 Vol.2, No.11, 1327-1334 (2010) doi:10.4236/health.2010.211198 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ Health Openly accessible at Gross domestic product and dietary pattern among 49 western countries with a focus on polyamine intake Phan Nguyen Thanh Binh1,2, Kuniyasu Soda3*, Masanobu Kawakami4 1Department of Community Nutrition, HCMC Nutrition Center, Ho Chi Minh City, Vietnam; 2Department of Food and Nutrition, Japan Women’s University, Tokyo, Japan; 3Department of Cardiovascular Research Institute, Saitama Medical Center, Jichi Medical University, Saitama, Japan; *Corresponding Author: soda@jichi.ac.jp; 4Department of Internal Medicine, Saitama Medical Center, Jichi Medical University, Saitama, Japan. Received 7 August 2010; revised 21 August 2010; accepted 6 September 2010. ABSTRACT Socioeconomic status is known to affect dietary profile, and differences in food habits and choice may affect polyamine intake due to significant variations in the concentrations of the poly- amines spermine, spermidine, and putrescine present in different foods. The relationship be- tween gross domestic product (GDP) and die- tary profile, with a focus on polyamine intake, was investigated for 49 different European and other Western countries. The data for food sup- ply and GDP were collected from the database of the United Nations and the International Mo- netary Fund, respectively, and the amount of polyamine intake from food was estimated us- ing polyamine concentrations listed in published sources. Countries were divided equally accor- ding to GDP values into two categories, higher and lower, and the amount and composition of food polyamines as well as dietary profile were compared. Higher GDP countries supply animal products and seafood in greater amounts than lower GDP countries; however, whole milk sup- ply per calorie was higher in lower GDP than higher GDP countries. While crops supply was relatively higher in lower GDP countries, fruit supply was greater in higher GDP countries. Higher GDP was associated with increased amount of spermine and putrescine per total calorie, although spermidine amount per calorie was similar between higher and lower GDP countries. GDP, as an indicator of countries’ socioeconomic status, is associated with the amount and the composition of polyamines as well as dietary pattern. Keywords: GDP; Dietary Pattern; Polyamine Intake; Western Countries 1. INTRODUCTION Socioeconomic status, defined by economic activities and social life, is closely associated with individual health as well as the public disease burden, which would in- clude cardiovascular disease [1-3], type 2 diabetes [4-5] and some cancers [6-7]. At a national level, gross domes- tic product (GDP) per capita is considered to reflect the socioeconomic status of the country and is consistently related to health conditions, namely, wealthier countries generally have healthier populations [8-10]. Among the many factors that are involved in the association be- tween socioeconomic status and health, dietary pattern is considered to be one of the most important. A number of studies have shown an association between socioeco- nomic status and dietary pattern as well as lifestyle [11, 12]. Among many nutrients and non-nutrients in foods, recent studies have brought light the importance of food polyamines, because recent studies have shown many biological activities of polyamine and beneficial effects for the health of mammals [13-15]. Polyamines, sper- mine, spermidine, and putrescine are polycations syn- thesized in almost all cells. Polyamines have been shown to be absorbed from the intestinal lumen and distributed to organs and tissues in the whole body [16-17]. Because foods are comprised of cellular components from vari- ous organisms, the majority of foods contain polyamines but their concentration is wide-ranging [18-20]. Since diets are built from a wide variety of foods and are also affected by different methods of processing and cooking, a community’s diet is influenced and shaped by multi- dimensional factors, including socioeconomic status [21]. Therefore, the amount of polyamine intake must vary considerably between regions. In the present study, in order to investigate the asso- 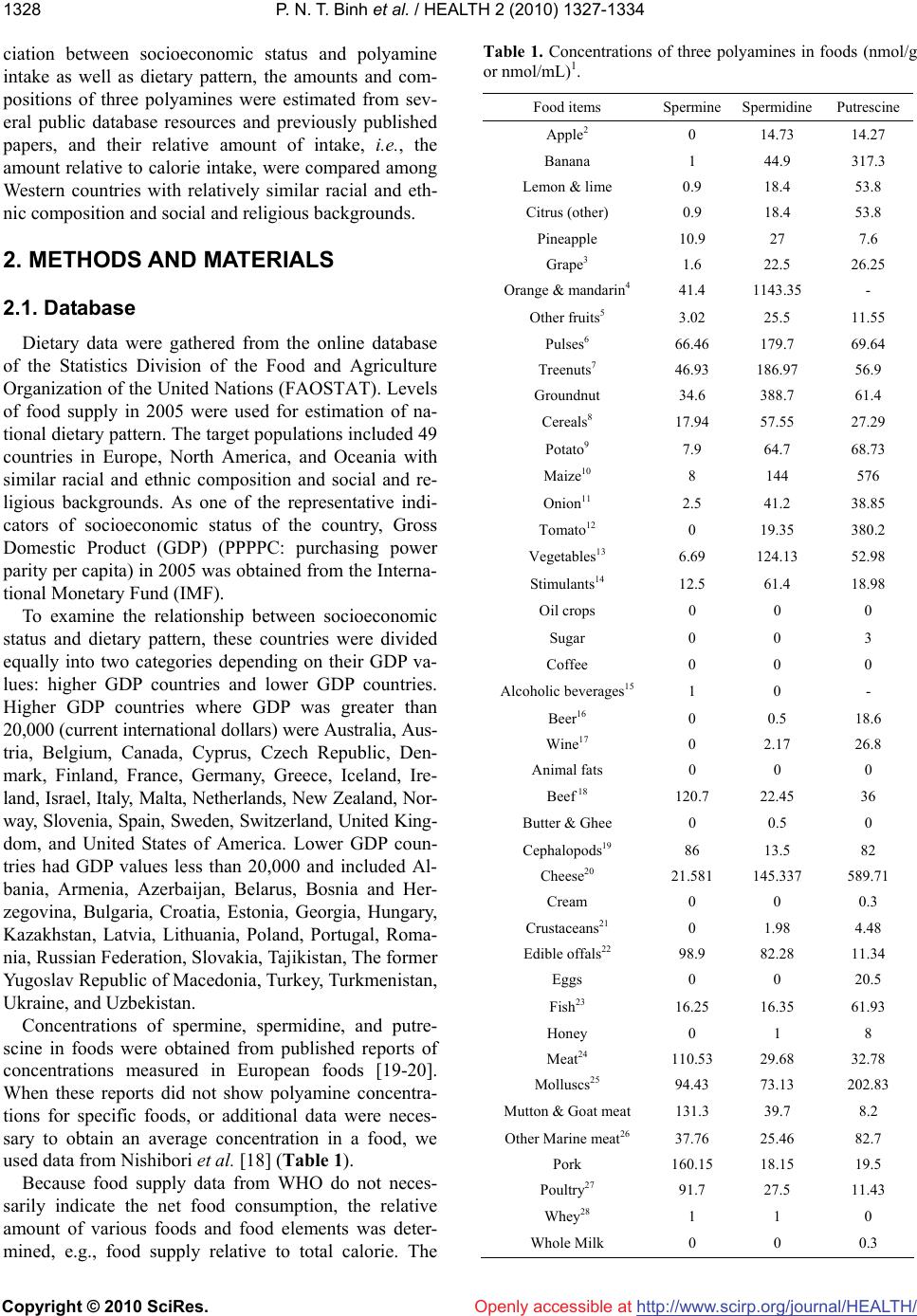 P. N. T. Binh et al. / HEALTH 2 (2010) 1327-1334 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 1328 ciation between socioeconomic status and polyamine intake as well as dietary pattern, the amounts and com- positions of three polyamines were estimated from sev- eral public database resources and previously published papers, and their relative amount of intake, i.e., the amount relative to calorie intake, were compared among Western countries with relatively similar racial and eth- nic composition and social and religious backgrounds. 2. METHODS AND MATERIALS 2.1. Database Dietary data were gathered from the online database of the Statistics Division of the Food and Agriculture Organization of the United Nations (FAOSTAT). Levels of food supply in 2005 were used for estimation of na- tional dietary pattern. The target populations included 49 countries in Europe, North America, and Oceania with similar racial and ethnic composition and social and re- ligious backgrounds. As one of the representative indi- cators of socioeconomic status of the country, Gross Domestic Product (GDP) (PPPPC: purchasing power parity per capita) in 2005 was obtained from the Interna- tional Monetary Fund (IMF). To examine the relationship between socioeconomic status and dietary pattern, these countries were divided equally into two categories depending on their GDP va- lues: higher GDP countries and lower GDP countries. Higher GDP countries where GDP was greater than 20,000 (current international dollars) were Australia, Aus- tria, Belgium, Canada, Cyprus, Czech Republic, Den- mark, Finland, France, Germany, Greece, Iceland, Ire- land, Israel, Italy, Malta, Netherlands, New Zealand, Nor- way, Slovenia, Spain, Sweden, Switzerland, United King- dom, and United States of America. Lower GDP coun- tries had GDP values less than 20,000 and included Al- bania, Armenia, Azerbaijan, Belarus, Bosnia and Her- zegovina, Bulgaria, Croatia, Estonia, Georgia, Hungary, Kazakhstan, Latvia, Lithuania, Poland, Portugal, Roma- nia, Russian Federation, Slovakia, Tajikistan, The former Yugoslav Republic of Macedonia, Turkey, Turkmenistan, Ukraine, and Uzbekistan. Concentrations of spermine, spermidine, and putre- scine in foods were obtained from published reports of concentrations measured in European foods [19-20]. When these reports did not show polyamine concentra- tions for specific foods, or additional data were neces- sary to obtain an average concentration in a food, we used data from Nishibori et al. [18] (Table 1). Because food supply data from WHO do not neces- sarily indicate the net food consumption, the relative amount of various foods and food elements was deter- mined, e.g., food supply relative to total calorie. The Table 1. Concentrations of three polyamines in foods (nmol/g or nmol/mL)1. Food items Spermine Spermidine Putrescine Apple2 0 14.73 14.27 Banana 1 44.9 317.3 Lemon & lime 0.9 18.4 53.8 Citrus (other) 0.9 18.4 53.8 Pineapple 10.9 27 7.6 Grape3 1.6 22.5 26.25 Orange & mandarin4 41.4 1143.35 - Other fruits5 3.02 25.5 11.55 Pulses6 66.46 179.7 69.64 Treenuts7 46.93 186.97 56.9 Groundnut 34.6 388.7 61.4 Cereals8 17.94 57.55 27.29 Potato9 7.9 64.7 68.73 Maize10 8 144 576 Onion11 2.5 41.2 38.85 Tomato12 0 19.35 380.2 Vegetables13 6.69 124.13 52.98 Stimulants14 12.5 61.4 18.98 Oil crops 0 0 0 Sugar 0 0 3 Coffee 0 0 0 Alcoholic beverages15 1 0 - Beer16 0 0.5 18.6 Wine17 0 2.17 26.8 Animal fats 0 0 0 Beef 18 120.7 22.45 36 Butter & Ghee 0 0.5 0 Cephalopods19 86 13.5 82 Cheese20 21.581 145.337 589.71 Cream 0 0 0.3 Crustaceans21 0 1.98 4.48 Edible offals22 98.9 82.28 11.34 Eggs 0 0 20.5 Fish23 16.25 16.35 61.93 Honey 0 1 8 Meat24 110.53 29.68 32.78 Molluscs25 94.43 73.13 202.83 Mutton & Goat meat 131.3 39.7 8.2 Other Marine meat26 37.76 25.46 82.7 Pork 160.15 18.15 19.5 Poultry27 91.7 27.5 11.43 Whey28 1 1 0 Whole Milk 0 0 0.3 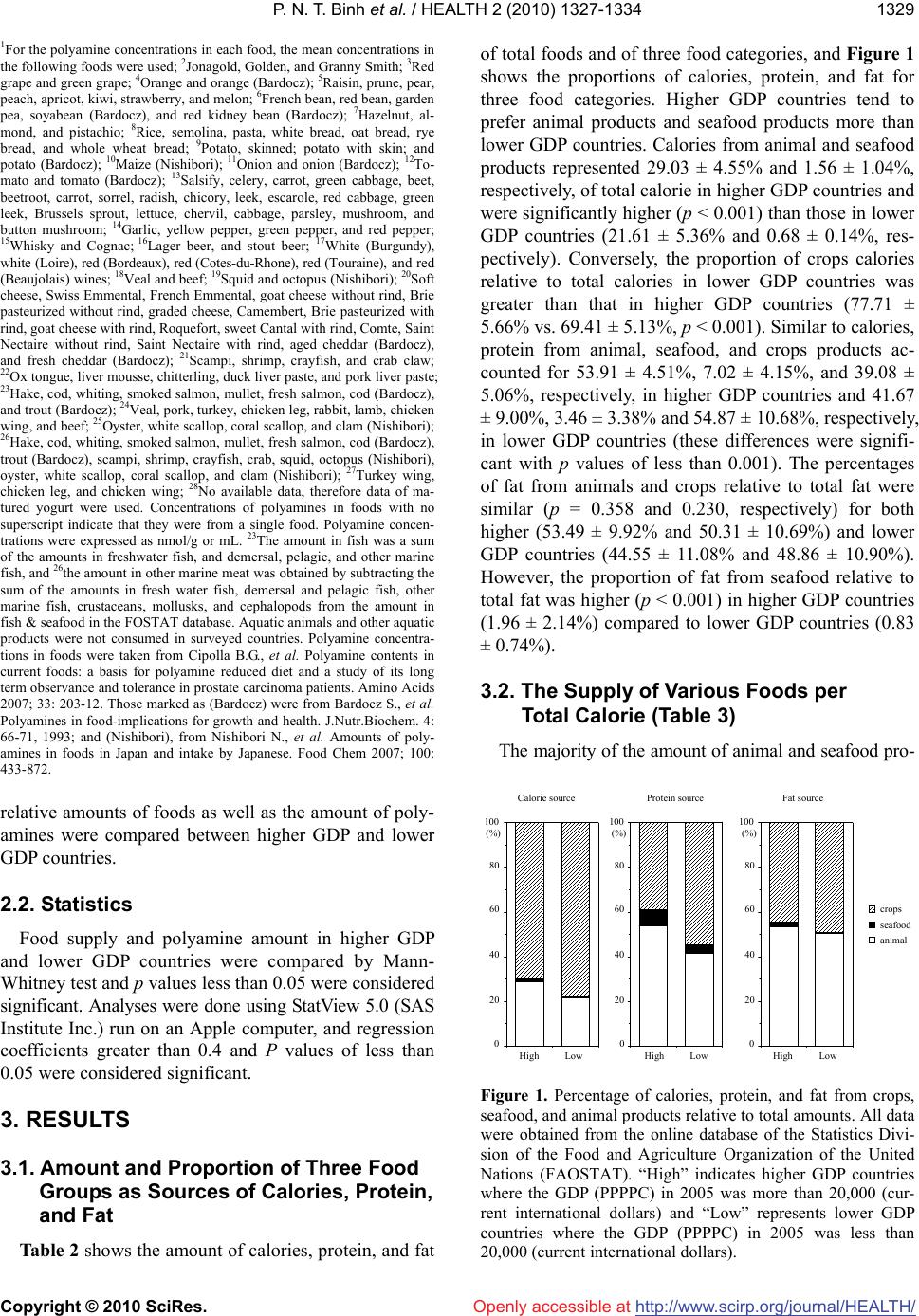 P. N. T. Binh et al. / HEALTH 2 (2010) 1327-1334 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 1329 Openly accessible at 1For the polyamine concentrations in each food, the mean concentrations in the following foods were used; 2Jonagold, Golden, and Granny Smith; 3Red grape and green grape; 4Orange and orange (Bardocz); 5Raisin, prune, pear, peach, apricot, kiwi, strawberry, and melon; 6French bean, red bean, garden pea, soyabean (Bardocz), and red kidney bean (Bardocz); 7Hazelnut, al- mond, and pistachio; 8Rice, semolina, pasta, white bread, oat bread, rye bread, and whole wheat bread; 9Potato, skinned; potato with skin; and potato (Bardocz); 10Maize (Nishibori); 11Onion and onion (Bardocz); 12To- mato and tomato (Bardocz); 13Salsify, celery, carrot, green cabbage, beet, beetroot, carrot, sorrel, radish, chicory, leek, escarole, red cabbage, green leek, Brussels sprout, lettuce, chervil, cabbage, parsley, mushroom, and button mushroom; 14Garlic, yellow pepper, green pepper, and red pepper; 15Whisky and Cognac; 16Lager beer, and stout beer; 17White (Burgundy), white (Loire), red (Bordeaux), red (Cotes-du-Rhone), red (Touraine), and red (Beaujolais) wines; 18Veal and beef; 19Squid and octopus (Nishibori); 20Soft cheese, Swiss Emmental, French Emmental, goat cheese without rind, Brie pasteurized without rind, graded cheese, Camembert, Brie pasteurized with rind, goat cheese with rind, Roquefort, sweet Cantal with rind, Comte, Saint Nectaire without rind, Saint Nectaire with rind, aged cheddar (Bardocz), and fresh cheddar (Bardocz); 21Scampi, shrimp, crayfish, and crab claw; 22Ox tongue, liver mousse, chitterling, duck liver paste, and pork liver paste; 23Hake, cod, whiting, smoked salmon, mullet, fresh salmon, cod (Bardocz), and trout (Bardocz); 24Veal, pork, turkey, chicken leg, rabbit, lamb, chicken wing, and beef; 25Oyster, white scallop, coral scallop, and clam (Nishibori); 26Hake, cod, whiting, smoked salmon, mullet, fresh salmon, cod (Bardocz), trout (Bardocz), scampi, shrimp, crayfish, crab, squid, octopus (Nishibori), oyster, white scallop, coral scallop, and clam (Nishibori); 27Turkey wing, chicken leg, and chicken wing; 28No available data, therefore data of ma- tured yogurt were used. Concentrations of polyamines in foods with no superscript indicate that they were from a single food. Polyamine concen- trations were expressed as nmol/g or mL. 23The amount in fish was a sum of the amounts in freshwater fish, and demersal, pelagic, and other marine fish, and 26the amount in other marine meat was obtained by subtracting the sum of the amounts in fresh water fish, demersal and pelagic fish, other marine fish, crustaceans, mollusks, and cephalopods from the amount in fish & seafood in the FOSTAT database. Aquatic animals and other aquatic products were not consumed in surveyed countries. Polyamine concentra- tions in foods were taken from Cipolla B.G., et al. Polyamine contents in current foods: a basis for polyamine reduced diet and a study of its long term observance and tolerance in prostate carcinoma patients. Amino Acids 2007; 33: 203-12. Those marked as (Bardocz) were from Bardocz S., et al. Polyamines in food-implications for growth and health. J.Nutr.Biochem. 4: 66-71, 1993; and (Nishibori), from Nishibori N., et al. Amounts of poly- amines in foods in Japan and intake by Japanese. Food Chem 2007; 100: 433-872. relative amounts of foods as well as the amount of poly- amines were compared between higher GDP and lower GDP countries. 2.2. Statistics Food supply and polyamine amount in higher GDP and lower GDP countries were compared by Mann- Whitney test and p values less than 0.05 were considered significant. Analyses were done using StatView 5.0 (SAS Institute Inc.) run on an Apple computer, and regression coefficients greater than 0.4 and P values of less than 0.05 were considered significant. 3. RESULTS 3.1. Amount and Proportion of Three Food Groups as Sources of Calories, Protein, and Fat Table 2 shows the amount of calories, protein, and fat of total foods and of three food categories, and Figure 1 shows the proportions of calories, protein, and fat for three food categories. Higher GDP countries tend to prefer animal products and seafood products more than lower GDP countries. Calories from animal and seafood products represented 29.03 ± 4.55% and 1.56 ± 1.04%, respectively, of total calorie in higher GDP countries and were significantly higher (p < 0.001) than those in lower GDP countries (21.61 ± 5.36% and 0.68 ± 0.14%, res- pectively). Conversely, the proportion of crops calories relative to total calories in lower GDP countries was greater than that in higher GDP countries (77.71 ± 5.66% vs. 69.41 ± 5.13%, p < 0.001). Similar to calories, protein from animal, seafood, and crops products ac- counted for 53.91 ± 4.51%, 7.02 ± 4.15%, and 39.08 ± 5.06%, respectively, in higher GDP countries and 41.67 ± 9.00%, 3.46 ± 3.38% and 54.87 ± 10.68%, respectively, in lower GDP countries (these differences were signifi- cant with p values of less than 0.001). The percentages of fat from animals and crops relative to total fat were similar (p = 0.358 and 0.230, respectively) for both higher (53.49 ± 9.92% and 50.31 ± 10.69%) and lower GDP countries (44.55 ± 11.08% and 48.86 ± 10.90%). However, the proportion of fat from seafood relative to total fat was higher (p < 0.001) in higher GDP countries (1.96 ± 2.14%) compared to lower GDP countries (0.83 ± 0.74%). 3.2. The Supply of Various Foods per Total Calorie (Table 3) The majority of the amount of animal and seafood pro- High 0 20 40 60 80 100 Calorie source animal seafood crops Protein sourceFat source Low High 0 20 40 60 80 100 Low High 0 20 40 60 80 100 Low (%) (%)(%) Figure 1. Percentage of calories, protein, and fat from crops, seafood, and animal products relative to total amounts. All data were obtained from the online database of the Statistics Divi- sion of the Food and Agriculture Organization of the United Nations (FAOSTAT). “High” indicates higher GDP countries where the GDP (PPPPC) in 2005 was more than 20,000 (cur- rent international dollars) and “Low” represents lower GDP countries where the GDP (PPPPC) in 2005 was less than 20,000 (current international dollars). 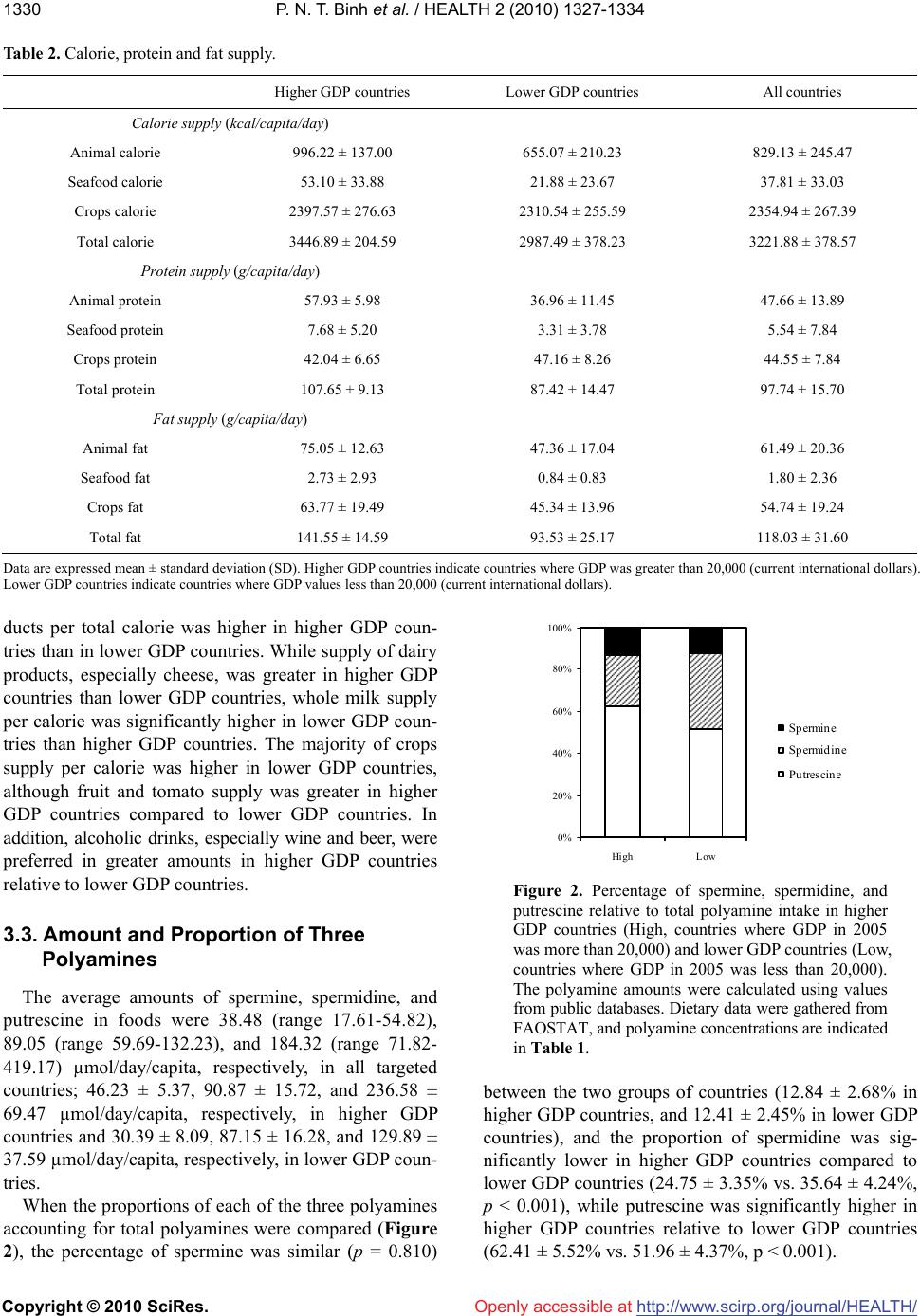 P. N. T. Binh et al. / HEALTH 2 (2010) 1327-1334 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 1330 Table 2. Calorie, protein and fat supply. Higher GDP countries Lower GDP countries All countries Calorie supply (kcal/capita/day) Animal calorie 996.22 ± 137.00 655.07 ± 210.23 829.13 ± 245.47 Seafood calorie 53.10 ± 33.88 21.88 ± 23.67 37.81 ± 33.03 Crops calorie 2397.57 ± 276.63 2310.54 ± 255.59 2354.94 ± 267.39 Total calorie 3446.89 ± 204.59 2987.49 ± 378.23 3221.88 ± 378.57 Protein supply (g/capita/day) Animal protein 57.93 ± 5.98 36.96 ± 11.45 47.66 ± 13.89 Seafood protein 7.68 ± 5.20 3.31 ± 3.78 5.54 ± 7.84 Crops protein 42.04 ± 6.65 47.16 ± 8.26 44.55 ± 7.84 Total protein 107.65 ± 9.13 87.42 ± 14.47 97.74 ± 15.70 Fat supply (g/capita/day) Animal fat 75.05 ± 12.63 47.36 ± 17.04 61.49 ± 20.36 Seafood fat 2.73 ± 2.93 0.84 ± 0.83 1.80 ± 2.36 Crops fat 63.77 ± 19.49 45.34 ± 13.96 54.74 ± 19.24 Total fat 141.55 ± 14.59 93.53 ± 25.17 118.03 ± 31.60 Data are expressed mean ± standard deviation (SD). Higher GDP countries indicate countries where GDP was greater than 20,000 (current international dollars). Lower GDP countries indicate countries where GDP values less than 20,000 (current international dollars). ducts per total calorie was higher in higher GDP coun- tries than in lower GDP countries. While supply of dairy products, especially cheese, was greater in higher GDP countries than lower GDP countries, whole milk supply per calorie was significantly higher in lower GDP coun- tries than higher GDP countries. The majority of crops supply per calorie was higher in lower GDP countries, although fruit and tomato supply was greater in higher GDP countries compared to lower GDP countries. In addition, alcoholic drinks, especially wine and beer, were preferred in greater amounts in higher GDP countries relative to lower GDP countries. 0% 20% 40% 60% 80% 100% Hi ghL ow Sp ermine Spermidine Putresci ne Figure 2. Percentage of spermine, spermidine, and putrescine relative to total polyamine intake in higher GDP countries (High, countries where GDP in 2005 was more than 20,000) and lower GDP countries (Low, countries where GDP in 2005 was less than 20,000). The polyamine amounts were calculated using values from public databases. Dietary data were gathered from FAOSTAT, and polyamine concentrations are indicated in Table 1. 3.3. Amount and Proportion of Three Polyamines The average amounts of spermine, spermidine, and putrescine in foods were 38.48 (range 17.61-54.82), 89.05 (range 59.69-132.23), and 184.32 (range 71.82- 419.17) µmol/day/capita, respectively, in all targeted countries; 46.23 ± 5.37, 90.87 ± 15.72, and 236.58 ± 69.47 µmol/day/capita, respectively, in higher GDP countries and 30.39 ± 8.09, 87.15 ± 16.28, and 129.89 ± 37.59 µmol/day/capita, respectively, in lower GDP coun- tries. between the two groups of countries (12.84 ± 2.68% in higher GDP countries, and 12.41 ± 2.45% in lower GDP countries), and the proportion of spermidine was sig- nificantly lower in higher GDP countries compared to lower GDP countries (24.75 ± 3.35% vs. 35.64 ± 4.24%, p < 0.001), while putrescine was significantly higher in higher GDP countries relative to lower GDP countries (62.41 ± 5.52% vs. 51.96 ± 4.37%, p < 0.001). When the proportions of each of the three polyamines accounting for total polyamines were compared (Figure 2), the percentage of spermine was similar (p = 0.810) Openly accessible at 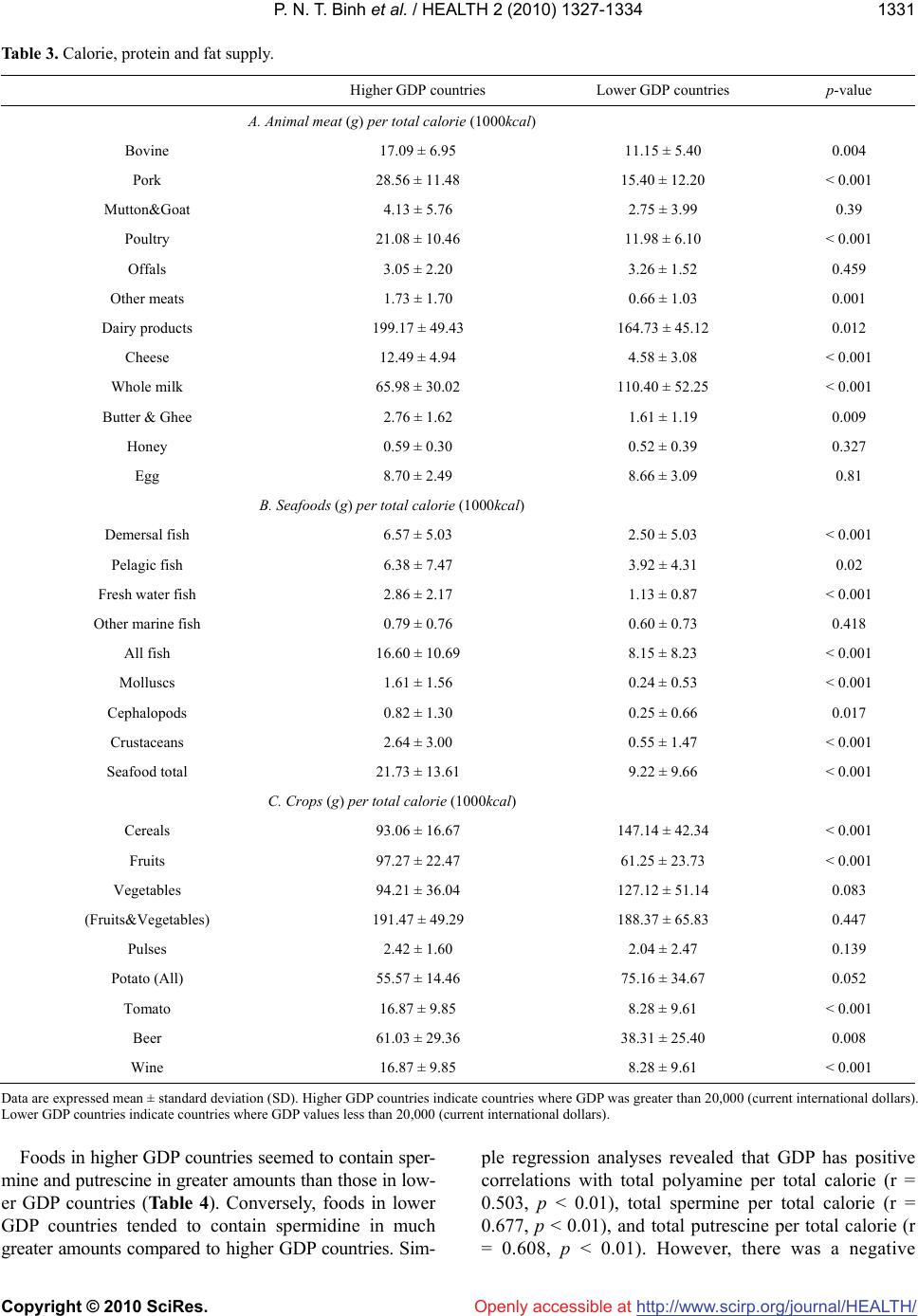 P. N. T. Binh et al. / HEALTH 2 (2010) 1327-1334 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 1331 Table 3. Calorie, protein and fat supply. Higher GDP countries Lower GDP countries p-value A. Animal meat (g) per total calorie (1000kcal) Bovine 17.09 ± 6.95 11.15 ± 5.40 0.004 Pork 28.56 ± 11.48 15.40 ± 12.20 < 0.001 Mutton&Goat 4.13 ± 5.76 2.75 ± 3.99 0.39 Poultry 21.08 ± 10.46 11.98 ± 6.10 < 0.001 Offals 3.05 ± 2.20 3.26 ± 1.52 0.459 Other meats 1.73 ± 1.70 0.66 ± 1.03 0.001 Dairy products 199.17 ± 49.43 164.73 ± 45.12 0.012 Cheese 12.49 ± 4.94 4.58 ± 3.08 < 0.001 Whole milk 65.98 ± 30.02 110.40 ± 52.25 < 0.001 Butter & Ghee 2.76 ± 1.62 1.61 ± 1.19 0.009 Honey 0.59 ± 0.30 0.52 ± 0.39 0.327 Egg 8.70 ± 2.49 8.66 ± 3.09 0.81 B. Seafoods (g) per total calorie (1000kcal) Demersal fish 6.57 ± 5.03 2.50 ± 5.03 < 0.001 Pelagic fish 6.38 ± 7.47 3.92 ± 4.31 0.02 Fresh water fish 2.86 ± 2.17 1.13 ± 0.87 < 0.001 Other marine fish 0.79 ± 0.76 0.60 ± 0.73 0.418 All fish 16.60 ± 10.69 8.15 ± 8.23 < 0.001 Molluscs 1.61 ± 1.56 0.24 ± 0.53 < 0.001 Cephalopods 0.82 ± 1.30 0.25 ± 0.66 0.017 Crustaceans 2.64 ± 3.00 0.55 ± 1.47 < 0.001 Seafood total 21.73 ± 13.61 9.22 ± 9.66 < 0.001 C. Crops (g) per total calorie (1000kcal) Cereals 93.06 ± 16.67 147.14 ± 42.34 < 0.001 Fruits 97.27 ± 22.47 61.25 ± 23.73 < 0.001 Vegetables 94.21 ± 36.04 127.12 ± 51.14 0.083 (Fruits&Vegetables) 191.47 ± 49.29 188.37 ± 65.83 0.447 Pulses 2.42 ± 1.60 2.04 ± 2.47 0.139 Potato (All) 55.57 ± 14.46 75.16 ± 34.67 0.052 Tomato 16.87 ± 9.85 8.28 ± 9.61 < 0.001 Beer 61.03 ± 29.36 38.31 ± 25.40 0.008 Wine 16.87 ± 9.85 8.28 ± 9.61 < 0.001 Data are expressed mean ± standard deviation (SD). Higher GDP countries indicate countries where GDP was greater than 20,000 (current international dollars). Lower GDP countries indicate countries where GDP values less than 20,000 (current international dollars). Foods in higher GDP countries seemed to contain sper- mine and putrescine in greater amounts than those in low- er GDP countries (Table 4). Conversely, foods in lower GDP countries tended to contain spermidine in much greater amounts compared to higher GDP countries. Sim- ple regression analyses revealed that GDP has positive correlations with total polyamine per total calorie (r = 0.503, p < 0.01), total spermine per total calorie (r = 0.677, p < 0.01), and total putrescine per total calorie (r = 0.608, p < 0.01). However, there was a negative 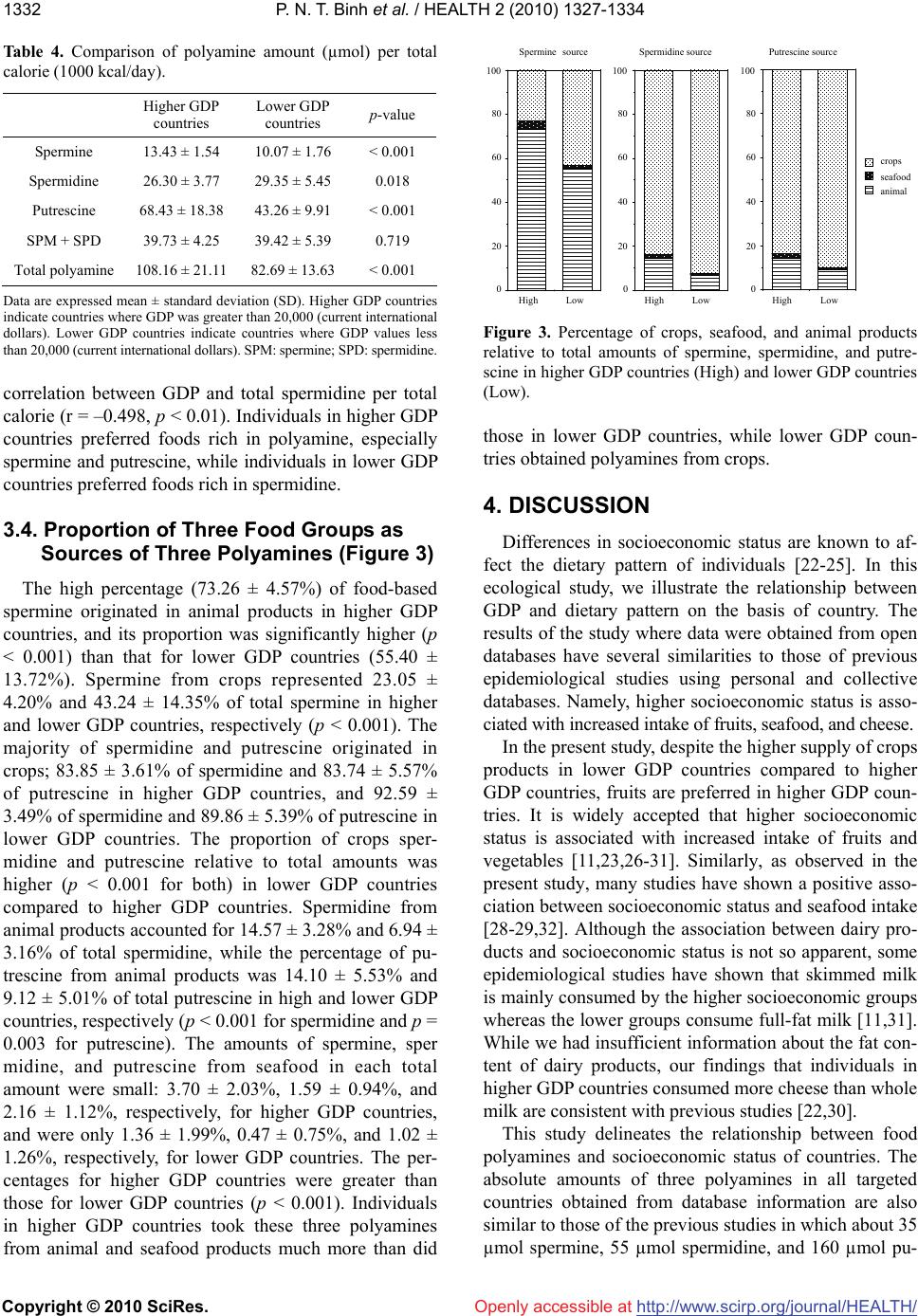 P. N. T. Binh et al. / HEALTH 2 (2010) 1327-1334 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 1332 Openly accessible at Table 4. Comparison of polyamine amount (µmol) per total calorie (1000 kcal/day). Higher GDP countries Lower GDP countries p-value Spermine 13.43 ± 1.54 10.07 ± 1.76 < 0.001 Spermidine 26.30 ± 3.77 29.35 ± 5.45 0.018 Putrescine 68.43 ± 18.38 43.26 ± 9.91 < 0.001 SPM + SPD 39.73 ± 4.25 39.42 ± 5.39 0.719 Total polyamine 108.16 ± 21.1182.69 ± 13.63 < 0.001 Data are expressed mean ± standard deviation (SD). Higher GDP countries indicate countries where GDP was greater than 20,000 (current international dollars). Lower GDP countries indicate countries where GDP values less than 20,000 (current international dollars). SPM: spermine; SPD: spermidine. correlation between GDP and total spermidine per total calorie (r = –0.498, p < 0.01). Individuals in higher GDP countries preferred foods rich in polyamine, especially spermine and putrescine, while individuals in lower GDP countries preferred foods rich in spermidine. 3.4. Proportion of Three Food Groups as Sources of Three Polyamines (Figure 3) The high percentage (73.26 ± 4.57%) of food-based spermine originated in animal products in higher GDP countries, and its proportion was significantly higher (p < 0.001) than that for lower GDP countries (55.40 ± 13.72%). Spermine from crops represented 23.05 ± 4.20% and 43.24 ± 14.35% of total spermine in higher and lower GDP countries, respectively (p < 0.001). The majority of spermidine and putrescine originated in crops; 83.85 ± 3.61% of spermidine and 83.74 ± 5.57% of putrescine in higher GDP countries, and 92.59 ± 3.49% of spermidine and 89.86 ± 5.39% of putrescine in lower GDP countries. The proportion of crops sper- midine and putrescine relative to total amounts was higher (p < 0.001 for both) in lower GDP countries compared to higher GDP countries. Spermidine from animal products accounted for 14.57 ± 3.28% and 6.94 ± 3.16% of total spermidine, while the percentage of pu- trescine from animal products was 14.10 ± 5.53% and 9.12 ± 5.01% of total putrescine in high and lower GDP countries, respectively (p < 0.001 for spermidine and p = 0.003 for putrescine). The amounts of spermine, sper midine, and putrescine from seafood in each total amount were small: 3.70 ± 2.03%, 1.59 ± 0.94%, and 2.16 ± 1.12%, respectively, for higher GDP countries, and were only 1.36 ± 1.99%, 0.47 ± 0.75%, and 1.02 ± 1.26%, respectively, for lower GDP countries. The per- centages for higher GDP countries were greater than those for lower GDP countries (p < 0.001). Individuals in higher GDP countries took these three polyamines from animal and seafood products much more than did High 0 20 40 60 80 100 Spermine source animal seafood crops Spermidine sourcePutrescine source Low High 0 20 40 60 80 100 Low High 0 20 40 60 80 100 Low Figure 3. Percentage of crops, seafood, and animal products relative to total amounts of spermine, spermidine, and putre- scine in higher GDP countries (High) and lower GDP countries (Low). those in lower GDP countries, while lower GDP coun- tries obtained polyamines from crops. 4. DISCUSSION Differences in socioeconomic status are known to af- fect the dietary pattern of individuals [22-25]. In this ecological study, we illustrate the relationship between GDP and dietary pattern on the basis of country. The results of the study where data were obtained from open databases have several similarities to those of previous epidemiological studies using personal and collective databases. Namely, higher socioeconomic status is asso- ciated with increased intake of fruits, seafood, and cheese. In the present study, despite the higher supply of crops products in lower GDP countries compared to higher GDP countries, fruits are preferred in higher GDP coun- tries. It is widely accepted that higher socioeconomic status is associated with increased intake of fruits and vegetables [11,23,26-31]. Similarly, as observed in the present study, many studies have shown a positive asso- ciation between socioeconomic status and seafood intake [28-29,32]. Although the association between dairy pro- ducts and socioeconomic status is not so apparent, some epidemiological studies have shown that skimmed milk is mainly consumed by the higher socioeconomic groups whereas the lower groups consume full-fat milk [11,31]. While we had insufficient information about the fat con- tent of dairy products, our findings that individuals in higher GDP countries consumed more cheese than whole milk are consistent with previous studies [22,30]. This study delineates the relationship between food polyamines and socioeconomic status of countries. The absolute amounts of three polyamines in all targeted countries obtained from database information are also similar to those of the previous studies in which about 35 µmol spermine, 55 µmol spermidine, and 160 µmol pu-  P. N. T. Binh et al. / HEALTH 2 (2010) 1327-1334 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 1333 Openly accessible at trescine were estimated to be consumed [33], and those in higher GDP countries were also similar to those of previous reports for higher GDP countries, Britain, Italy, Spain, Sweden, and Netherlands in which 350 to 500 µmol polyamines were estimated to be consumed [34]. The present study shows that individuals in higher GDP countries prefer foods rich in polyamine, especially spermine and putrescine, much more than those in lower GDP countries. Increased spermine supply in higher GDP countries seems due mainly to the increased supply of animal meat, in which spermine is abundant. Increased putrescine supply in higher GDP countries seems to be due to the increased supply of vegetables and fruit, where the putrescine concentration is high. This ecological study showed that socioeconomic sta- tus is associated not only with dietary pattern but also with the amount and proportion of polyamines. The dif- ference in food choice is considered to have some role in the prevalence of several diseases [35-47], and our pre- vious studies showed that increased polyamine intake contributes to decreases in age-associated pathological changes in mice [13]. Therefore, increased polyamine in- take may have some role on the difference in the preva- lence of diseases associated with socioeconomic dispar- ity. However, this is an ecological study and data do not necessarily indicate the personal food consumption, so, there may be confounding factor(s) between polyamine amount and socioeconomic status. Further analyses us- ing personal database are desired. 5. ACKNOWLEDGEMENTS Statement of conflicts of interest and funding: We have no conflict of interest to disclose. Sources of funding: This research received no specific grant from any funding agency in the public, commercial, or non-profit sectors. REFERENCES [1] Cooper, R., Cutler, J., Desvigne-Nickens, P., Fortmann, S.P., Friedman, L., Havlik, R., Hogelin, G., Marler, J., McGovern, P., Morosco, G., Mosca, L., Pearson, T., Stamler, J., Stryer, D. and Thom, T. (2000) Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the national conference on cardiovascular disease pre- vention. Circulation, 102(25), 3137-3147. [2] Kaplan, G.A. and Keil, J.E. (1993) Socioeconomic fac- tors and cardiovascular disease: A review of the literature. Circulation, 88(4), 1973-1998. [3] Rooks, R.N., Simonsick, E.M., Miles, T., Newman, A., Kritchevsky, S.B., Schulz, R. and Harris, T. (2002) The association of race and socioeconomic status with car- diovascular disease indicators among older adults in the health, aging, and body composition study. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 57(4), S247-256. [4] Tang, M., Chen, Y. and Krewski, D. (2003) Gender-re- lated differences in the association between socioeco- nomic status and self-reported diabetes. International Jour- nal of Epidemiology, 32(3), 381-385. [5] Everson, S.A., Maty, S.C., Lynch, J.W. and Kaplan, G.A. (2002) Epidemiologic evidence for the relation between socioeconomic status and depression, obesity, and diabe- tes. Journal of Psychosomatic Research, 53(4), 891-895. [6] Mullins, C.D., Cooke, J.L., Wang, J., Shaya, F.T., Hsu, D.V. and Brooks, S. (2004) Disparities in prevalence rates for lung, colorectal, breast, and prostate cancers in Medicaid. Journal of the National Medical Association, 96, 809-816. [7] Ward, E., Jemal, A., Cokkinides, V., Singh, G.K., Cardi- nez, C., Ghafoor, A. and Thun, M. (2004) Cancer dispari- ties by race/ethnicity and socioeconomic status. CA: A Cancer Journal for Clinicians, 54(2), 78-93. [8] Janssen, F., Kunst, A.E. and Mackenbach, J.P. (2006) Association between gross domestic product throughout the life course and old-age mortality across birth cohorts: parallel analyses of seven European countries, 1950-1999. Social Science & Medicine, 63(1), 239-254. [9] Tresserras, R., Canela, J., Alvarez, J., Sentis, J. and Sa- lleras, L. (1992) Infant mortality, per capita income, and adult illiteracy: An ecological approach. American Jour- nal of Public Health, 82, 435-438. [10] Beckfield, J. (2004) Does income inequality harm health? New cross-national evidence. Journal of Health and So- cial Behavior, 45(3), 231-248. [11] James, W.P., Nelson, M., Ralph, A. and Leather, S. (1997) Socioeconomic determinants of health. The contribution of nutrition to inequalities in health. British Medical Jour- nal, 314(7093), 1545-1549. [12] Mullie, P., Clarys, P., Hulens, M. and Vansant, G. (2010) Dietary patterns and socioeconomic position. European Journal of Clinical Nutrition, 64(3), 231-238. [13] Soda, K., Kano, Y., Sakuragi, M., Takao, K., Lefor, A. and Konishi, F. (2009) Long-term oral polyamine intake increases blood polyamine concentrations. Journal of Nu- tritional Science and Vitaminology, 55(4), 361-366. [14] Soda, K., Dobashi, Y., Kano, Y., Tsujinaka, S. and Ko- nishi, F. (2009) Polyamine-rich food decreases age-as- sociated pathology and mortality in aged mice. Experi- mental Gerontology, 44(11), 727-732. [15] Soda, K., Kano, Y., Nakamura, T., Kasono, K., Kawa- kami, M. and Konishi, F. (2005) Spermine, a natural poly- amine, suppresses LFA-1 expression on human lym- phocyte. The Journal of Immunology, 175(1), 237-245. [16] Bardocz, S., Brown, D.S., Grant, G. and Pusztai, A. (1990) Luminal and basolateral polyamine uptake by rat small intestine stimulated to grow by Phaseolus vulgaris lectin phytohaemagglutinin in vivo. Biochimica et Biophysica Acta, 1034, 46-52. [17] Nishimura, K., Araki, N., Ohnishi, Y. and Kozaki, S. (2001) Effects of dietary polyamine deficiency on Try- panosoma gambiense infection in rats. Experimental Pa- rasitology, 97(2), 95-101. [18] Nishibori, N., Fujihara, S. and Akatuki, T. (2007) Amounts of polyamines in foods in Japan and intake by Japanese. Food Chemistry, 100, 491-497.  P. N. T. Binh et al. / HEALTH 2 (2010) 1327-1334 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 1334 [19] Cipolla, B.G., Havouis, R. and Moulinoux, J.P. (2007) Polyamine contents in current foods: A basis for polyamine reduced diet and a study of its long term observance and tolerance in prostate carcinoma patients. Amino Acids, 33, 203-212. [20] Bardócz, S., Grant, G., Brown, D.S., Ralph, A. and Pusz- tai, A. (1993) Polyamines in food - Implications for growth and health. The Journal of Nutritional Biochemistry, 4(2), 66-71. [21] Gedrich, K. (2003) Determinants of nutritional behaviour: A multitude of levers for successful intervention? Appe- tite, 41(3), 231-238. [22] Sanchez-Villegas, A., Martinez, J.A., Prattala, R., Toledo, E., Roos, G. and Martinez-Gonzalez, M.A. (2003) A sys- tematic review of socioeconomic differences in food ha- bits in Europe: Consumption of cheese and milk. Euro- pean Journal of Clinical Nutrition, 57(8), 917-929. [23] Groth, M.V., Fagt, S. and Brondsted, L. (2001) Social determinants of dietary habits in Denmark. European Journal of Clinical Nutrition, 55(11), 959-966. [24] Hulshof, K.F., Brussaard, J.H., Kruizinga, A.G., Telman, J. and Lowik, M.R. (2003) Socio-economic status, die- tary intake and 10 y trends: The Dutch National Food Consumption Survey. European Journal of Clinical Nu- trition, 57(1), 128-137. [25] Shahar, D., Shai, I., Vardi, H., Shahar, A. and Fraser, D. (2005) Diet and eating habits in high and low socioeco- nomic groups. Nutrition, 21, 559-566. [26] Roos, G., Johansson, L., Kasmel, A., Klumbiene, J. and Prattala, R. (2001) Disparities in vegetable and fruit con- sumption: European cases from the north to the south. Public Health Nutrition, 4(1), 35-43. [27] Irala-Estevez, J.D., Groth, M., Johansson, L., Oltersdorf, U., Prattala, R. and Martinez-Gonzalez, M.A. (2000) A systematic review of socio-economic differences in food habits in Europe: Consumption of fruit and vegetables. European Journal of Clinical Nutrition, 54(9), 706-714. [28] Galobardes, B., Morabia, A. and Bernstein, M.S. (2001) Diet and socioeconomic position: Does the use of differ- ent indicators matter? International Journal of Epidemi- ology, 30(2), 334-340. [29] Johansson, L.R., Solvoll, K., Bjorneboe, G.E. and Drevon, C.A. (1998) Intake of very-long-chain n-3 fatty acids re- lated to social status and lifestyle. European Journal of Clinical Nutrition, 52(10), 716-721. [30] Roos, E., Prattala, R., Lahelma, E., Kleemola, P. and Pietinen, P. (1996) Modern and healthy: Socioeconomic differences in the quality of diet. European Journal of Clinical Nutrition, 50, 753-760. [31] Smith, A.M. and Baghurst, K.I. (1992) Public health implications of dietary differences between social status and oc- cupational category groups. Journal of Epidemi- ology & Community Health, 46(4), 409-416. [32] Barberger-Gateau, P., Jutand, M.A., Letenneur, L., Lar- rieu, S., Tavernier, B. and Berr, C. (2005) Correlates of regular fish consumption in French elderly community dwellers: Data from the Three-City study. European Jour- nal of Clinical Nutrition, 59(7), 817-825. [33] Zoumas-Morse, C., Rock, C.L., Quintana, E.L., Neu- houser, M.L., Gerner, E.W. and Meyskens, F.L. (2007) Development of a polyamine database for assessing die- tary intake. Journal of the American Dietetic Association, 107(6), 1024-1027. [34] Bardocz, S., Duguid, T.J., Brown, D.S., Grant, G., Pusztai, A., White, A. and Ralph, A. (1995) The importance of dietary polyamines in cell regeneration and growth. Brit- ish Journal of Nutrition, 73(6), 819-828. [35] Bosetti, C., Pelucchi, C. and La Vecchia, C. (2009) Diet and cancer in Mediterranean countries: Carbohydrates and fats. Public Health Nutrition, 12(Special Issue 9A), 1595- 1600. [36] Martinez-Gonzalez, M.A. and Sanchez-Villegas, A. (2004) The emerging role of Mediterranean diets in cardiovas- cular epidemiology: Monounsaturated fats, olive oil, red wine or the whole pattern? European Journal of Epide- miology, 19(1), 9-13. [37] Fung, T.T., Willett, W.C., Stampfer, M.J., Manson, J.E. and Hu, F.B. (2001) Dietary patterns and the risk of coro- nary heart disease in women. Archives of Internal Medi- cine, 161(15), 1857-1862. [38] Hu, F.B., Rimm, E.B., Stampfer, M.J., Ascherio, A., Spie- gelman, D. and Willett, W.C. (2000) Prospective study of major dietary patterns and risk of coronary heart disease in men. American Journal of Clinical Nutrition, 72(4), 912-921. [39] Fung, T., Hu, F.B., Fuchs, C., Giovannucci, E., Hunter, D.J., Stampfer, M.J., Colditz, G.A. and Willett, W.C. (2003) Major dietary patterns and the risk of colorectal cancer in women. Archives of Internal Medicine, 163, 309-314. [40] Handa, K. and Kreiger, N. (2002) Diet patterns and the risk of renal cell carcinoma. Public Health Nutrition, 5(6), 757-767. [41] Chen, H., Ward, M.H., Graubard, B.I., Heineman, E.F., Markin, R.M., Potischman, N.A., Russell, R.M., Weisen- burger, D.D. and Tucker, K.L. (2002) Dietary patterns and adeno - Carcinoma of the esophagus and distal stomach. American Journal of Clinical Nutrition, 75(1), 137-144. [42] Terry, P., Suzuki, R., Hu, F.B. and Wolk, A. (2001) A prospective study of major dietary patterns and the risk of breast cancer. Cancer Epidemiology, Biomarkers & Prevention, 10, 1281-1285. [43] Terry, P., Hu, F.B., Hansen, H. and Wolk, A. (2001) Pro- spective study of major dietary patterns and colorectal cancer risk in women. American Journal of Epidemiol- ogy, 154(12), 1143-1149. [44] Slattery, M.L., Boucher, K.M., Caan, B.J., Potter, J.D. and Ma, K.N. (1998) Eating patterns and risk of colon cancer. American Journal of Epidemiology, 148(1), 4-16. [45] Schulze, M.B., Hoffmann, K., Kroke, A. and Boeing, H. (2003) Risk of hypertension among women in the EPIC- Potsdam Study: Comparison of relative risk estimates for exploratory and hypothesis-oriented dietary patterns. Ame- rican Journal of Epidemiology, 158(4 ), 365- 373. [46] He, K., Rimm, E.B., Merchant, A., Rosner, B.A., Stamp- fer, M.J., Willett, W.C. and Ascherio, A. (2002) Fish consumption and risk of stroke in men. The Journal of the American Medical Association, 288(24), 3130-3136. [47] van Dam, R.M., Rimm, E.B., Willett, W.C., Stampfer, M.J. and Hu, F.B. (2002) Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Annals of Internal Medicine, 136(3), 201-209.
|