 Open Journal of Respiratory Diseases, 2013, 3, 52-62 http://dx.doi.org/10.4236/ojrd.2013.32009 Published Online May 2013 (http://www.scirp.org/journal/ojrd) Genetic Implications in COPD. The Current Knowledge Ioannis Sotiriou, Demosthenes Makris Intensive Care Unit, University Hospital of Larisa, Larisa, Greece Email: giasotiriou@yahoo.gr Received December 28, 2012; revised January 30, 2013; accepted February 10, 2013 Copyright © 2013 Ioannis Sotiriou, Demosthenes Makris. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Chronic Obstructive Pulmonary Disease (COPD) is a multifactorial disease in the pathogenesis of which contributes a variety of causative factors including genetic and environmental ones. They may also be interactions of genetic suscep- tibilities and environmental influences. Towards to that in the pathogenesis of the disease except smoking, it seems to have a great impact the genetic predisposition of the individuals suffering from that serious progressive disease. Re- garding to these observations and findings very interesting studies have been conducted in order to elucidate the impli- cations of different genes, and their polymorphisms in disease aetiology. This is a review which elucidates the impact of genetic susceptibility in COPD. Keywords: Association Studies; Chronic Obstructive Pulmonary Disease; Genetics; Genes; Polymorphisms 1. Introduction Chronic Obstructive Pulmonary Disease (COPD) is a progressive disease which is characterized by alteration in normal lung architecture and by a non-fully reversible airflow limitation. The airflow limitation is usually pro- gressive and associated with an inflammatory response of the lungs to noxious particles or gases. Chronic inflam- mation causes structural changes and narrowing of the airways. Moreover, it causes obstruction of the small airways (obstructive bronchiolitis) leading to the secon- dary destruction of the lung parenchyma (emphysema) [1]. Although cigarette smoking is the major environ- mental risk factor for the development of chronic ob- structive pulmonary disease (COPD), the fact that only 20% of the smokers develop the disease, suggests that genetic factors influence COPD susceptibility. Firstly, there is substantial variability in the development of chronic airflow obstruction among cigarette smokers [2, 3]. Secondly, studies conducted among families, mem- bers of which were free from lung diseases, have demon- strated familial accumulation of spirometric measures, suggesting that genetic factors influence to varying de- grees the integrity of the pulmonary function [4,5]. Addi- tionally familial studies with members suffering from COPD, demonstrated serious disturbances of pulmonary function tests (PFT’s), regarding the degree of airway obstruction, in first-degree relatives of patients with COPD compared with control subjects, suggesting the impact of genetic influences in COPD [6,7]. Last but not least, severe A1-antitrypsin deficiency is a proven ge- netic factor which causes pulmonary emphysema in a small group of patients with chronic obstructive pulmo- nary disease. 2. Evolutionary Technologies In the era of genetic analysis and conduction of studies, in order to elucidate and simultaneously to confirm a plausible association between genes and the disease pathophysiology, arises the substantial need to develop molecular methods in order to facilitate, at laboratory scale the genetic aspect of the disease. With regard to that the current applied methods and genome technolo- gies include the polymerase chain reaction (PCR) and microarreys. 2.1. Applicable Methods in Genetic Analyses Polymerase Chain Reaction (PCR) PCR presents an important development in DNA tech- nology that has enormous implications for basic research and genetic diagnostics. This method allows the multi- plication of selected DNA sequences (with a relatively simple enzyme reaction) to produce a large number of copies for a limited time. This allows the analysis of a specific piece of DNA without prior cloning, which sig- nificantly increases the speed of analysis. It is necessary C opyright © 2013 SciRes. OJRD  I. SOTIRIOU, D. MAKRIS 53 to synthesize chemically two small oligonucleotide prim- ers (usually 15 - 30 nucleotides in length), based on the known sequence of the region. Because of the fact that the primers are presented in large excess, every launcher will find a sequence complementary to the overall mix- ture of DNA and will be hybridised in situ. Subsequently, it is also added an enzyme that can withstand high tem- peratures up to 95˚C, called Taq polymerase. This en- zyme synthesizes a DNA chain by the end of the primer using DNA as the original matrix. This whole process can be done in an easy way round, with each cycle last- ing only a few minutes. 30 - 40 cycles can be performed without having to add new enzymes or primers. The re- sult is an exponential increase of the amount of DNA in the region between the two primers. The practical effect is that it can begin the experimental phase in nanograms amounts of DNA, PCR can take place, and then the sam- ple is analyzed on agarose gel to produce a specific band, which corresponds exactly to the distance between the primers. The primers are added to double-stranded DNA, which is then denaturated to monoclonal DNA through a thermal procedure. As a technique the major advantages are: quick (speed) and easy to perform, DNA cloning by PCR can be performed in a few hours, using relatively unsophisticated equipment, sensitive, PCR is capable to amplify sequences from minute amounts of target DNA, even the DNA from a single cell and robust, PCR permits amplification of specific sequences from biological sub- strates in which the DNA is badly degraded or embedded in a medium from which conventional DNA isolation is problematic. Major disadvantages are extremely liable to contamination, high degree of operator skill required, not easy to set up a quantitative assay, and finally a positive result may be difficult to interpret. 2.2. DNA Microarreys DNA microarrays can be used to detect diversities in the levels of gene expression in different populations of cells on a genome-wide level. DNA microarrays rely on the hybridization properties of nucleic acids to monitor DNA or RNA abundance on a genomic scale in different types of cells. Since the initial application of this a very prom- ising technology in the 90’s faced a wide scope of use in almost all the biomedical fields. A DNA microarray is a collection of microscopic DNA spots attached to a solid surface. Microarrays could be applied as a method to measure diversities in expression levels, to point single nucleotide polymorphisms (SNPs) or to genotype or re- sequence mutant genomes. It is well established the no- tion that the pathophysiology of COPD extends beyond the scope of the cigarette smoke as the cornerstone of the inflammatory response unlocks in the lung cell substrates. Thus, numerous studies focused on the application of the microarreys technology to elucidate the complexity of pathological pathways of this progressive disease. With regard to this issue a study conducted and the results drew out a quite wide scope of diversity which could be address to the variability of the gene expression among the dif- ferent individuals [8]. Despite its role as a useful tool in genome analysis it is engaged with advantages and draw- backs. Advantages of DNA microarray tests include high throughput (lots of information with one test), and good coverage of the genome with the chips that have larger numbers of test spots. Disadvantages include the incom- plete coverage, which can lead to false normal results, and the ability to test only for unbalanced rearrangements (duplications and deletions), and not balanced transloca- tions or inversions. However being high throughput and cost effective most of the elements for this technology, it still suffers from lack of perfect processing methods and acceptable sensitivity as much as real time PCR. 2.3. Microsatellites Microsatellites also widely known as Simple Sequence Repeats (SSRs) or short tandem repeats (STRs), are repeating sequences of 2 - 6 base pairs of DNA [9]. They are classified in simple and composite ones. Sim- ple microsatellites contain only one kind of repeat se- quences and composite contains more than one type of repeats. Microsatellites present advantages as markers. They are abundant, high polymorphic and evenly dis- tributed. They are used as molecular markers in genet- ics and other studies. They can also be used to study gene duplication or deletion. Microsatellites are also predictors of SNP density as regions of thousands of nucleotides flanking microsatellites. They have an in- creased or decreased density of SNPs depending on the microsatellite sequence [10]. As a molecular technol- ogy they present a wide variability. Their variability is due to a higher rate of mutation compared to other neutral loci of DNA. These high amplitudes of muta- tion can be explained more frequently by slipped strand mispairing (slippage) during DNA replication on a sin- gle DNA strand. Microsatellites are a proven molecular method which present a high level of versatility as a plausible marker in pathobiology of COPD, but are not free of limitations. For their application previous ge- netic information is necessary, a great amount of up- front work demands, and last but not least drawbacks could be revealed regarding the PCR of microsatellites. With regard to the role of microsatellites as a marker presenting a crucial impact in the clinical course of COPD, there is evidence drawn out from studies. The results enforced the notion that the microsatellite DNA instability (MSI) is strongly associated with increased exacerbation frequency in COPD patients, and reveals that a somatic mutation may hold a key role in the pathobiology of the disease [11,12]. Copyright © 2013 SciRes. OJRD  I. SOTIRIOU, D. MAKRIS 54 3. Data on the Genetic Basis of the COPD The genetic basis of COPD has been investigated using association studies and candidate gene polymorphisms, which may have an important role in pathogenesis of that multifactorial disease. Until now they have studied sev- eral genes that may be associated with the development of COPD. Genes include proteases, anti-proteases, anti- oxidants, xenobiotic metabolizing enzymes, and also in- flammatory metabolites. The core of the numerous inves- tigations and researches is to shed more light upon the diversities of the genetic susceptibility that occur among different ethnic groups. It is crucial to reveal and to asso- ciate the potentially clinically relevant observations for development the optimal therapies of tomorrow today. 4. Genes Impact in COPD The Human Genome Project, the technological advances in single nucleotide polymorphism (SNP) genotyping, and the DNA sequencing have enriched our research arsenal of useful tools for highlighting potential candi- date genes in the complex pathogenesis of the disease. Considering the diversity of diseases such as chronic obstructive pulmonary disease, which are influenced by various genetic factors, environmental factors, as well as interactions between genes and between genes and envi- ronment, it is imperative to carry out more studies in or- der to illuminate the pathophysiology of the disease. The most commonly applied approach is to select a candidate gene from known or suspected COPD pathophysiology, and to test genetic variants within that gene for associa- tion to COPD. Candidate genes selected from gene asso- ciation studies. A large number of genetic association studies conducted in order to evaluate the distribution of variants within candidate genes selected based on COPD pathophysiology (Table 1). 4.1. A-1-Antithrypsin in Genetics of COPD The genetic factor that has been extensively studied is the lack of an A1 antitrypsin glycoprotein belonging to the group of protease inhibitors of serine (protease inhibitor, PI or SEPRINA 1) encoding a1AT. It is synthesized in the liver and then it moves into the systemic circulation. Through the inhibition of neutrophil elastase it protects the lung parenchyma from destruction. The normal M allele is associated with normal levels a1AT. The S allele is associated with mild lowering a1AT. The G allele is associated with significant lowering a1AT and occurs with a frequency >1% among Caucasians. This rare autosomal recessive disorder occurs at a higher rate in Northern European. People with two G alleles or one Z and one non-viable allele are characterized as PiZ which is the most common form of severe a1AT. Sandford et al., in the Lung Health Study showed that the PiZ allele a1AT gene in heterozygous state is the largest proven genetic factor for the development of pulmonary emphy- sema [13]. In order to identify a possible correlation of descent affinity of the airflow and whether it correlates with the presence of genetic predisposition affecting a possible loci in chromosomes, the Framingham study assessed 1578 members of 330 families (linkage studies), showed that loci affecting peak values of FVC and FEV1 are located on chromosomes 21, 4 and 6 [14]. These evi- dences enforce the hypothesis that genetic variability might be involved in the clinical diverse presentation of the disease among different individuals or populations. 4.2. Genes and Oxidative Stress Pivotal role in pathophysiology of COPD has the oxida- tive stress. Towards to this Connett et al. conducted a study to highlight a possible genetic predisposition in antioxidant gene polymorphisms and susceptibility to a rapid decline in lung function among smokers. The in- vestigated polymorphisms were polymorphisms of anti- oxidant enzyme glutathione S-transferase (GST) and in particular GSTM1, GSTT1 and GSTP1. The study re- vealed that the combination of family history of COPD with the presence of genotype GSTP1 105ILe/ILe is as- sociated with the rapid decline in lung function [15]. The protein that encodes the Microsomal epoxide hydrolase 1 (mEPHX1) gene plays an important antioxidant role in the lungs. There is in vitro evidence that the polymor- phism in the gene promoter region reduces the upregula- tion of heme oxygenase-1 in response to reactive oxygen species in cigarette smoke. Also, the combination of family history of COPD and the presence in homozygous state of the haplotype His113/His139 of the microsomal epoxide hydrolase (mEPHX) gene is associated with ac- celerated decline in lung function in smokers. At last but not least, the HMOX 1 gene (GT) n alleles revealed no association with rapidly decline lung function [15]. The protective role of the EPHX1 Tyr113His polymorphism in the pathogenetic pathways of COPD has been empha- sized by the case-control study, conducted by Brogger J. et al. [16]. Hu G. et al. in a study reviewing meta analy- sis results concerning the association of antioxidant genes in pathogenesis of COPD, concluded that EPHX1 113 and EPHX1 139 are both genetically relative factors for susceptibility of COPD in Asian population, espe- cially the fast activity phenotype EPHX1.This observa- tion did not appeared in Caucasians who are more sus- ceptible in the risk for developing COPD concerning the slow activity phenotype of the EPHX 1 in contrast to the Asians. These facts underline that the susceptibility in COPD depends upon the diverse ethnic gene-environ- ment interactions [17]. Analyses the same aspect of the oxidative stress and the plausible role of the EPHX 1 as a protective mechanism against it Lakhdar R. et al. in an Copyright © 2013 SciRes. OJRD 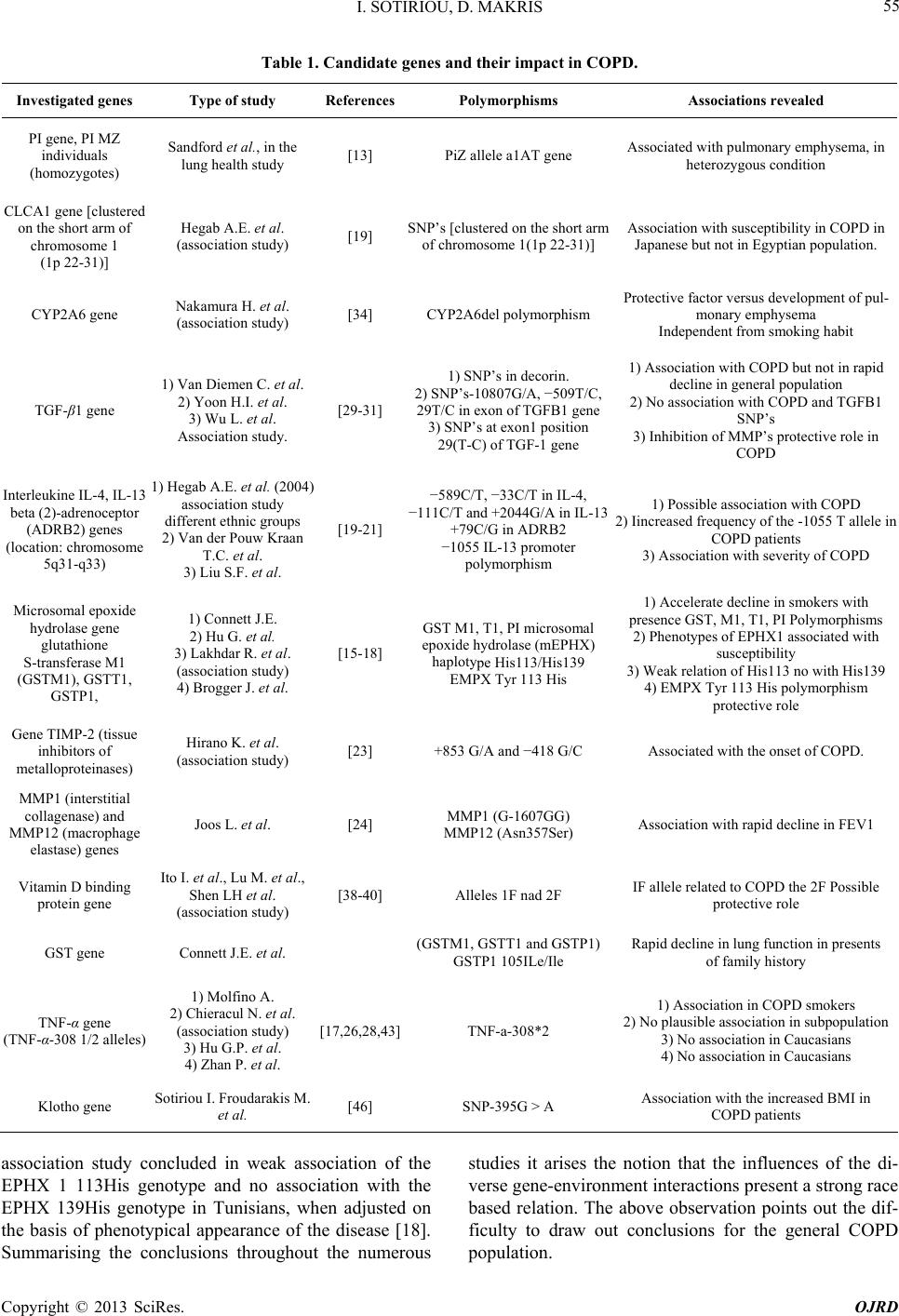 I. SOTIRIOU, D. MAKRIS Copyright © 2013 SciRes. OJRD 55 Table 1. Candidate genes and their impact in COPD. Investigated genes Type of study ReferencesPolymorphisms Associations revealed PI gene, PI MZ individuals (homozygotes) Sandford et al., in the lung health study [13] PiZ allele a1AT gene Associated with pulmonary emphysema, in heterozygous condition CLCA1 gene [clustered on the short arm of chromosome 1 (1p 22-31)] Hegab A.E. et al. (association study) [19] SNP’s [clustered on the short arm of chromosome 1(1p 22-31)] Association with susceptibility in COPD in Japanese but not in Egyptian population. CYP2A6 gene Nakamura H. et al. (association study) [34] CYP2A6del polymorphism Protective factor versus development of pul- monary emphysema Independent from smoking habit TGF-β1 gene 1) Van Diemen C. et al. 2) Yoon H.I. et al. 3) Wu L. et al. Association study. [29-31] 1) SNP’s in decorin. 2) SNP’s-10807G/A, −509T/C, 29T/C in exon of TGFB1 gene 3) SNP’s at exon1 position 29(T-C) of TGF-1 gene 1) Association with COPD but not in rapid decline in general population 2) No association with COPD and TGFB1 SNP’s 3) Inhibition of MMP’s protective role in COPD Interleukine IL-4, IL-13 beta (2)-adrenoceptor (ADRB2) genes (location: chromosome 5q31-q33) 1) Hegab A.E. et al. (2004) association study different ethnic groups 2) Van der Pouw Kraan T.C. et al. 3) Liu S.F. et al. [19-21] −589C/T, −33C/T in IL-4, −111C/T and +2044G/A in IL-13 +79C/G in ADRB2 −1055 IL-13 promoter polymorphism 1) Possible association with COPD 2) Iincreased frequency of the -1055 T allele in COPD patients 3) Association with severity of COPD Microsomal epoxide hydrolase gene glutathione S-transferase M1 (GSTM1), GSTT1, GSTP1, 1) Connett J.E. 2) Hu G. et al. 3) Lakhdar R. et al. (association study) 4) Brogger J. et al. [15-18] GST M1, T1, PI microsomal epoxide hydrolase (mEPHX) haplotype His113/His139 EMPX Tyr 113 His 1) Accelerate decline in smokers with presence GST, M1, T1, PI Polymorphisms 2) Phenotypes of EPHX1 associated with susceptibility 3) Weak relation of His113 no with His139 4) EMPX Tyr 113 His polymorphism protective role Gene TIMP-2 (tissue inhibitors of metalloproteinases) Hirano K. et al. (association study) [23] +853 G/A and −418 G/C Associated with the onset of COPD. MMP1 (interstitial collagenase) and MMP12 (macrophage elastase) genes Joos L. et al. [24] MMP1 (G-1607GG) MMP12 (Asn357Ser) Association with rapid decline in FEV1 Vitamin D binding protein gene Ito I. et al., Lu M. et al., Shen LH et al. (association study) [38-40] Alleles 1F nad 2F IF allele related to COPD the 2F Possible protective role GST gene Connett J.E. et al. (GSTM1, GSTT1 and GSTP1) GSTP1 105ILe/Ile Rapid decline in lung function in presents of family history TNF-α gene (TNF-α-308 1/2 alleles) 1) Μolfino A. 2) Chieracul N. et al. (association study) 3) Hu G.P. et al. 4) Zhan P. et al. [17,26,28,43]TNF-a-308*2 1) Association in COPD smokers 2) No plausible association in subpopulation 3) No association in Caucasians 4) No association in Caucasians Klotho gene Sotiriou I. Froudarakis M. et al. [46] SNP-395G > A Association with the increased BMI in COPD patients association study concluded in weak association of the EPHX 1 113His genotype and no association with the EPHX 139His genotype in Tunisians, when adjusted on the basis of phenotypical appearance of the disease [18]. Summarising the conclusions throughout the numerous studies it arises the notion that the influences of the di- verse gene-environment interactions present a strong race based relation. The above observation points out the dif- ficulty to draw out conclusions for the general COPD population.  I. SOTIRIOU, D. MAKRIS 56 4.3. Interleukins in COPD Genetics Interleukins are a group of cytokines (secreted pro- teins/signaling molecules) expressed by leucocytes. They promote the development and differentiation of T, B, and haematopoietic cells. IL-13 is a pleiotropic cytokine that may be important in the regulation of the inflammatory and immune responses. It inhibits inflammatory cytokine production and synergises with IL-2 in regulating inter- feron-gamma synthesis. The sequences of IL-4 and IL-13 are distantly related. Interleukin (IL)-4, IL-13 and beta-2- adrenoceptor (ARDB2) are involved with the hyperre- sponsiveness of the airways that by itself constitutes a danger factor for COPD. A study that has been carried out in this field included two different ethnic group populations, has proven that the polymorphism ARDB2 + 79 C/G is probably involved with the pathogenesis of COPD [19]. Investigating the impact of the IL-13 in the pathogenesis of COPD a case control study concluded that the polymorphisms in promotor of the gene IL-13 may increase the risk of COPD [20]. The same conclu- sion has been reached out from a case-control study conducted in Taiwanese population [21]. The ADRB2 are specific receptors on the cell membrane and simulta- neously therapeutic target in the treatment of COPD. To elucidate the role of these receptors in different geno- typic expression of the COPD patients a case control study concluded that the Gly16 polymorphism of beta-2- adrenoceptor may increase susceptibility to develop COPD, and Gln27 beta2 adrenoceptor polymorphism may be associated with the severity of COPD in Chinese population [22]. Current evidence comes from Asian populations only, and the necessity for further investiga- tion is crucial in order to reach out conclusive results for the COPD patients as a whole. 4.4. Protease/Antiprotease Imbalance. Metalloproteinases in COPD Another crucial factor in the pathophysiology and patho- genesis of COPD is the metalloproteinases. The metallo- proteinases are endopeptidases. Their catalytic center is stabilized by zinc ions (Zn) and calcium (Ca). They have the capacity to degrade all proteins and proteoglycans of the extracellular matrix. Their action is inhibited by natural tissue inhibitors of metalloproteinases (TIMP’s). The imbalance between proteases-antiproteases represents another important field in the pathogenesis of the COPD. Polymorphisms of the gene TIMP-2 (tissue inhibitors of metalloproteinases) +853 GIA and −418 G/C, in a study conducted among 88 patients with COPD and 40 healthy, was found to be associated with the onset of COPD [23]. On the other hand patients with a rapid decline in FEV1 have polymorphisms in the genes MMP1 (G-1607GG) and MMP12 (Asn357Ser) [24]. The results are the evidence of susceptibility of the lung parenchyma in cigarette smoke among specific population. The in- vestigated polymorphisms suggest high affinity to the progress of the pulmonary functional decline. However, further investigation must be conducted in order to elucidate the susceptibility of this evidence per se or in correlation with other factors which influence the natural history of the disease. TNF-a It is generally accepted that COPD is associated with an abnormal inflammatory response. This extends beyond the lung to systemic manifestations. The inflammatory process having a leading role in the pathophysiology of COPD, lists a variety of inflammatory mediators. TNF-a mediated inflammation is thought to play a key role in both the respiratory and systemic features of COPD. The TNF-308 polymorphism is a biallelic restriction fragment length polymorphism (RFLP) originally described by Wilson [25]. The alleles of TNF-a-308-1 and TNF-a- 308-2, in section 308 of the promotor of TNF-a gene are related, in part, with extensive emphysema like lesions in smokers, as they are demonstrated by a study in Japanese patients. On the other hand, in the study of Chieracul N. et al. no association was revealed among the Thai popu- lation [26]. A similar result has been reached in the study of Hu GP et al. who demonstrated that the TNF2 allele is a risk factor for COPD among Asians but no association of TNFa-308 polymorphism and COPD was revealed in Caucasians [27]. The same results reached out in a meta-analysis of Zhan P. et al. [28]. The studies that have been conducted to this direction had ambiguous results. A possible explanation for this is the genetic diversity of the studied populations and the various interactions of exogenous causative factors, and the environmental in- fluences on gene expression. 4.5. The Cytokine TGF-β1 The TGF-β1 (transforming growth factor-beta1) is a cy- tokine with diverse actions in cell proliferation, differen- tiation and inflammation, simultaneously. Plus it is an- other potential candidate gene which appears to have a pivotal role in the pathogenesis of COPD. Wu L et al. conducted an association study carried out in order to derive a relationship between TGF-β1 gene and COPD. They concluded that this gene could conceivably act to prevent the degradation of elastin by inhibiting the ex- pression of matrix metalloproteases. It is also possible that TGF-β1 may acts to promote the synthesis of elastin and, as a result, it can have a role in repairing the loss of elastic fibres that occurs as a result of smoking. From this perspective it may be associated with the pathogenesis of COPD as a factor against the inflammation pathways in the lung tissue [29]. In addition Van Diemen et al. have revealed that SNP’s in decorin alone or in combination Copyright © 2013 SciRes. OJRD  I. SOTIRIOU, D. MAKRIS 57 with the TGF β-1 does not disrupt the balance of those genes expression. Also TGF-β1 is associated with COPD but not with severe decline in FEV1 in general popula- tion [30]. On the other hand Yoon HI et al. concluded that there is no significant association between the SNP’s −10807G/A, −509T/C, 29T/C in exon of TGFB1 gene, and the presence of COPD [31]. Despite this current evi- dence from previous studies results are inconclusive. These evidence based observations once more underline the plausible impact of the genetic diversity in the ex- pression for the pathogenesis of the disease combined with the degree in gene-by-gene and gene-environment interaction. 4.6. CFTR and COPD The role of the CFTR in the pathophysiology of COPD still remains a future field to elucidate. In patients with brochitic predominant phenotype, known as “blue bloat- ers”, has not been clarified completely whether the muta- tions of transmembrane regulator transfer of cystic fibro- sis (CFTR), are associated with COPD. However a link has been reported on chromosome 22. The study that implicates the impact that CFTR-dependent ceramide signalling may have in lung injury and emphysema has been conducted by Bodas et al. [32]. Ceramides is a fam- ily of lipid molecules. A ceramide is composed of sphin- gosine and a fatty acid. Ceramides are found in high concentrations within the cells membrane. They are one of the component lipids that make up sphingomyelin, one of the major lipids in the lipid bilayer. It is unclear how membrane-CFTR may modulate ceramide signaling in lung injury and emphysema. Cftr(+/+) and Cftr(−/−) mice and cells were used to evaluate the CFTR-depend- ent ceramide signaling in lung injury. The results indicate that inhibition of de novo ceramide synthesis may be effective in disease states with low CFTR expression like emphysema and chronic lung injury, but not in complete absence of lipid-raft CFTR as in ΔF508-cystic fibrosis. The data demonstrate the critical role of membrane-lo- calized CFTR in regulating ceramide accumulation and inflammatory signaling in lung injury and emphysema [33]. 4.7. The CYP2A6 Genotypes and CYP2A6del Polymorphism CYP2A6 is another molecular structure. It is member of the enzyme system cytochrome P450, which metabolizes nicotine to cotinine. It appears to have important role in the pathogenesis of COPD. The polymorphism CYP2A6del acts as a protective factor against the development of pulmonary emphysema, regardless of smoking habit, as was highlighted by the study of Nakamura H et al. [34]. Despite the current evidence, the results are confounding and the avoidance of such factor depends strongly in the well patients’ classification and the stratification of the severity of the pulmonary tissue injuries. 4.8. Vitamin D Binding Protein (VDBP) Scientific interest has in recent years the emergence of a possible association of polymorphisms VDBP (vitamin d-binding protein) in the pathophysiology of COPD and particularly in pulmonary emphysema. Towards to this direction association studies have been conducted to achieve this goal. The VDBP, also known as Gc-globulin, is a protein with molecular weight of 55 kda, which is produced in the liver and connects the extracellular actin to endotoxin with the addition of vitamin D. Is a precur- sor of macrophage activating factor (maf) and enhances the neutrophil chemotactic properties of c5 derived pep- tides. This function is prevented by neutrophil elastase inhibitors, suggesting a relationship between the prote- ase-antiprotease pathway and inflammation [35-37]. Subsequently, Ito et al. performed a case control study, in which they found that the prevalence of the Gc 1F homozygotes was significantly greater in patients with copd than among controls, yielding a significant associa- tion of the studied polymorphism with the susceptibility of the disease and with the severity as well [38]. In the case-control study Lu M et al. concluded that allele 1F is one of the risk factors for COPD associated with smok- ing. 1F homozygote may increase the risk of copd. On the other hand, allele 2 might have a protective effect on pathogenesis of COPD [39]. Shen LH et al. revealed similar results regarding to the role of F1 and F2 alleles, found significantly higher proportion of VDBP-1F ho- mozygosity in COPD patients, while the frequency of VDBP-2 homozygosity was significantly lower in COPD patients, which seemed to suggest that VDBP-2 homo- zygocity provided a protective effect. The study con- ducted in a limited genetic uniform chinese han popula- tion, due to lower incidence of the 1F haplotype among the caucasians [40]. These data are consistent with stud- ies conducted in the same population regarding to the different polymorphisms, a fact that underlines the high affinity of gene-by gene and gene-by-environment inter- actions in the pathobiological progress of the disease. 4.9. Klotho Gene Kuro-o et al. cloned a mouse gene from a transgenic mouse model with several age-related disorders. This new gene named Klotho, is involved in the suppression of different aging phenotypes [41]. The Klotho gene is or- chestrated by 5 exons (those of the transcribed regions of a gene present in the mature mRNA and usually contain coding information) and lists more than 50 kb on chro- mosome 13q12. Spans approximately 50 kb of genomic Copyright © 2013 SciRes. OJRD  I. SOTIRIOU, D. MAKRIS 58 cDNA, encodes both membrane and secreted forms, en- codes type I membrane protein not a helicase (Figure 1). Defect in the expression of the Klotho gene in mice mod- els, led to the appearance of a syndrome resembling to the human pre-ageing, emphysema, reduction of life ex- pectancy, subfertility, arteriosclerosis, skin atrophy and osteoporosis. The (kl−/−) show short life expectancy be- ginning of pulmonary emphysema that resembles hu- man pulmonary emphysema both histologically and functionally [42]. Studies conducted in humans have shown already the correlation between gene polymor- phisms and osteoporosis, and coronary heart disease (CAD: Coronary Artery Disease), the blood pressure, stroke episode and longevity. Mice that were deficient (lack) of Klotho protein showed extensive and progres- sive atherosclerosis in combination with medial calcifi- cation of the aorta as well as thickening of the inner layer of mid-range, arteries [43]. Study that analyzed the func- tional KL-VS allele of the Klotho, gene concluded that it affects the duration of life. The homozygous in this allele subjects showed a decline in survival rate of 2.6% in the age of ≥65 years. Also, smoking carriers of this allele had increased risk of occult coronary artery disease, which reinforces the strong interaction of gene-envi- ronment. Finally, in this study the carriers of the allele with normal blood pressure had higher risk of latent coronary heart disease than the hypertensive patients not carrying the allele [44]. Another study analyzed the SNPs (single nucleotide polymorphisms) of the gene Klotho for any correlation with bone density. The study included population of 1187 white women and 215 postmeno- pausal Japanese women. The result was that the popula- tion of white women two polymorphisms, one in the promotor of the G-395A and the other in exon 4, C-1818T, and their haplotypes, as well, were signifi- cantly associated with bone mineral density in elderly postmenopausal females (≥65 years), but not in younger premenopausal or postmenopausal female [45]. A recent study based on the histological changes in animals, hy- pothesised that genetic variations likely to regulate the levels of expression of the Klotho gene might be involved in the manifestation of COPD. Thus, the aim of this study was to assess the detect and distribution of −395G > A variant alleles between patients with COPD and their possible relation with clinical parameters such as severity of the disease, lung function, age, or BMI. The poly- Figure 1. The Klotho gene. morphism −395G > A of the Klotho gene was detected in the COPD patients. No association was found, except with the BMI, with the parameters studied such as lung function, the GOLD stage, the age and the severity of smoking. Considering that Klotho gene is a metabolic gene, question is raised whether the mechanism of em- physema induction of the Klotho deficient gene is through a possible metabolic path in patients with COPD [46]. 5. Gene Therapies and Pharmacogenetics in COPD Considering the diversity of conclusion and final end- points from numerous studies to date, with regard to ge- netic susceptibility as a plausible factor in the develop- ment and progression of COPD, a need for a comment in gene therapy for the disease is raised. The subject de- serves a de profundum mention, and the analysis of such promising field of research needs a whole review per se. for those reasons the review regarding this subject will be brief in nature. Pharmacogenetics in COPD is a very promising field of research waiting to be strongly eluci- date. Several studies have been conducted towards to that direction. They’ve launched from the mostly prescribed therapy of COPD the short-acting β-agonists (SABA’s), the long-acting β-agonist (LABA’s) and anticholinergic agents. They focused on different genes with possible association with COPD such as beta-2-adrenoceptor (ardb2), genotyping single nucleotide polymorphisms (SNP’s). Two of the most commonly coding variants arg16gly and gln27glu, were examined. In Japanese population with diverse severity stage of the disease was found decreased bronchodilator effect in alleles [47]. But in a study conducted in caucasians the results were in contrary indicating no association of both alleles and bronchodilator response [48]. Another trial included asian population, investigated the results of the combina- tion of different pharmaceutical agents concluded in no association in bronchodilator effects or a significant change functional parameters at the end of the treatment period [49]. Another study concluded in increased pul- monary functional parameters with regard to the arg16gly haplotype, after treatment with tiotropium [50]. Despite the current evidence of previous studies results are in- conclusive, and the diversity of the studied population regarding the race and the disease severity make really difficult the effort to draw out firm conclusions. Gene therapy is a fundamentally different treatment approach based on intervention techniques in gene expression in pathological tissues. The aim is to transfer nucleic acid (DNA or RNA) with specific vectors within the cell to terminate the pathogenetic process. The development of this technology is being tested. The strategies are: a nor- Copyright © 2013 SciRes. OJRD  I. SOTIRIOU, D. MAKRIS 59 mal gene into the genome randomly enters and replaces a non-functional gene (replacement gt) or interferes with the cell cycle of the cell. Intervention in a gene expres- sion: induction or repression (knockout gt) by introduc- ing dominant gene or antisence on or small interfering RNA’s. The introduction of genes converts the prodrug to the toxic metabolites (suicide gt). The specific immune responses (immunomodulatory gt) stimulation could be achieved by vaccination with genetically modified cells. The main concern for this therapy strategy is the suffi- ciency of the diverse vectors, adenovirus vectors, retro- virus or lentivirus vectors and even aa vectors to reach and activate the cellular flora of the lung tissue. Thus the route of the application of those transfer molecules either through airways or intravenously even the combination of both may be of advance for targeting the pulmonary parenchyma. However the strategy is yet in its infancy regarding chronic obstructive lung disease. 6. Discussion Chronic obstructive pulmonary disease (COPD) is, with- out doubt, a disease which employs and will employ in more extensive profile the global scientific community in the coming years. As the harmful exogenous factors that have a serious impact on the natural course of disease are multiplied, the more urgent is the need for more de pro- fundum research focusing on the causative factors of the disease beyond the cornerstone agent, the cigarette smoke. The fact that only a small fraction of smokers develop the disease (about 15%), suggests that except environmental factors, genetic susceptibility also have a possible important role in the pathophysiology and pathogenesis of COPD [51]. Numerous investigations have been conducted which were aimed to highlight the importance of genetic aspect of this dismal disease. The last 15 - 20 years have been studied various genes and polymorphisms with a common denominator to elucidate their possible association with the disease. Towards this direction the more extensively studied and proven ge- netic factor is the a-1-antithrypcin deficiency [13,14]. The accumulating evidence of these data guided physi- cians to develop more efficient diagnostic algorithms and to provide optimal therapeutic procedures. But still the treatment tools, available to date, are insufficient to per- form panacea of the disease. Studies that have been im- plicated the oxidative stress as a crucial impact factor in lung inflammatory pathways and COPD concluded that several antioxidant genes such as gst (gstm1, gstt1 and gstp1), microsomal epoxide hydrolase 1 (mephx1) [15-18] and Klotho gene [52] exert an important role regarding the rapid decline in pulmonary function. However, some inconclusive results drawn out from previous studies are due to diversities in population that have been studied. With regard to the role of inflammatory mediators and cytokines, such as il-4, il-13, beta-2-adrenoceptor (ardb2), the protease/antiprotease imbalance, the tgf-β1 factor, the tnf-a factor despite the ambiguous results from the stud- ies conducted towards plausible association between genes encoded those factors and the onset or the progres- sive severity of copd, the conclusion is that susceptibility of the lung tissue depends not only on the exogenous noxious substances but on the genetic based host de- fending mechanisms as well. Promising field in the research era towards genetics and COPD is the further investigation of somatic muta- tions of lung epithelial barrier cells (lebc’s) and the mi- crosatellite instability (msi), on specific chromosomal loci of the related to the disease genes, since cigarette smoke may provoke the genesis of somatic mutation on lebc’s due to oxidative stress. In particularly, the genes that have presented high affinity to the pathobiological mechanisms of the COPD (Klotho gene, antioxidant genes) [11,12,53]. The future avenue in COPD pharma- cogenetics is constellating by the emergence of fully and carefully designed protocols assessing the specific pa- tient phenotype, clinical evaluation and radiographic as- sessment for the disease extension, plus the collection of blood samples for DNA determination. When all the above become the routine clinical practice then we will can consider the pharmacogenetics as a crucial tool in the physicians quiver for optimal and personalized therapy. Towards the future of the novel and more efficient thera- pies for this multifactorial disease the common notion is to enforce the research interest to genetic susceptibility. The necessity of further investigation with regard to gene therapy is demanded. The scope of the research has to be focused on the most efficient cell vehicles, in order to develop more accurate and efficient biological therapy strategies for COPD. Antioxidant genes, specific cells lines which present in abundance in the lung epithelium, such as neutrophils could be the core of the future thera- peutic targets in maintenance or even the regression of such a dismal disease. 7. Conclusion COPD is a multifactorial disease with many complex and multifaceted pathophysiology, associated with cigarette smoking that generally manifests with increasing age. Several studies have been conducted in order to under- stand the mechanisms involved in disease. Although some progress has been made in COPD genetics, there are many areas that require more investigation. Regard- ing the inflammation pathways and the extrapulmonary- systemic manifestation of the disease, novel approaches will likely also be essential in the identification of COPD genetic determinants, including biomarkers of the patho- physiologic processes involved in COPD. Interpretation Copyright © 2013 SciRes. OJRD  I. SOTIRIOU, D. MAKRIS 60 of the results of the association studies is a demanding procedure due to the heterogeneity of different pheno- types in genetics. Genome-wide association studies may contribute the best insights towards the identification of the susceptibility genes in COPD. Finally, epigenetics and gene therapies have a pivotal role as an impact factor in the genetics of COPD and they need to be considered in concert with genetic findings. REFERENCES [1] “Definition and Mechanisms Underlying Airflow Limita- tion in COPD, Risk Factors in COPD,” In: Global Initia- tive for COPD, GOLD Guidelines, 2006. [2] B. Burrows, R. J. Knudson, M. G. Cline and M. D. Le- bowitz, “Quantitative Relationships between Cigarette Smoking and Ventilatory Function,” American Review Respiratory Disease, Vol. 115, No. 2, 1977, pp. 195-205. [3] C. Fletcher, R. Peto, C. Tinker and F. E. Speizer, “Factors Related to the Development of Airflow Obstruction,” In: The Natural History of Chronic Bronchitis and Emphy- sema, Oxford University Press, Oxford, 1976, pp. 70-105. [4] S. Redline, P. V. Tishler, B. Rosner, F. I. Lewitter, M. Vandenburgh, S. T. Weis and F. E. Speizer, “Genotypic and Phenotypic Similarities in Pulmonary Function among Family Members of Adult Monozygotic and Dizygotic Twins,” American Journal of Epidemiology, Vol. 129, No. 4, 1989, pp. 827-836. [5] F. I. Lewitter, I. B. Tager, M. McGue, P. V. Tishler and F. E. Speizer, “Genetic and Environmental Determinants of Level of Pulmonary Function,” American Journal of Epidemiology, Vol. 120, No. 4, 1984, pp. 518-529. [6] F. Kueppers, R. D. Miller, H. Gordon, N. G. Hepper and K. Offord, “Familial Prevalence of Chronic Obstructive Pulmonary Disease in a Matched Pair Study,” American Journal of Medicine, Vol. 63, No. 3, 1977, pp. 336-342. doi:10.1016/0002-9343(77)90270-4 [7] B. H. Cohen, et al., “Chronic Obstructive Pulmonary Disease: A Challenge in Genetic Epidemiology,” Ameri- can Journal of Epidemiology, Vol. 112, No. 2, 1980, pp. 274-288. [8] N. R. Hackett, et al., “Variability of Antioxidant-Related Gene Expression in the Airway Epithelium of Cigarette Smokers,” American Journal of Respiratory Cell and Molecular Biology, Vol. 29, No. 3, 2003, pp. 331-343. doi:10.1165/rcmb.2002-0321OC [9] P. Turnpenny and S. Ellard, “Emery’s Elements of Medi- cal Genetics,” 12th Edition, Elsevier Churchill Livingstone, Edinburg, 2005. [10] M. A. Varela and W. Amos, “Heterogeneous Distribution of SNPs in the Human Genome: Microsatellites as Pre- dictors of Nucleotide Diversity and Divergence,” Ge- nomics, Vol. 95, No. 3, 2010, pp. 151-159. doi:10.1016/j.ygeno.2009.12.003 [11] D. Makris, et al., “Microsatellite DNA Instability and COPD Exacerbations,” European Respiratory Journal, Vol. 32, No. 3, 2008, pp. 612-618. doi:10.1183/09031936.00169307 [12] N. M. Siafakas, et al., “Microsatellite DNA Instability in COPD,” Chest, Vol. 116, No. 1, 1999, pp. 47-51. doi:10.1378/chest.116.1.47 [13] A. J. Sandford, T. Chaqani, T. D. Weir, J. E. Connet, N. R. Anthonisen and P. D. Pare, “Susceptibility Genes for Rapid Decline of Lung Function in the Lung Health Study,” American Journal of Respiratory and Critical Care Medicine, Vol. 163, No. 2, 2001, pp. 469-473. doi:10.1164/ajrccm.163.2.2006158 [14] O. Joost, et al., “Genetic Loci Influencing Lung Function: A Genome-Wide Scan in Framingham Study,” American Journal of Respiratory and Critical Care Medicine, Vol. 165, No. 6, 2002, pp. 795-799. doi:10.1164/ajrccm.165.6.2102057 [15] J. Q. He, J. Ruan, J. E. Connett, N. R. Anthonisen, P. D. Pare and A. J. Sandford, “Antioxidant Gene Polymor- phisms and Susceptibility to a Rapid Decline in Lung Function in Smokers,” American Journal of Respiratory and Critical Care Medicine, Vol. 166, No. 3, 2002, pp. 323-328. doi:10.1164/rccm.2111059 [16] J. Brogger, V. M. Steen, H. J. Eiken, A. Gulsvik and P. Bakke, “Genetic Association between COPD and Poly- morphisms in TNF, ADRB2 and EPHX1,” European Respiratory Journal, Vol. 27, No. 4, 2006, pp. 682-688. doi:10.1183/09031936.06.00057005 [17] G. Hu, Z. Shi, J. Hu, G. Zou, G. Peng and P. Ran, “Asso- ciation between Polymorphisms of Microsomal Epoxide Hydrolase and COPD: Results from Meta-Analyses,” Respirology, Vol. 13, No. 6, 2008, pp. 837-850. doi:10.1111/j.1440-1843.2008.01356.x [18] R. Lakhdar, et al., “Microsomal Epoxide Hydrolase Gene Polymorphisms and Susceptibility to Chronic Obstructive Pulmonary Disease in the Tunisian Population,” Genetic Testing and Molecular Biomarkers, Vol. 14, No. 6, 2010, pp. 857-863. doi:10.1089/gtmb.2009.0168 [19] A. E. Hegab, et al., “Polymorphisms of IL4, IL13, and ADRB2 Genes in COPD,” Chest, Vol. 126, No. 6, 2004, pp. 1832-1839. doi:10.1378/chest.126.6.1832 [20] T. C. Van der Pouw Kraan, et al., “Chronic Obstructive Pulmonary Disease Is Associated with the -1055 IL-13 Promoter Polymorphism,” Genes and Immunity, Vol. 3, No. 7, 2002, pp. 436-439. doi:10.1038/sj.gene.6363896 [21] S. F. Liu, et al., “Il13 Promoter (-1055) Polymorphisms Associated with Chronic Obstructive Pulmonary Disease in Taiwanese,” Experimental Lung Research, Vol. 35, No. 10, 2009, pp. 807-816. doi:10.3109/01902140902893644 [22] L. I. Ho, et al., “Polymorphism of the Beta(2)-Adreno- ceptor in COPD in Chinese Subjects,” Chest, Vol. 120, No. 5, 2001, pp. 1493-1499. doi:10.1378/chest.120.5.1493 [23] K. Hirano, et al., “Tissue Inhibitor of Metalloproteinases-2 Gene Polymorphisms in Chronic Obstructive Pulmonary Disease,” European Respiratory Journal, Vol. 18, No. 5, 2001, pp. 748-752. doi:10.1183/09031936.01.00102101 [24] L. Joos, et al., “The Role of Matrix Metalloproteinase Polymorphisms in the Rate of Decline in Lung Function,” Human Molecular Genetics, Vol. 11, No. 5, 2002, pp. Copyright © 2013 SciRes. OJRD  I. SOTIRIOU, D. MAKRIS 61 569-576. doi:10.1093/hmg/11.5.569 [25] A. G. Wilson, F. S. di Giovine, A. I. Blakemore and G. W. Duff, “Single Base Polymorphism in the Tumour Necro- sis Factor Alpha Gene Is Detectable by NcoI Restriction of PCR Product,” Human Molecular Genetics, Vol. 1, No. 5, 1992, p. 353. doi:10.1093/hmg/1.5.353 [26] N. Chieracul, P. Wongwisutikul, S. Vejbaesya and K. Chotvilaiwan, “Tumor Necrosis Factor-Alpha Gene Pro- moter Polymorphism Is Not Associated with Smoking- Related COPD in Thailand,” Respirology, Vol. 10, No. 1, 2005, pp. 36-39. doi:10.1111/j.1440-1843.2005.00626.x [27] G. P. Hu, G. Y. Peng, J. X. Hu and P. X. Pan, “Associa- tion of Tumor Necrosis Factor Alpha 308 G/A Gene Promoter Polymorphism with the Presence of Chronic Obstructive Pulmonary Disease: A Meta-Analysis,” Chi- nese Journal of Tuberculosis and Respiratory Diseases, Vol. 30, No. 8, 2007, pp. 588-594. [28] P. Zhan, et al., “TNF-308 Gene Polymorphism Is Associ- ated with COPD Risk among Asians: Meta-Analysis of Data for 6118 Subjects,” Molecular Biology Reports, Vol. 38, No. 1, 2011, pp. 219-227. doi:10.1007/s11033-010-0098-y [29] L. Wu, J. Chau, et al., “Transforming Growth Factor-β1 Genotype and Susceptibility to Chronic Obstructive Pul- monary Disease,” Thorax, Vol. 59, No. 2, 2004, pp. 126- 129. doi:10.1136/thorax.2003.005769 [30] C. C. Van Diemen, D. S. Postma, J. M. Vonk, M. Bru- inenberg, I. M. Nolte and H. M. Boezen, “Decorin and TGF-Beta1 Polymorphisms and Development of COPD in a General Population,” Respiratory Research, Vol. 16, No. 7, 2006, p. 89. doi:10.1186/1465-9921-7-89 [31] H. I. Yoon, et al., “Lack of Association between COPD and Transforming Growth Factor-Beta1 (TGFB1) Genetic Polymorphisms in Koreans,” International Journal of Tuberculosis and Lung Disease, Vol. 10, No. 5, 2006, pp. 504-509. [32] M. Bodas, T. Min, S. Mazur and N. Vij, “Critical Modi- fier Role of Membrane-Cystic Fibrosis Transmembrane Conductance Regulator-Dependent Ceramide Signaling in Lung Injury and Emphysema,” Journal of Immunology, Vol. 186, No. 1, 2011, pp. 602-613. doi:10.4049/jimmunol.1002850 [33] N. Minematsu, H. Nakamura, et al., “Association of CYP2A6 Deletion Polymorphisms with Smoking Habit and Development of Pulmonary Emphysema,” Thorax, Vol. 58, No. 7, 2003, pp. 623-628. doi:10.1136/thorax.58.7.623 [34] N. Yamamoto, S. Homma, et al., “Vitamin D3 Binding Protein (Groupspecific Component) Is a Precursor for the Macrophage-Activating Signal Factor from Lysophos- phatidylcholine-Treated Lymphocytes,” Proceedings of the National Academy Science USA, Vol. 88, No. 19, 1991, pp. 8539-8543. doi:10.1073/pnas.88.19.8539 [35] R. R. Kew, R. O. Webster, et al., “Gc-Globulin (Vitamin D-Binding Protein) Enhances the Neutrophil Chemotactic activity of C5a and C5a des Arg,” Journal of Clinical In- vestigation, Vol. 82, No. 1, 1998, pp. 364-369. doi:10.1172/JCI113596 [36] S. J. Di Martino, A. B. Shah, G. Trujillo and R. R. Kew, “Elastase Controls the Binding of the Vitamin D-Binding Protein (Gc-Globulin) to Neutrophils: A Potential Role in the Regulation of C5a Co-Chemotactic Activity,” The Journal of Immunology, Vol. 166, No. 4, 2001, pp. 2688- 2694. [37] I. Ito, et al., “Risk and Severity of COPD Is Associated with the Group-Specific Component of Serum Globulin 1F Allele,” Chest Journal, Vol. 125, No. 1, 2004, pp. 63- 70. doi:10.1378/chest.125.1.63 [38] M. Lu, B. Yang and Y. Y. Cai, “The Relationship be- tween Vitamin D Binding Protein Gene Polymorphism and Chronic Obstructive Pulmonary Disease,” Chinese Journal of Internal Medicine, Vol. 43, No. 2, 2004, pp. 117-120. [39] L. H. Shen, et al., “Association of Vitamin D Binding Protein Variants with Susceptibility to Chronic Obstruc- tive Pulmonary Disease,” Journal of International Medi- cal Research, Vol. 38, No. 3, 2010, pp. 1093-1098. [40] M. Kuro-o, Y. Matsumura, et al., “Mutation of the Mouse Klotho Gene Leads to a Syndrome Resembling Aging,” Nature, Vol. 390, No. 6655, 1997, pp. 45-51. doi:10.1038/36285 [41] N. A. Molfino, “Genetics of COPD,” Chest Journal, Vol. 125, No. 5, 2004, pp. 1929-1940. doi:10.1378/chest.125.5.1929 [42] Y. Saito, T. Nakamura, Y. Ohyama, et al., “In Vivo Klotho Gene Delivery Protects Against Endothelial Dys- function in Multiple Risk Factor Syndrome,” Biochemical and Biophysical Research Communications, Vol. 276, No. 2, 2000, pp. 767-772. doi:10.1006/bbrc.2000.3470 [43] D. E. Arking, et al., “Klotho Allele Status and the Risk of Early-Onset Occult Coronary Artery Disease” The Ame- rican Journal of Human Genetics, Vol. 72, No. 5, 2003, pp. 1154-1161. doi:10.1086/375035 [44] K. Kawano, N. Ogata, et al., “Klotho Gene Polymor- phisms Associated with Bone Density of Aged Post- menopausal Women,” Journal of Bone and Mineral Re- search, Vol. 17, No. 10, 2002, pp. 1744-1751. doi:10.1359/jbmr.2002.17.10.1744 [45] I. Sotiriou, M. Froudarakis, et al., “Klotho Gene Poly- morphism -395G>A in Patients with Chronic Obstructive Pulmonary Disease (COPD),” Pne umon, Vol. 23, No. 4, 2010, pp. 342-347. [46] N. Hizawa, et al., “β2-Adrenergic Receptor Genetic Polymorphisms and Short-Term Bronchodilator Responses in Patients with COPD,” Chest Journal, Vol. 132, No. 5, 2007, pp. 1485-1492. doi:10.1378/chest.07-1103 [47] M. Mokry, P. Joppa, et al., “β2-Adrenergic Receptor Haplotype and Bronchodilator Response to Salbutamol in Patients with Acute Exacerbations of COPD,” Medical Science Monitor, Vol. 14, No. 8, 2008, pp. 392-398. [48] W. J. Kim, Y. M. Oh, et al., “Lung Function Response to 12-Week Treatment with Combined Inhalation of Long- Acting β2 Agonist and Glucocorticoid According to ADRB2 Polymorphism in Patients with Chronic Obstruc- tive Pulmonary Disease,” Lung, Vol. 186, No. 6, 2008, pp. 381-386. doi:10.1007/s00408-008-9103-9 [49] N. Umeda, T. Yoshikawa, H. Kanazawa, K. Hirata and S. Copyright © 2013 SciRes. OJRD  I. SOTIRIOU, D. MAKRIS Copyright © 2013 SciRes. OJRD 62 Fujimoto, “Association of β2-Adrenoreceptor Genotypes with Bronchodilatory Effect of Tiotropium in COPD,” Respirology, Vol. 13, No. 3, 2008, pp. 346-352. doi:10.1111/j.1440-1843.2008.01259.x [50] N. M. Siafakas and E. G. Tzortzaki, “Few Smokers De- velop COPD. Why?” Respiratory Medicine, Vol. 96, No. 8, 2002, pp. 615-624. doi:10.1053/rmed.2002.1318 [51] T. Nagai, K. Yamada, et al., “Cognition Impairment in the Genetic Model of Aging Klotho Gene Mutant Mice: A Role of Oxidative Stress,” The FASEB Journal, Vol. 17, No. 1, 2003, pp. 50-52. [52] E. G. Tzortzaki and N. M. Siafakas, “A Hypothesis for Initiation of COPD,” European Respiratory Journal, Vol. 34, No. 2, 2009, pp. 310-315. doi:10.1183/09031936.00067008
|