or db2,4 DNA of the diabody expression plasmids was linearized with the restriction enzyme AhdI and then transfected into BHK cells by the calcium phosphate co-precipitation method [33]. Selection of stable producing cells was per- formed in the presence of 800 µg/ml hygromycin B in the cell culture medium. 2.7. Production of CD3 × VEGFR-2 Antibody bs2,5 in Murine Myeloma Cells For adaption to serum-free culture, 2.2 × 106 cells were cultured in a T75 flask in 25 ml IMDM with 3% FCS. The preadapted cells were then cultured to a cell density of 5 × 105 cells/ml in 25 ml serum-free CD Hybridoma Media (Gibco, Invitrogen) with 7.5 mM glutamine and 1 mg/ml G418; 1/250 v/v cholesterol lipid concentrate. For antibody production, 2 × 105 cells/ml in 75 ml medium per T175 flask were inoculated. Cell culture supernatants (600 ml total volume) were harvested, filtered and dia- lyzed against PBS pH 8.0. The supernatants were centri- fuged and filtered to remove insoluble precipitates. The recombinant antibody was purified using an anti-c-myc agarose column (Sigma-Aldrich). 2.8. Production of CD3 × VEGFR-2 Antibody db2,5 in BHK21 Cells BHK21 cells were stably transfected with DNA of the plasmid pSecTag/HygroA (Invitrogen) containing the co- ding sequence of the diabody db2,5. For the production of db2,5 in BHK21, the cells were cultured in Pro-CHO 5 media supplemented with 7.5 mM glutamine and 0.8 Copyright © 2013 SciRes. OPEN ACCESS  A. Kopacek et al. / Advances in Bioscience and Biotechnology 4 (2013) 654-664 657 mg/ml hygromycin B. 280 ml of cell culture super- natant from eight T175 flasks were harvested and dial- yzed against 50 mM NaH2PO4; 300 mM NaCl; pH 8.0 at 4˚C. The dialyzed cell culture supernatants were purified using Co2+ based affinity resins. 2.9. Purification of the CD3 × VEGFR-2 Antibody bs2,5 The bispecific antibody bs2,5 was produced in Sp2/0- Ag14 murine myeloma cells and secreted in the super- natant. A 5 ml column (MoBiTec) was packed with 1.5 ml anti-c-myc agarose. The column was washed with 7.5 ml elution buffer (0.1 M ammonium hydroxide, pH 11.2 and 7.5 ml PBS binding buffer, pH 8.0). The flow-through was reloaded three times. The column was washed until the flow-through solution reached a lower OD280 nm of 0.01. bs2,5 was eluted using 50 mM NaH2PO4; 300 mM NaCl; 250 mM imidazole; pH 8.0. 1 ml fractions were collected, neutralized by the addition of 90 µl 1 N acetic acid and dialyzed against PBS pH 7.0 at 4˚C. Protein con- centrations were determined using the Micro BCA assay method (Thermo Fisher Scientific) [34]. 2.10. SDS PAGE and Immune-Blot Analysis Proteins were separated using 10% SDS-PAGE. Proteins were fixed with 40% ethanol; 10% acetic acid and were silver stained according to the manufacturer’s specifi- cations (GE Healthcare). Alternatively, proteins were transferred to polyvin ylidene fluoride (PVDF) me mbranes (BioRad, Hercules, USA) using the SemiDry method [35]. Membranes were blocked for one hour in blocking buffer (5% Blotting Grad Blocker Non-Fat Dry Milk in PBS; BioRad) at room temperature (RT). Subsequently, the membranes were incubated with a primary antibody (mouse anti-c-myc (9E10): sc-40, Santa Cruz, Heidelberg, Germany; His-Tag monoclonal antibody, Merck Milli- pore, Darmstadt, Germany or E tag antibody, GE Health- care); all 1:1.000 diluted in 3 ml of 2.5% blocking buffer) overnight at RT. The membranes were washed three times with PBS/0.05% Tween 20, then incubated with a peroxidase labeled secondary antibody in 3 ml of 2.5% blocking buffer for one hour at RT (peroxidase-conju- gated AffiniPure goat anti-mouse IgG (H + L), Dianova; diluted 1:1.000). Then, the membrane was washed three times with PBS/0.05% Tween 20 and once with distilled water and subsequently the membrane was dried. The activit y of peroxidase wa s visualized u sing DAB reac tion according to the manufacturer’s specifications (SIG MAFASTTM DAB; Sigma-Aldrich). 2.11. Purification of Bispecific CD3 × VEGFR-2 Diabody db2,5 The bispecific antibody db2,5 was produced in BHK21 cells and secreted into the cell culture supernatant. The purification of serum-free ProCHO 5 supernatant was performed with TALONTMSuperflow Metal (Co2+) affinity resins (Clontech). A 5 ml column with 1 ml resin volume was equilibrated with binding buffer containing 5 mM imidazole. 280 ml of cell culture supernatant was dial- yzed in 50 mM NaH2PO4; 300 mM NaCl; pH 8.0. The flow-through was loaded on the column five-times in a row. The column wa s washed using 10 ml b inding buff er with 5 mM imidazole and the bound protein db2,5 was eluted with 10 ml elution buffer (50 mM NaH2PO4, 300 mM NaCl; 250 mM imidazole pH 8.0). Fractions of 1 ml were collected and dialyzed three times against PBS pH 7.0 at 4˚C. Determination of protein concentration and protein purity with silver stained SDS-PAGEs or by Western blot analysis were carried out as described for the purification of bs2,5 antibody. 2.12. Antigen Binding of CD3 × VEGFR-2 Antibodies bs2,5 and db2,5 To examine the binding of bispecific antibodies bs2,5 and db2,5, different antigen expressing cell lines were used: The VEGFR-2 expressing PAE-KDR, the VEGFR-1 expressing PAE-FLT-1, Jurkat and the NCAM ex- pressing TE671 cell line. 105 cells of each cell typ e were used in 100 µL FACS buffer (PBS; 2% FCS) per well of a 96-well round bottom plate. The antibodies were di- luted in a volume of 40 µl PBS and added to the respec- tive wells so that concentrations resulted in total of 0.2 µg/ml of each antibody. The plate was incubated for 30 min at 4˚C, centrifuged (1000 g; 5 min; 4˚C) and the supernatant was discarded. After one wash step with 200 µl FACS buffer, the cells were incubated either with primary anti-c-myc antibody 1:200 diluted in 100 µl of FACS buffer or with b uffer on ly for 30 min at 4˚C. Cells incubated with only the second phycoerythrin (PE) la- beled antibody served as negative controls. The solu- tions were removed via centrifugation and one wash step as mentioned above. The second PE labeled antibody (goat anti-mouse IgG Fc-RPE, Dianova, Hamburg, Ger- many, 1:200 diluted in 100 µl of FACS buffer) was ad- ded and wells with negative samples were only filled with buffer. After a 30 min incubation period at 4˚C, the cells were washed once and resuspended in 200 µL FACS buffer. Dead cells were stained with PI (Appli- chem, Darmstadt, Germany). 20000 antibody stained cells per sample were analyzed with flow cytomety (BD FACSCalibur, BD Bioscience, San Jose, USA using the software BD CellQuestTM Pro, BD Bioscience). Viable cells were gated after staining with PI and the emission of each fluorochrome was compensated using the soft- ware FlowJow (Tree Star Inc., Ashland, USA). CD3 expressing Jurkat or NCAM expressing TE671 Copyright © 2013 SciRes. OPEN ACCESS 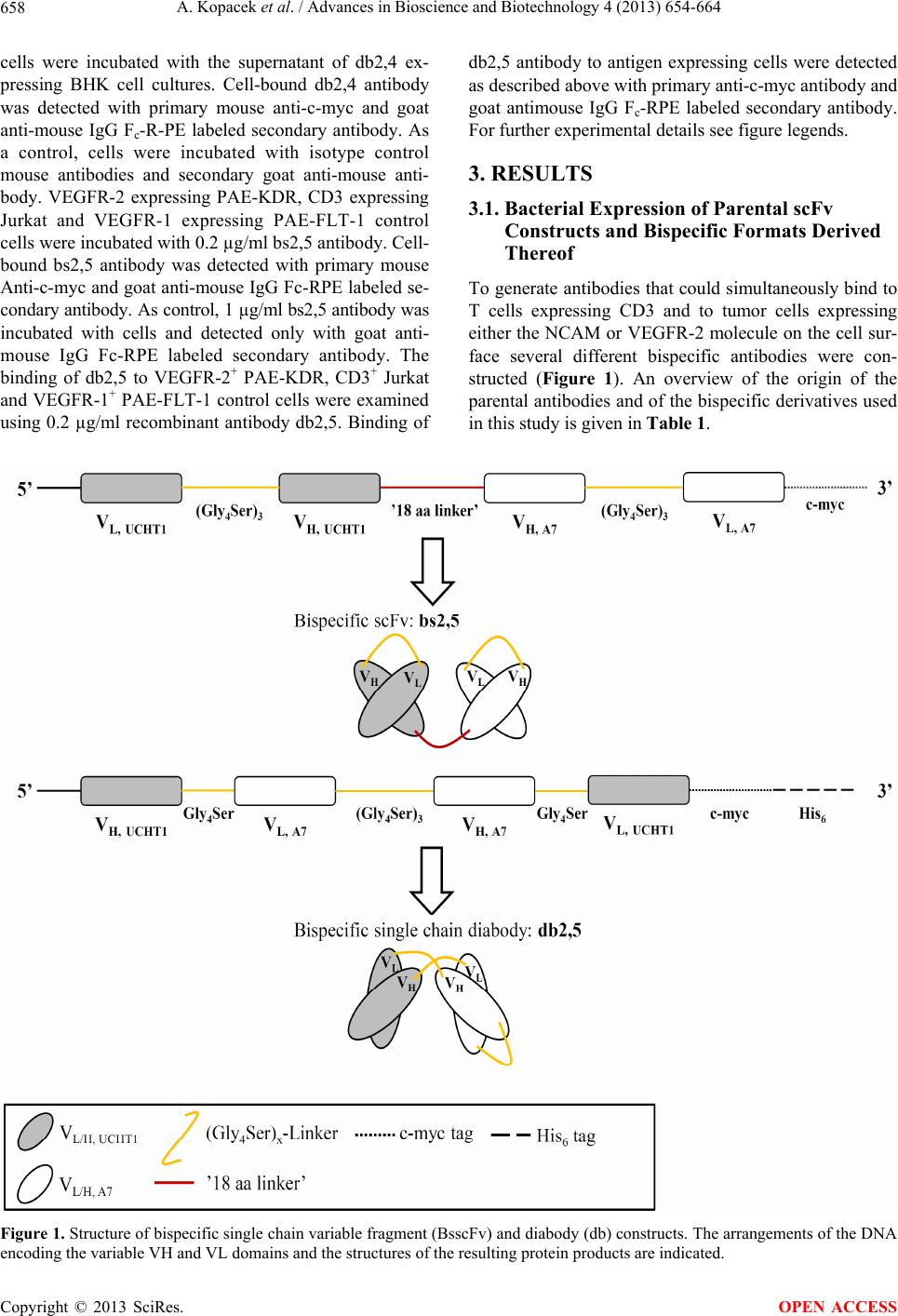 A. Kopacek et al. / Advances in Bioscience and Biotechnology 4 (2013) 654-664 Copyright © 2013 SciRes. 658 cells were incubated with the supernatant of db2,4 ex- pressing BHK cell cultures. Cell-bound db2,4 antibody was detected with primary mouse anti-c-myc and goat anti-mouse IgG Fc-R-PE labeled secondary antibody. As a control, cells were incubated with isotype control mouse antibodies and secondary goat anti-mouse anti- body. VEGFR-2 expressing PAE-KDR, CD3 expressing Jurkat and VEGFR-1 expressing PAE-FLT-1 control cells were incubated with 0.2 µg/ml bs2,5 antibody. Cell- bound bs2,5 antibody was detected with primary mouse Anti-c-myc and goat anti-mouse IgG Fc-RPE labeled se- condary antibody. As control, 1 µg/ml bs2,5 antibody was incubated with cells and detected only with goat anti- mouse IgG Fc-RPE labeled secondary antibody. The binding of db2,5 to VEGFR-2+ PAE-KDR, CD3+ Jurkat and VEGFR-1+ PAE-FLT-1 control cells were examined using 0.2 µg/ml recombinant antibody db2,5. Binding of db2,5 antibody to antigen expressing cells were detected as described above with primary anti-c-myc antibody and goat antimouse IgG Fc-RPE labeled secondary antibody. For further experimental details see figure legends. 3. RESULTS 3.1. Bacterial Expression of Parental scFv Constructs and Bispecific Formats Derived Thereof To generate antibodies that could simultaneously bind to T cells expressing CD3 and to tumor cells expressing either the NCAM or VEGFR-2 molecule on the cell sur- face several different bispecific antibodies were con- structed (Figure 1). An overview of the origin of the parental antibodies and of the bispecific derivatives used in this study is given in Table 1. Figure 1. Structure of bispecific single chain variable fragment (BsscFv) and diabody (db) constructs. The arrangements of the DNA encoding the variable VH and VL domains and the structures of the resulting protein products are indicated. OPEN ACCESS  A. Kopacek et al. / Advances in Bioscience and Biotechnology 4 (2013) 654-664 659 Table 1. Recombinant antibody constructs and expression. Construct Antigen specificity Parental constructs Species*FormatTag Expression system Protein expression CD3/ NCAM or VEGFR binding Reference db2,4 CD3xNCAM UCHT1xD29 h scdb myc-hisE. coli + +/+ This study db1,3 CD3xNCAM OKT3xERIC1 m scdb myc-hisE. coli n.d. This study bs1,3 CD3xNCAM OKT3xERIC1 m bsscFvmyc E. co li n.d. This study db2,5 CD3xVEGFR-2 UCHT1xA7 hxm scdb myc-hisE. coli + +/+ This study db1,3 CD3xNCAM OKT3xERIC1 m scdb myc-hisBHK21 n.d. This study db2,4 CD3xNCAM UCHT1xD29 h scdb myc-hisBHK21 + +/+ This study db2,5 CD3xVEGFR-2 UCHT1xA7 hxm scdb myc-his BHK21 + +/+ This study bs1,3 CD3xNCAM OKT3xERIC1 m bsscFvmyc SP2/0 This study bs2,4 CD3xNCAM UCHT1xD29 h bsscFvmyc SP2/0 + +/+ This study bs2,5 CD3xVEGFR-2 UCHT1xA7 hxm bsscFvmyc SP2/0 + +/+ This study OKT3 CD3 m sc E. coli + This study OKT3 CD3 m sc BHK21 + This study UCHT1 CD3 h sc E. coli + This study ERIC1 NCAM m sc E. coli n.d. This study ERIC1 NCAM m sc BHK21 n.d. This study D29 NCAM h sc E. coli + This study D29 NCAM h sc BHK21 + This study A7 VEGFR-2 m sc E. coli + This study A7 VEGFR-2 m sc BHK21 + This study OKT3 CD3 m Monoclonal [36] UCHT1 CD3 h Monoclonal IgG2a [28] ERIC1 NCAM m Monoclonal [37] D29 NCAM h ERIC1 derivative [38] A7 VEGFR-2 m phage display [27] The parental anti-CD3 scFv constructs OKT3 and the humanized UCHT1 as well as the anti-NCAM scFv ERIC1 and its humanized derivative D29 were expressed in E. coli. Proteins present in the culture supernatant and from the periplasmatic fraction were separated by SDS-PAGE and stained by decoration with tag-specific antibodies. The expression of scFv OKT3, scFv UCHT1 and scFv D29 in the periplasm was within the expected range (Figure 2(a), lanes 2 and B, clone 1-4) compared to the marginal expression levels of scFv ERIC1 (Figure 2(b), lane 3). Expression of the OKT3 × ERIC1 diabody was neither detectable in the periplasmatic fraction nor in the culture supernatant (Figure 3, lane 2). The scFv UCHT1 and UCHT1 × A7 diabody could be expressed in the periplasm of E. coli bacteria (Figure 4, lanes 7 and 8, respectively). Therefore, with the exceptio n of the anti- NCAM antibody ERIC1 derivatives all bispecific pro- teins examined could be expressed in the periplasm of bacteria. 3.2. Purification of CD3 × VEGFR-2 Antibody bs2,5 from Myeloma Cells For potential animal studies or future clinical applica- tions mammalian cell expression would be desirable to avoid the danger of immune reactive bacterial contami- nations. Murine bs2,5 producer cells were grown in se- rum-free medium. The bs2,5 protein present in the culture supernatant was purified by anti-myc affinity column chromatography and ch aracterized by SDS PAGE and by Copyright © 2013 SciRes. OPEN ACCESS  A. Kopacek et al. / Advances in Bioscience and Biotechnology 4 (2013) 654-664 660 (a) (b) Figure 2. Expression of scFv OKT3, ERIC1 and D29 in bacte- ria. E. coli HB2152 transformed with plasmid DNA encoding the antibody constructs indicated were cultured and media su- pernatant and periplasmatic fractions were analyzed by the Western Blotting procedure. The recombinant proteins were detected by hybridization with mouse anti-E-tag antibody, fol- lowed by incubation with peroxidase labeled goat anti-mouse antibody. (a) scFv OKT3 expressed in E. coli. lane 1, culture supernatant; lane 2, periplasmatic fraction; lane 3, periplas- matic fraction of ERIC1 expressing E. coli; lane 4, purified scFvA7. (b) Periplasmatic fraction of native E. coli HB2152 and of scFvD29 expressing clones 1 to 4. The position of the 28 kDa protein marker and the expected position of the scFv pro- tein are indicated. Figure 3. Expression of the OKT3 x ERIC1 diabody db1,3 in bacteria. Cell culture supernatant and periplasmic fractions were characterized as described in Figure 2. Lane 1, cell culture supernatant; lane 2, periplasmatic fraction; lane 3, affinity pu- rified scFv A7. immunoblot analysis. The recombinant protein could be detected in the load and in the eluted fractions but not in the flow-through or wash fractions (Figure 5 and data not shown). Overall, 49 µg of affinity-purified bs2,5 were obtained from 600 ml cell culture supernatant. This data showed that the protein was secreted into the cell culture medium and could be visualized as a single band by sil- ver stained PAGE. 3.3. Purification of CD3 × VEGFR-2 Bispecific Antibody db2,5 Since the anti-c-myc affinity colu mns were limited in the protein binding capacity the His-tagged diabody db2,5 Figure 4. Expression of scFv UCHT1 and UCHT1 × A7 di- abody db2,5 in bacteria. Antibodies were expressed and ana- lyzed as described in the legend of Figure 2. Lane 1, E. coli HB2152; lane 2, culture supernatant from E. coli HB2152 transformed with DNA of the scFv A7 expression plasmid; lane 3, supernatant scFv UCHT1; lane 4, supernatant UCHT1 × A7 diabody, lane 5, periplasmatic fraction of E. coli HB2152; lane 6, periplasmatic fraction of scFv A7 (positive control); lane 7, periplasmatic fraction of scFv UCHT1; lane 8, periplasmatic fraction of UCHT1 × A7 diabody; lane 9, affinity purified scFv A7. (a) (b) Figure 5. Analysis of affinity purified CD3 × VEGFR-2 bispecific antibody bs2,5. bs2,5 expressing myeloma cell culture supernatant was purified with a 1.5 ml anti-c-myc agarose affinity column and eluted fractions with the highest protein concentrations were combined. (a) Pro- teins se- parated by SDS-PAGE and silver stained. Lane 1, molecular weight markers; lane 2, 0.5 µg of purified bs2,5; lane 3, 1 µg of bs2,5, (b) Western blot immunoassay: 0.5 µg and 1 µg of purified bs2,5 shown in A were blotted onto a PVDF membrane and recombinant bs2,5 protein was detected using mouse anti-c-myc antibody and per- oxidase conjugated AffiniPure goat anti-mouse IgG anti- bodies. Lane 1, molecular weight markers; lane 2, 0.5 µg bs2,5; lane 3, 1.0 µg bs2,5. protein was purified using either Ni2+-NTA agarose or TALONTMSuperflow Metal (Co2+) affinity resins. In both cases, a band corresponding to the expected molecular weight of 56 kDa was detected by immuno-blot analysis with anti-His antibod y, indicating the bind ing of db2,5 to either of the two resins (data not shown). Copyright © 2013 SciRes. OPEN ACCESS  A. Kopacek et al. / Advances in Bioscience and Biotechnology 4 (2013) 654-664 661 However, in comparison to the Ni2+-NTA agarose the Co2+ purification protocol appeared more efficient (not shown). Subsequently, db2,5 in the cell culture super- natant was purified with a Co2+ affinity column. The purity and identity of the recombinant antibody was confirmed with silver stained SDS-PAGE and by im- munoblot analysis (Figure 6). The db2,5 could be iso- lated in a homogeneous form without any apparent deg- radation produ c t s. 3.4. Binding of CD3 × NCAM Antibody db2,4 to CD3 and NCAM Antigen Expressing Cells The binding of the bispecific db2,4 antibody present in the cell culture supernatant to CD3 and NCAM was test- ed with CD3+ (Jurkat) or NCAM+ (TE671) expressing cells, respectively. The diabody binds nearly to 100% of both living, gated cell typ es (Figures 7(a) and (b)). No binding was detected to the NCAM negative LS cells and to VEGFR-2 expressing PAE/KDR cells (Fig- ures 7(c) and (d)). This result indicates that comparative- ly low amounts of db2,4 antibody could efficiently label both CD3 and NCAM expressing cells. 3.5. Binding of CD3 × VEGFR-2 Antibody bs2,5 to CD3 and VEGFR-2 Expressing Target Cells The binding of the bispecific bs2,5 antibody to the two cognate antigens was tested with CD3+ or VEGFR-2+ ex- pressing cells, respectively. This antibody binds to 50% of the VEGFR-2+ cells and to 76% of the CD3+ cells (Figures 8 (a) and (b), black lines). (a) (b) Figure 6. Purification of CD3 × VEGFR-2 antibody db2,5 db2,5 antibody was purified by Co2+ affinity chromatography. The proteins were separated by SDS-PAGE and silver stained (a). Lane 1, Molecular weight markers; lane 2, 0.5 µg db2,5; lane 3, 1.0 µg db2,5. (b) Immunoblot as described in Figure 5. Lane 1, molecu lar weight markers; lane 2, 0.5 µg bs2,5; lane 3, 1.0 µg bs2,5. No binding of bs2,5 to the control cell line was detected (Figure 8(c), black graph). These results confirmed the specific binding of bs2,5 antibody to VEGFR-2+ and CD3+ cells. 3.6. Binding of CD3 × VEGFR-2 Antibody db2,5 to CD3 and VEGFR-2 Expressing Cells The binding of db2,5 to VEGFR-2 or CD3 antigens ex- pressed on mammalian cells was detected by FACS ana- lysis (Figures 9(a) and (b), black graphs). The recombi- nant antibody db2,5 reacted with 66% of living, gated VEGFR-2+ cells and with 76% of CD3+ cells (Figures 9(a) and (b), Jurkat and PAE-KDR cells). Figure 7. Binding of CD3 × NCAM antibody db2,4 to CD3 and NCAM expressing target cells. FACS analysis of CD3 (A) or NCAM (B) expressing cells after incubation with the su- pernatant of db2,4 expressing BHK cell cultures. Cell-bound db2,4 antibody was detected with mouse anti-c-myc antibody and goat anti-mouse IgG Fc-R-PE labeled antibody (red line). Cells incubated with isotype control mouse antibodies and se- condary goat anti-mouse antibody served as a control (black line). LS (C) cells and PAE/KDR cells (D) that did not express CD3 or NCAM were analyzed in the same way. Figure 8. Binding of CD3 × VEGFR-2 antibody bs2,5 to CD3 and VEGFR-2 expressing cells. Cells expressingVEGFR-2 (A), CD3 (B) or VEGFR-1 (C) were incubated with affinity purified bs2,5 antibody produced in murine myeloma cells. Cell-bound bs2,5 antibody was detected with primary mouse an ti-c-myc and goat anti-mouse IgG Fc-R PE labeled secondary antibody. Cell-bound bs2,5 antibody was detected with (black) or without (grey) primary antibody. Copyright © 2013 SciRes. OPEN ACCESS  A. Kopacek et al. / Advances in Bioscience and Biotechnology 4 (2013) 654-664 662 Figure 9. Analysis of CD3 × VEGFR-2 antibody db2,5 bind- ing to CD3 and VEGFR-2 expressing cells. The binding of affinity purified db2,5 antibody produced in BHK cells to VEGFR-2 (a), CD3 (b) or VEGFR-1 (c) expressing cells was examined as described in the legend of Figure 8. Cells were stained either with (black) or without (grey) primary antibody and with PE labeled secondary antibody. 4. DISCUSSION Bispecific antibodies are preferentially investigated for their potential use in cancer immunotherapy due to their high potential to activate T cell cytotoxicity whereas con- ventional antibo dies solely activate cells primarily of the monocytic family that express Fc receptors. Much re- mains to be investigated to improve the clinical efficacy. On the one hand, there is a considerable effort required to obtain suitable parental monospecific antibodies with appropriate specificities, to isolate the DNA sequences encoding the binding domains, to humanize the relevant amino acid sequences and to assemble the binding do- mains. On the other hand, these manipulations may result in a reduced or even ineffective binding to the respective antigens. Even if the resu lting protein is effective in vitro, short half-lives in the human circulation, cross reactivity with normal cells expressing the target antigen or inef- fective access to the tumor cells in vivo may limit the clinical success. To further investigate this approach no- vel bispecific antibodies and their respective monospeci- fic precursors were cloned and expressed in bacteria and mammalian cells. In addition to antibody sequences of murine origin, humanized versions that are expected to be less immunogenic in patients were constructed. Here, with the exception of the ERIC1 construct, all mono- specific and bispecific constructs in combination with either bacterial or mammalian expression systems yield- ed useful amounts of apparently intact and fully functio- nal recombinant antibodies. Even though in bacteria full length antibodies cannot be properly expressed, assem- bled and folded , the fo ld ing of th e function al sing le ch ain constructs appeared more robust and they could be ex- pressed in either bacteria or mammalian cells. Binding of db2,5 antibody to PAE-FLT-1 cells that did not express CD3 or VEGFR-2 was not detected (Figure 9(c)). These results indicated bispecific binding of db2,5 antibodies to VEGFR-2+ cells and to CD3+ cells. Interestingly, the scFv domain ERIC1 could not be expressed satisfactorily in bacteria. Similarly, it could not be expressed as a bi- valent construct in either bacteria or mammalian cells. This could suggest that the folding of this domain was distorted in the scFv fragment and in all recombinant derivatives thereof that were investigated in this study. One explanation may be that the functionality of this binding site is sensitive to changes in the surrounding peptide sequences. This idea is supported by the different properties of individual constructs of D29, the humanized derivative of ERIC1. D29 could be expressed as a scF v in bacteria and binding to NCAM could be demonstrated (data not shown). Similarly, both bispecific single chain and single chain diabody UCHT1 × D29 derivatives of D29 could be expressed in mammalian cells. However, whereas the scFv D29 and the scdb bound to the NCAM antigen, only weak binding of the corresponding bispeci- fic single chain antibody could be detected (data not shown). It could therefore be envisioned, that by optimi- zation of the linker sequences or by in vitro mutagenesis and affinity selection the proper expression and binding could be restored [7,39]. The bispecific CD3 × VEGFR-2 in the single chain and diabody format could be ex- pressed in bacteria and mammalian cells and both prod- ucts showed robust binding to both antigens. Therefore, these molecules appear promising for further evaluation in cell culture cytotoxicity assays and in appropriate ani- mal tumor models. 5. ACKNOWLEDGEMENTS We thank Ms. Astrid Hans for excellent technical assistance. We kindly acknowledge the financial support of AK by a grant of the Clotten- Stiftung, Freiburg, Germany. PPM was supported by the Deutsche For- schungsgemeinschaft DFG grant SFB599. REFERENCES [1] Holliger, P. and Hudson, P.J. (2005) Engineered antibody fragments and the rise of single domains. Nature Bio- technology, 23, 1126-1136. doi:10.1038/nbt1142 [2] Buhler, P., Wolf, P., Gierschner, D., Schaber, I., Katzen- wadel, A., Schultze-Seemann, W., Wetterauer, U., Tacke, M., Swamy, M., Schamel, W.W. and Elsasser-Beile, U. (2008) A bispecific diabody directed against prostate- specific membrane antigen and CD3 induces T-cell me- diated lysis of prostate cancer cells. Cancer Immunology, Immunotherapy, 57, 43-52. doi:10.1007/s00262-007-0348-6 [3] Baeuerle, P.A. and Reinhardt, C. (2009) Bispecific T-cell engaging antibodies for cancer therapy. Cancer Research, 69, 4941-4944. doi:10.1158/0008-5472.CAN-09-0547 [4] Thakur, A. and Lum, L.G. (2010) Cancer therapy with bispecific antibodies: Clinical experience. Current Opi- nion of Molecular Therapy, 12, 340-349. Copyright © 2013 SciRes. OPEN ACCESS  A. Kopacek et al. / Advances in Bioscience and Biotechnology 4 (2013) 654-664 663 [5] Beckman, R.A., Weiner, L.M. and Davis, H.M. (2007) Antibody constructs in cancer therapy: protein engineer- ing strategies to improve exposure in solid tumors. Can- cer, 109, 170-179. doi:10.1002/cncr.22402 [6] Toleikis, L. and Frenzel, A. (2012) Cloning single-chain antibody fragments (ScFv) from hyrbidoma cells. Me- thods of Molecular Biology, 907, 59-71. [7] Hoogenboom, H.R. (2005) Selecting and screening recom- binant antibody libraries. Nature Biotechnology, 23, 1105- 1116. doi:10.1038/nbt1126 [8] Bradbury, A.R., Sidhu, S., Dübel, S. and McCafferty, J. (2011) Beyond natural antibodies: The power of in vitro display technologies. Nature Biotechnology, 29, 245-254. doi:10.1038/nbt.1791 [9] Amann, M., D’Argouges, S., Lorenczewski, G., Bris- chwein, K., Kischel, R., Lutterbuese, R., Mangold, S., Rau, D., Volkland, J., Pflanz, S., Raum, T., Munz, M., Kufer, P., Schlereth, B., Baeuerle, P.A. and Friedrich, M. (2009) Antitumor activity of an EpCAM/CD3-bispecific BiTE antibody during long-term treatment of mice in the absence of T-cell anergy and sustained cytokine release. Journal of Immunotherapy, 32, 452-464. doi:10.1097/CJI.0b013e3181a1c097 [10] Asano, R., Kawaguchi, H., Watanabe, Y., Nakanishi, T., Umetsu, M., Hayashi, H., Katayose, Y., Unno, M., Kudo, T. and Kumagai, I. (2008) Diabody-based recombinant formats of humanized IgG-like bispecific antibody with effective retargeting of lymphocytes to tumor cells. Jour- nal of Immunotherapy, 31, 752-761. doi:10.1097/CJI.0b013e3181849071 [11] Buhler, P., Molnar, E., Dopfer, E.P., Wolf, P., Gierschner, D., Wetterauer, U., Schamel, W.W. and Elsasser-Beile, U. (2009) Target-dependent T-cell activation by coligation with a PSMA x CD3 diabody induces lysis of prostate cancer cells. Journal of Therapy, 32, 565-573. [12] Kellner, C., Bruenke, J., Stieglmaier, J., Schwemmlein, M., Schwenkert, M., Singer, H., Mentz, K., Peipp, M., Lang, P., Oduncu, F., Stockmeyer, B. and Fey, G.H. (2008) A novel CD19-directed recombinant bispecific antibody de- rivative with enhanced immune effector functions for hu- man leukemic cells. Journal of Immunotherapy, 31, 871- 884. doi:10.1097/CJI.0b013e318186c8b4 [13] Lutterbuese, R., Raum, T., Kischel, R., Lutterbuese, P., Schlereth, B., Schaller, E., Mangold, S., Rau, D., Meier, P., Kiener, P.A., Mulgrew, K., Oberst, M.D., Hammond, S.A., Baeuerle, P.A. and Kufer, P. (2009) Potent control of tumor growth by CEA/CD3-bispecific single-chain an- tibody constructs that are not competitively inhibited by soluble CEA. Journal of Immunotherapy, 32, 341-352. doi:10.1097/CJI.0b013e31819b7c70 [14] Sebastian, M., Kiewe , P., Sc huette, W. , Brust , D., Peschel, C., Schneller, F., Ruhle, K.H., Nilius, G., Ewert, R., Lo- dziewski, S., Passlick, B., Sienel, W., Wiewrodt, R., Ja- ger, M., Lindhofer, H., Friccius-Quecke, H. and Schmittel, A. (2009) Treatment of malignant pleural effusion with the trifunctional antibody catumaxomab (Removab) (anti- EpCAM x Anti-CD3): results of a phase 1/2 study. Jour- nal of Immunotherapy, 32, 195-202. doi:10.1097/CJI.0b013e318195b5bb [15] Xiong, D., Xu, Y., Liu, H., Peng, H., Shao, X., Lai, Z., Fan, D., Yang, M., Han, J., Xie, Y., Yang, C. and Zhu, Z. (2002) Efficient inhibition of human B-cell lymphoma xenografts with an anti-CD20 x anti-CD3 bispecific dia- body. Cancer Letters, 1, 77, 29-39. doi:10.1016/S0304-3835(01)00758-3 [16] Jensen, M., Ernestus, K., Kemshead, J., Klehr, M., Von Bergwelt-Baildon, M.S., Schinkothe, T., Schultze, J.L. and Berthold, F. (2003) The bi-specific CD3 x NCAM antibody: A model to preactivate T cells prior to tumour cell lysis. Clinical and Experimental Immunology, 134, 253-263. doi:10.1046/j.1365-2249.2003.02300.x [17] Grosse-Hovest, L., Brandl, M., Dohlsten, M., Kalland, T., Wilmanns, W. and Jung, G. (1999) Tumor-growth inhibi- tion with bispecific antibody fragments in a syngeneic mouse melanoma model: The role of targeted T-cell co- stimulation via CD28. International Journal of Cancer, 80, 138-144. doi:10.1002/(SICI)1097-0215(19990105)80:1<138::AID- IJC25>3.0.CO;2-J [18] Jung, G., Brandl, M., Eisner, W., Fraunberger, P., Rei- fenberger, G., Schlegel, U., Wiestler, O. and Wilmanns, W. (2001) Local immunotherapy of glioma patients with a combination of 2 bispecific antibody fragments and resting autologous lymphocytes: Evidence for in situ T- cell activation and therapeutic efficacy. International Jour- nal of Cancer, 91, 225-230. doi:10.1002/1097-0215(200002)9999:9999<::AID-IJC10 38>3.3.CO;2-7 [19] Chames, P. and Baty, D. (2009) Bispecific antibodies for cancer therapy: The light at the end of the tunnel? Mabs, 1, 539-547. doi:10.4161/mabs.1.6.10015 [20] Verma, R., Boleti, E. and George, A.J. (1998) Antibody en- gineering: Comparison of bacterial, yeast, insect and mam- malian expre ssion systems. Journal of Immunological Me- thods, 216, 165-181. doi:10.1016/S0022-1759(98)00077-5 [21] Jostock, T. and Knopf, H.P. (2012) Mammalian stable ex- pression of biotherapeutics. Methods in Molecular Bio- logy, 899, 227-238. doi:10.1007/978-1-61779-921-1_15 [22] Young, C.L., Britton, Z.T. and Robinson, A.S. (2012) Re- combinant protein expression and purification: A com- prehensive review of affinity tags and microbial appli- cations. Biotechnological Journal, 7, 620-634. doi:10.1002/biot.201100155 [23] Jensen, M. and Berthold, F. (2007) Targeting the neural cell adhesion molecule in cancer. Cancer Letters, 258, 9- 21. doi:10.1016/j.canlet.2007.09.004 [24] Spannuth, W.A., Nick, A.M., Jennings, N.B., Armaiz- Pena, G.N., Mangala, L.S., Danes, C.G., Lin, Y.G., Mer- ritt, W.M., Thake r, P.H., Kamat, A.A., Han, L.Y., Tonra, J.R., Coleman, R.L., Ellis, L.M. and Sood, A.K. (2009) Functional significance of VEGFR-2 on ovarian cancer cells. International Journal of Cancer, 124, 1045-1053. doi:10.1002/ijc.24028 [25] Rapisarda, A. and Melillo, G. (2012) Role of the VEGF/ VEGFR axis in cancer biology and therapy. Advanced Cancer Research, 114, 237-267. [26] Masood, R., Cai, J., Zheng, T., Smith, D.L., Hinton, D.R. Copyright © 2013 SciRes. OPEN ACCESS  A. Kopacek et al. / Advances in Bioscience and Biotechnology 4 (2013) 654-664 Copyright © 2013 SciRes. 664 OPEN ACCESS and Gill, P.S. (2001) Vascular endothelial growth factor 8VEGF) is an autocrine growth factor for VEGF recep- tor-positive human tumors. Blood, 98, 1904-1913. doi:10.1182/blood.V98.6.1904 [27] Böldicke, T., Tesar, M., Griesel, C., Rohde, M., Grone, H. J., Waltenberger, J., Kollet, O., Lapidot, T., Yayon, A. and Weich, H. (2001) Anti-VEGFR-2 scFvs for cell iso- lation. Single-chain antibodies recognizing the human va- scular endothelial growth factor receptor-2 (VEGFR-2/ flk-1) on the surface of primary endothelial cells and pre- selected CD34+ cells from cord blood. Stem Cells, 19, 24-36. doi:10.1634/stemcells.19-1-24 [28] Frankel, A.E., Zuckero, S.L., Mankin, A.A., Grable, M., Mitchell, K., Lee, Y.J., Neville, D.M. and Woo, J.H. (2009) Anti-CD3 recombinant diphtheria immunotoxin therapy of cutaneous T cell lymphoma. Current Drug Targets, 10, 104-109. doi:10.2174/138945009787354539 [29] Grosse-Hovest, L., Hartlapp, I., Marwan, W., Brem, G., Rammensee, H. and Jung, G. (2003) A recombinant bis- pecific single-chain antibody induces targeted, supra-ago- nistic CD28-stimulation and tumor cell killing. European Journal of Immunology, 33, 1334-1340. doi:10.1002/eji.200323322 [30] Böldicke, T., Somplatzki, S., Sergeev, G. and Mueller, P. P. (2012) Functional inhibition of transitory proteins by intrabody-mediated retention in the endoplasmatic reti- culum. Methods, 56, 338-350. doi:10.1016/j.ymeth.2011.10.008 [31] Minsky, A., Summers, R.G. and Knowles, J.R. (1986) Se- cretion of beta-lactamase into the periplasm of Escheri- chia coli: Evidence for a distinct release step associated with a conformational change. Proceedings of National Academy Science of the USA, 83, 4180-4184. doi:10.1073/pnas.83.12.4180 [32] Waltenberger, J., Claesson-Welsh, L., Siegbahn, A., Shi- buya, M. and Heldin, C. H. (1994) Different signal trans- duction properties of KDR and Flt1, two receptors for va- scular endothelial growth factor. Journal of Biological Chemistry, 269, 26988-26995. [33] Wigler, M., Sweet, R., Sim, G.K., Wold, B., Pellicer, A., Lacy, E., Maniatis, T., Silverstein, S. and Axel, R. (1979) Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell, 16, 777-785. doi:10.1016/0092-8674(79)90093-X [34] Smith, P.K., Krohn, R.I., Hermanson, G.T., Mallia, A.K., Gartner, F.H., Provenzano, M.D., Fujimoto, E.K., Goeke, N.M., Olson, B.J. and Klenk, D.C. (1985) Measurement of protein using bicinchoninic acid. Analytical Bioche- mistry, 150, 76-85. doi:10.1016/0003-2697(85)90442-7 [35] Burnette, W.N. (1981) “Western blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate-poly- acrylamide gels to unmodified nitrocellulose and radio- graphic detection with antibody and radioiodinated pro- tein A. Analytical Biochemistry, 112, 195-203. doi:10.1016/0003-2697(81)90281-5 [36] Kung, P., Goldstein, G., Reinherz, E.L. and Schlossman, S.F. (1979) Monoclonal antibo dies defining distinctive hu- man T cell surface antigens. Science, 206, 347-349. doi:10.1126/science.314668 [37] Bourne, S.P., Patel, K., Walsh, F., Popham, C.J., Coak- ham, H.B. and Kemshead, J.T. (1991) A monoclonal anti- body (ERIC-1), raised against retinoblastoma, that recog- nizes the neural cell adhesion molecule (NCAM) express- ed on brain and tumours arising from the neuroectoderm. Journal of Neurooncology, 10, 111-119. doi:10.1007/BF00146871 [38] Whittington, H.A., Hancoc k, J. and Kemshead, J. T. (2001) Generation of a humanised single chain Fv (Scfv) derived from the monoclonal Eric-1 recognising the human neural cell adhesion molecule. Medical and Pediatric Oncology, 36, 243-246. doi:10.1002/1096-911X(20010101)36:1<243::AID-MPO 1060>3.0.CO;2-5 [39] Pluckthun, A. (2012) Ribosome display: A perspective. Methods in Molecular Biology, 805, 3-28. doi:10.1007/978-1-61779-379-0_1
|