 Vol.2, No.11, 1280-1286 (2010) doi:10.4236/health.2010.211190 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ Health Openly accessible at Development of time-resolved immunofluorometric assays for the detection of house dust mite-allergic IgE in human sera Ratchanoo Phiphatchaipaisarn1, Jundee Rabablert1*, Kornkarn Bramarapravati2, Duangthep Thongdee1, Nares Wongpitoon3, Worawan Durongpisitkul3, Nat Malainual4 1Department of Biology, Faculty of Science, Silpakorn University, Nakorn Pathom, Thailand; *Corresponding Author: jundee@su.ac.th; 2Department of Preclinical Science, Faculty of Medicine, Thammasat University, Patumthani, Thailand; 3Department of Pediatric, Police General Hospital, Bangkok, Thailand; 4Department of Parasitology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand. Received 6 September 2010; revised 15 September 2010; accepted 16 September 2010. ABSTRACT Dermatophagoides farinae and D. pteronyssi- nus are the prevalent house dust mites (HDM) in tropical countries and are associated with aller- gic diseases. This investigation developed a time- resolved immunofluorometric assay (TR-IFMA) for the first time to detect specific IgE antibody in patients with skin prick test positive to HDM but no detectable IgE by other means. Levels of IgE to natural and recombinant HDM allergens were measured by TR-IFMA in 50 HDM-allergic patients and 19 healthy participants compared to sandwich enzyme-linked immunosorbent as- say (ELISA). A recombinant allergen, rDerf2, showed a 14 kDa band corresponding to broad range proteins of natural HDM.TR-IFMA showed sensitivity lower than 0.35 kUA/l. TR-IFMA em- ploying three HDM antigens showed good cor- relations with sandwich ELISA at R2 0.93-0.96. TR-IFMA detected HDM IgE in 62, 62, 25 percent of allergic patient serum sample compared to 28, 32, and 22 percent detected by ELISA result using three HDM allergen. TR-IFMA also detec- ted 26.3, 31.6, and 5.3 percent positive samples from 19 healthy participants while ELISA showed 0, 5.3, and 0 percents IgE positive samples. The use of rDerf2 as an HDM allergen for the assay was verified with no statistically different from other HDM allergens. TR-IFMA showed lower detection limit than ELISA and yielded higher sensitivity for serum of people with allergic sym- ptoms with no detectable HDM IgE. It is antici- pated that TR-IFMA for HDM-specific IgE detec- tion will play an important role in future diagno- sis of HDM allergy in clinical laboratories and for different research purposes. Keywords: House Dust Mites; Time-Resolved Immunofluorometric Assay; Allergy; IgE 1. INTRODUCTION House-dust mites (HDMs) represent one of the most important allergen sources for the development of aller- gic diseases worldwide, particularly asthma and allergic rhinitis [1]. Dermatophagoides farinae (Df, originally known as American HDM) and Dermatophagoides pte- ronyssinus (Dp, European HDM) are the predominant species in tropical and subtropical climates [2]. More than 80% of allergic patients are sensitized to mite aller- gens [3,4]. Positive skin prick tests (SPT) or serum IgE antibod- ies to HDMs are demonstrable in allergic patients. How- ever, if patients are allergic to histamine, skin prick test could lead to complicated immunoreaction harmful to the patients [5]. Enzyme-linked immunosorbent assay (ELISA) is the method used to quantify serum HDM-IgE in vitro in laboratories [6]. However, a more sensitive in vitro test is needed for people who have dust mite aller- gic clinical diagnosis but shown undetectable HDM-IgE in their serum. Monoclonal antibodies (mAbs) have been frequently used in allergy research. Their uses include the quantifi- cation of environmental allergen [7], allergens purifica- tion [8], and crystal structure [9]. Specificity for unique epitopes and unlimited in vitro production capability are the advantage of allergen-specific mAbs. The purpose of this study was to development a time- resolved immunofluorometric assay (TR-IFMA) for the measurement of HDM-specific IgE. The TRF is charac- terized by lower detection limits and greater specificity,  R. Phiphatchaipaisarn et al. / HEALTH 2 (2010) 1280-1286 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 1281 reproducibility and practicability [10]. Two group II HDM allergens and a group two recombinant vector were used for validation of the assay. 2. METERIALS AND METHODS 2.1. Reagents Commercial allergens were purchased from Allertech (ALK, UK). DELFIA® Wash, DELFIA® Assay Buffer, DELFIA® EU-labeled Streptavidin and DELFIA® En- hancement Solution were purchased from Perkin-Elmer Life Sciences (Wallac Oy, Turku, Finland). Monoclonal anti-group 2 antibodies (mAb 1D8 clone, Indoor biotec- nologies Ltd, Manchester, UK). Biotinylated rabbit-anti mouse IgG solution and Biotinylated-labeled Mouse anti-human IgE antibody purchased from SouthernBio- tech (Birmingham, USA). BCIP/NBT 1 Component Sub- strate and ABTS® Peroxidase 1 Component substrate So- lution were purchased from KPL (MD, USA). C96 Mi- croWell® plates were purchased from NuncTM (Roskilde, Denmark). The instrument used for measurement - DE- LFIA 1420 Automatic Immunoassay System - is the pro- duct of Perkin-Elmer Life Sciences (Wallac Oy, Turku, Finland). 2.2. HDM Extracts and Recombinants Mite Allergens Total HDM extracts of D. farinae (Df) and D. ptero- nyssinus (Dp) were prepared from Spent Mite medium from Siriraj Dust Mite Center, Bangkok, Thailand [7]. The extracts were stored at –20℃. Recombinant rDerf2 allergen was constructed with ligated cDNA in pPICZ vector. It was a kind gift from Assist. Prof. Dr. Surapon Piboonpocanun, the Institute of Molecular Biology and Genetics, Mahidol University, Thailand. The positive transformants were subjected to expression in Pichia pasroris [11,12]. The supernatants were stored at -20℃ until used. Proteins were separated by SDS-PAGE followed by transferring the fractionated proteins to a nitrocellulose membrane using method as described by the manufac- turer with a wet blotting apparatus (Bio-Rad, USA). The blotted membrane was blocked overnight at 4℃. The membrane was further incubated with Monoclonal anti- group 2 antibodies, followed by Biotinylated rabbit-anti mouse IgG solution (1:5000) and BCIP/NBT 1 Compo- nent Substrate. 2.3. Immunological Analysis 2.3.1. Enzyme-Linked Immuosorbent Assay (ELISA) Specific IgE to Df, rDerf2 and Dp were analyzed by the ImmunoCAP™ system by using the cut-off limit 0.35 kilo units of aprotinin-specific antibodies per litre (kUA/l), as recommended by the manufacturer. They were ana- lyzed by sandwich ELISA (will be referred to as ELISA thereafter) as previously described [13]. Wells were coated with 1D8 clone mAb and let incubated over-night. Then 1 ng/well allergens in PBS containing 1% BSA were add- ed. The wells were further incubated with serum (1:10), followed by biotinylated-labeled Mouse anti-human IgE antibody (1:1000), streptavidin-peroxidase and ABTS® Peroxidase 1 Component substrate. The result was meas- ured in a DELFIA 1420 Automatic Immunoassay System. 2.3.2. Time-Resolved Immunoflurometric Assay (TR-IFMA) Specific IgE to all three allergens were analyzed by the ImmunoCAP™ system by using the cut-off limit 0.35 kUA/l as recommended by the manufacturer. Df, recombinant Derf2 and Dp were analyzed by TR-IFMA [13]. Wells were coated with mAb and incubated over- night. After incubation, 1 ng/well allergens in PBS con- taining 1 % BSA were added. The wells were further incubated with serum (1:10), followed by biotinylated- labeled Mouse anti-human IgE antibody (1:1000), DEL- FIA® EU-labeled Streptavidin and DELFIA® enhance- ment solution. The result was measured in a DELFIA 1420 Automatic Immunoassay System. 2.4. Patients and Collection of Samples Fifty rhinitis patients with/without intermittent or per- sistent, mild-to-moderate asthma with positive result to Skin Prick Test (SPT) were selected for this investiga- tion by Dr. Nares Wongpitoon or Dr. Voravan Durong- pisitkul of Department of Pediatric, Police General Hos- pital, Bangkok, Thailand. As the control group, 19 heal- thy subjects with no history of allergic diseases were in- cluded. The study was approved by the Ethics committee of the Police general Hospital and written informed con- sent was obtained from all participants. Five milliliter of venous blood was taken from each participant. The blood was centrifuged within 6 hr of co- llection at 400 g for 30 min. The serum was then collected and stored frozen at –20℃. In order to quantify specific IgE levels, serum from three highest HDM-sensitized individuals as analyzed by ELISA and TR-IFMA were analyzed by the Immuno- CAP™ system at the laboratory of Faculty of Medicine, Mahidol University, Bangkok, Thailand. The IgE levels in several dilutions of the reference serum were analyzed. Fifth calibrator points in two-fold dilutions ranging 0.22-3.49, 0.22-3.49 and 0.28-4.55 kUA/l of Df, rDerf2 and Dp specific IgE respectively were made for use in 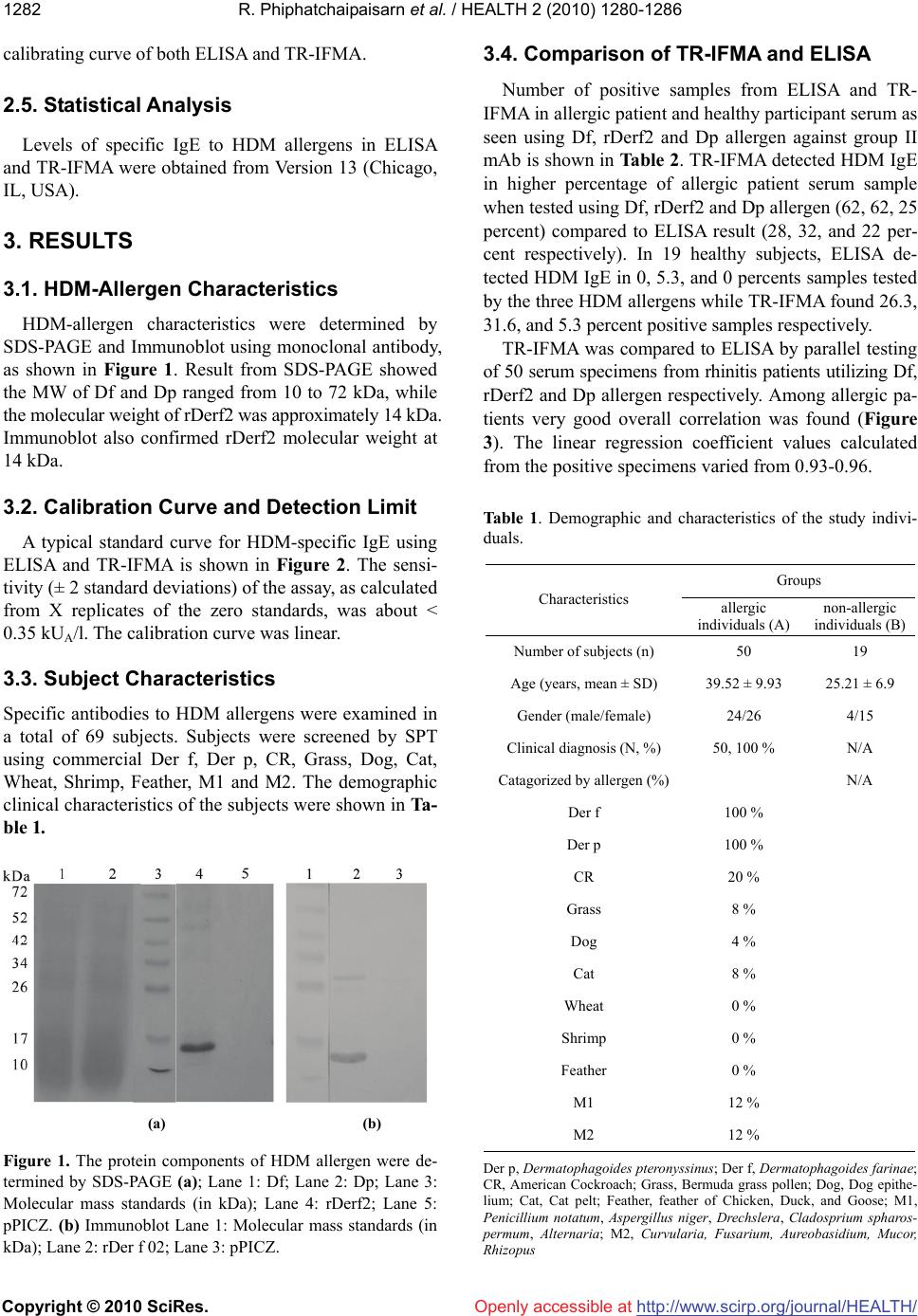 R. Phiphatchaipaisarn et al. / HEALTH 2 (2010) 1280-1286 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 1282 Openly accessible at calibrating curve of both ELISA and TR-IFMA. 2.5. Statistical Analysis Levels of specific IgE to HDM allergens in ELISA and TR-IFMA were obtained from Version 13 (Chicago, IL, USA). 3. RESULTS 3.1. HDM-Allergen Characteristics HDM-allergen characteristics were determined by SDS-PAGE and Immunoblot using monoclonal antibody, as shown in Figure 1. Result from SDS-PAGE showed the MW of Df and Dp ranged from 10 to 72 kDa, while the molecular weight of rDerf2 was approximately 14 kDa. Immunoblot also confirmed rDerf2 molecular weight at 14 kDa. 3.2. Calibration Curve and Detection Limit A typical standard curve for HDM-specific IgE using ELISA and TR-IFMA is shown in Figure 2. The sensi- tivity (± 2 standard deviations) of the assay, as calculated from X replicates of the zero standards, was about < 0.35 kUA/l. The calibration curve was linear. 3.3. Subject Characteristics Specific antibodies to HDM allergens were examined in a total of 69 subjects. Subjects were screened by SPT using commercial Der f, Der p, CR, Grass, Dog, Cat, Wheat, Shrimp, Feather, M1 and M2. The demographic clinical characteristics of the subjects were shown in Ta- ble 1. (a) (b) Figure 1. The protein components of HDM allergen were de- termined by SDS-PAGE (a); Lane 1: Df; Lane 2: Dp; Lane 3: Molecular mass standards (in kDa); Lane 4: rDerf2; Lane 5: pPICZ. (b) Immunoblot Lane 1: Molecular mass standards (in kDa); Lane 2: rDer f 02; Lane 3: pPICZ. 3.4. Comparison of TR-IFMA and ELISA Number of positive samples from ELISA and TR- IFMA in allergic patient and healthy participant serum as seen using Df, rDerf2 and Dp allergen against group II mAb is shown in Table 2. TR-IFMA detected HDM IgE in higher percentage of allergic patient serum sample when tested using Df, rDerf2 and Dp allergen (62, 62, 25 percent) compared to ELISA result (28, 32, and 22 per- cent respectively). In 19 healthy subjects, ELISA de- tected HDM IgE in 0, 5.3, and 0 percents samples tested by the three HDM allergens while TR-IFMA found 26.3, 31.6, and 5.3 percent positive samples respectively. TR-IFMA was compared to ELISA by parallel testing of 50 serum specimens from rhinitis patients utilizing Df, rDerf2 and Dp allergen respectively. Among allergic pa- tients very good overall correlation was found (Figure 3). The linear regression coefficient values calculated from the positive specimens varied from 0.93-0.96. Table 1. Demographic and characteristics of the study indivi- duals. Groups Characteristics allergic individuals (A) non-allergic individuals (B) Number of subjects (n) 50 19 Age (years, mean ± SD) 39.52 ± 9.93 25.21 ± 6.9 Gender (male/female) 24/26 4/15 Clinical diagnosis (N, %) 50, 100 % N/A Catagorized by allergen (%) N/A Der f 100 % Der p 100 % CR 20 % Grass 8 % Dog 4 % Cat 8 % Wheat 0 % Shrimp 0 % Feather 0 % M1 12 % M2 12 % Der p, Dermatophagoides pteronyssinus; Der f, Dermatophagoides farinae; CR, American Cockroach; Grass, Bermuda grass pollen; Dog, Dog epithe- lium; Cat, Cat pelt; Feather, feather of Chicken, Duck, and Goose; M1, Penicillium notatum, Aspergillus niger, Drechslera, Cladosprium spharos- permum, Alternaria; M2, Curvularia, Fusarium, Aureobasidium, Mucor, hizopus R 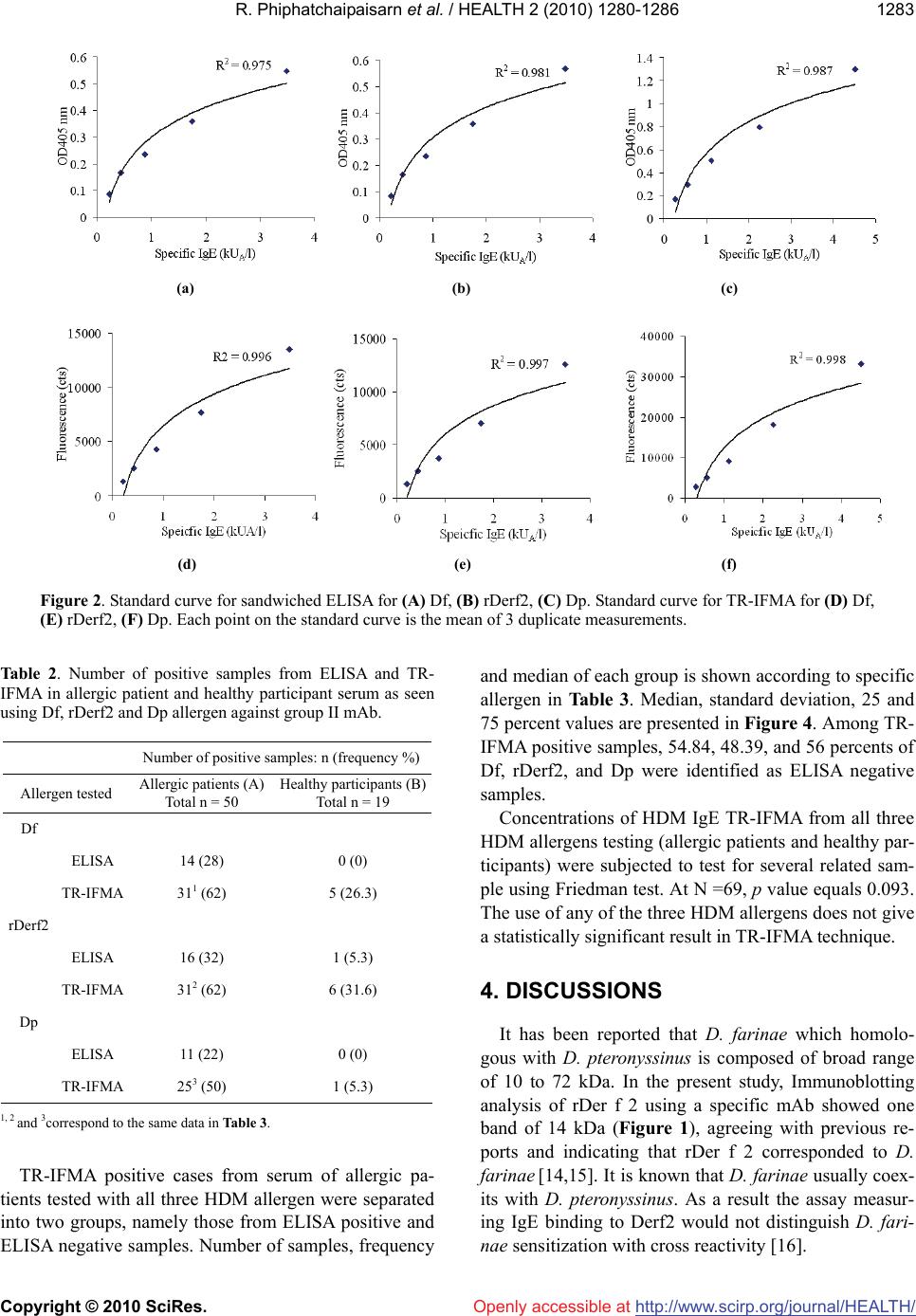 R. Phiphatchaipaisarn et al. / HEALTH 2 (2010) 1280-1286 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 1283 (a) (b) (c) (d) (e) (f) Figure 2. Standard curve for sandwiched ELISA for (A) Df, (B) rDerf2, (C) Dp. Standard curve for TR-IFMA for (D) Df, (E) rDerf2, (F) Dp. Each point on the standard curve is the mean of 3 duplicate measurements. Table 2. Number of positive samples from ELISA and TR- IFMA in allergic patient and healthy participant serum as seen using Df, rDerf2 and Dp allergen against group II mAb. Openly accessible at Number of positive samples: n (frequency %) Allergen tested Allergic patients (A) Total n = 50 Healthy participants (B) Total n = 19 Df ELISA 14 (28) 0 (0) TR-IFMA 311 (62) 5 (26.3) rDerf2 ELISA 16 (32) 1 (5.3) TR-IFMA 312 (62) 6 (31.6) Dp ELISA 11 (22) 0 (0) TR-IFMA 253 (50) 1 (5.3) 1, 2 and 3correspond to the same data in Table 3. TR-IFMA positive cases from serum of allergic pa- tients tested with all three HDM allergen were separated into two groups, namely those from ELISA positive and ELISA negative samples. Number of samples, frequency and median of each group is shown according to specific allergen in Table 3. Median, standard deviation, 25 and 75 percent values are presented in Figure 4. Among TR- IFMA positive samples, 54.84, 48.39, and 56 percents of Df, rDerf2, and Dp were identified as ELISA negative samples. Concentrations of HDM IgE TR-IFMA from all three HDM allergens testing (allergic patients and healthy par- ticipants) were subjected to test for several related sam- ple using Friedman test. At N =69, p value equals 0.093. The use of any of the three HDM allergens does not give a statistically significant result in TR-IFMA technique. 4. DISCUSSIONS It has been reported that D. farinae which homolo- gous with D. pteronyssinus is composed of broad range of 10 to 72 kDa. In the present study, Immunoblotting analysis of rDer f 2 using a specific mAb showed one band of 14 kDa (Figure 1), agreeing with previous re- ports and indicating that rDer f 2 corresponded to D. farinae [14,15]. It is known that D. farinae usually coex- its with D. pteronyssinus. As a result the assay measur- ing IgE binding to Derf2 would not distinguish D. fari- nae sensitization with cross reactivity [16].  R. Phiphatchaipaisarn et al. / HEALTH 2 (2010) 1280-1286 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 1284 Openly accessible at (a) (b) (c) Figure 3. Correlation between levels of specific IgE against Df (a), rDerf2 (b) and Dp (c) measured by ELISA and TR-IFMA. Figure 4. TR-IFMA positive cases from serum of allergic pa- tients tested with all three HDM allergens. Median, standard deviation, 25 and 75 percent values are presented. HDM aller- gens Df: 1) ELISA negative 2) ELISA positive rDerf2: 3) ELISA negative 4) ELISA positive Dp: 5) ELISA negative 6) ELISA positive. 4.1. Levels of Specific IgE Reactivity to HDM Allergens: Lower Detection Limit and Increase Testing Sensitivity The IgE reactivity profile for the sera from allergic patients (n = 50) and healthy participants (n = 19) was determined by three different allergens, namely Df, rDer f 2 and Dp measured by TR-IFMA compared with ELISA. As shown in Table 2, the efficacy of recombinant an- tigen (rDerf 2) and natural antigen (Df or Dp) were ex- amined in allergic patients and non-allergic individuals by both TR-IFMA and ELISA. In allergic patients, we found that only 28, 32, and 22 percents result was ob- tained by ELISA, while 62, 62, and 50 percent of patient serum reacted with Df, rDerf2, and Dp respectively us- ing TR-IFMA. The increase in rate of HDM IgE detec- tion was significant at 34, 30 and 28 percents for all three allergen respectively. This is the first indication Table 3. TR-IFMA positive cases in serum from allergic pa- tients categorized by ELISA result in Df, rDerf2 and Dp test- ing. TR-IFMA positive cases in symptomatic patients HDM allergens ELISA positive ELISA negative Df: total 31 samples1 n (frequency %) 14 (45.16) 17 (54.84) Median (kUA/l) 13.37 1.81* rDerf2: total 31 samples2 n (frequency %) 16 (51.61) 15 (48.39) Median (kUA/l) 16.67 2.74* Dp: total 25 samples3 n (frequency %) 11 (44) 14 (56) Median (kUA/l) 37.19 1.66* Mann-Whitney Rank Sum Test; p < 0.001* suggesting that TR-IFMA of all three HDM allergens show higher sensitivity for serum of people with allergic symptoms with no detectable HDM IgE. In addition, when ELISA showed 0, 5.3, and 0 percent positive sam- ples in healthy participant serum, TR-IFMA detected 26.3, 31.6, and 5.3 percent positive samples. That is the 26.3, 21.3 and 5.3 percent higher detection rate across the board. In 50 HDM-SPT positive allergic subjects, 48 per- cents of this group showed cross-reactive IgE binding to all three allergens measured by TR-IFMA, while only 22 percent showed cross-reactive IgE binding to all three allergens measured by ELISA. Additionally, cross-reac- tive IgE to two allergens was also observed by both TR- IFMA and ELISA. TR-IFMA showed 10, 2 and 0 per- cent cross-reactivity between Df/rDerf2, rDerf2/Dp and Df/Dp, while ELISA result showed 2, 0, and 0 percent respectively. In healthy participants there was no cross- reactivity report over all three antigens. Only 15.8 and 5.3  R. Phiphatchaipaisarn et al. / HEALTH 2 (2010) 1280-1286 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 1285 Openly accessible at percent cross-reactivity were reported for Df/rDerf2 and rDerf2/Dp. These numbers confirms TR-IFMA sensitivity over ELISA, and suggests that rDerf2 is a slightly better antigen as seen from cross-reactivity data. Table 3 and Figure 4 revealed lower detection limits and the sensitivity of TR-IFMA. All HDM allergens provided significantly different median between ELISA positive and negative samples in Mann-Whitney Rank Sum Test with p < 0.001. Figure 4 shows that even though ranges of all three ELISA negative groups show- ed overlap with the ELISA positive ones, one hundred percent of all three ELISA negative populations stayed within 40 percentile of the corresponding ELISA posi- tive population. TR-IFMA was able to showed real value of some ELISA negative samples beautifully in this de- monstration. This investigation employed three different HDM al- lergens, namely Df and Dp from natural sources, and a recombinant rDerf2. Result from the test for several re- lated sample using Friedman test showed that TR-IFMA result using all three allergens was not significantly dif- ferent from one another. Thus this investigation suggests that rDerf2 can be used as a HDM allergen in TR-IFMA. Since rDerf2 can be expressed in large quantity, this should ensure a homogeneous batch supply of HDM al- lergen for time to come. Immunoassays based on time-resolved fluorometry represents an attractive option that offers several advan- tages over other traditional techniques, including very high sensitivity, no use of radioactive reagents, stability of the reagents, low background interference and a wide test range [17, 18]. Additionally, the use of a specific mAb as capture antibody in this study avoided over estimation of allergens by reducing the cross-reactive epitopes rec- ognition. The use of specific polyclonal serum avoided the loss of detection produced by conformational changes affecting one or more epitopes. Hence, a specific anti- HDM polyclonal serum has been used as the secondary antibody in this investigation, unlike most of other im- munoassays described [19]. The time-resolved immunofluormetric assay develop- ed in this investigation provides sensitive and highly specific techniques for the determination of HDM-specific IgE in human sera. Measurements achieved with TR- IFMA and ELISA were highly correlated (R2 between 0.93-0.96). In regard to the limit of detection, the assay allows the detection of HDM-specific IgE as low as < 0.35 kUA/l, which is well below limits of detection re- ported by other techniques (Immunoblot, ELISA, RIA); TR-IFMA can therefore be considered as the most sensi- tive method for quantifying HDM-specific IgE. In addi- tion, the assay ranges of TR-IFMA is the wider than those of ELISA in our laboratory. Our data was consis- tent with previous reports [20,21]. It is clearly seen that TR-IFMA showed sensitivity to serum of allergic pa- tients with symptom whose serum were formerly HDM IgE negative using ELISA. The analysis of real samples from HDM positive allergic patients compared to HDM negative but positive to aeroallergen demonstrated that a broad range of HDM-specific IgE could be detected with the present techniques. 5. CONCLUSIONS TR-IFMA was developed for HDM allergen IgE test- ing and evaluated. Such improvements may be useful for screening of HDM allergy and other aeroallergens. TR- IFMA of all Df, rDerf2 and Dp showed lower detection limit than ELISA and yielded higher sensitivity for se- rum of people with allergic symptoms with no detectable HDM IgE. Recombinant HDM allergen, rDerf2, was pro- ven to be as good an allergen as its natural counterpart, namely Df and Dp. It is anticipated that TR-IFMA for HDM-specific IgE detection will play an important role in future diagnosis of HDM allergy in clinical laborato- ries and for different research purposes. 6. ACKNOWLEDGEMENTS This study was supported by the TRF (MRG-WII 505S061), a par- tial funding from Thailand Research Fund No. MRG-WII525S092 and DIG5180004. The authors thank Associate Professor Dr. Darawan Wanachiwanawin, Department of Parasitology, Faculty of Medicine Siriraj Hospital, Mahidol University for laboratory facility, and Assis- tant Professor Dr. Junya Pattaraarchachai, Faculty of Medicine, Tham- masat University for statistic analysis. REFERENCES [1] Arruda, L.K., Rizzo, M.C., Chapman, M.D., Fernandez- Caldas, E., Baggio, D., Platts-Mills, T.A. and Naspitz, C.K. (1991) Exposure and sensitization to dust mite al- lergens among asthmatic children in São Paulo, Brazil. Clinical & Experimental Allergy, 21(4), 433-439. [2] Colloff, M.J. (1998) Distribution and abundance of dust mites within homes. Allergy, 53, 24-27. [3] Nadchatram, M. (2005) House dust mites, our intimate associates. Tropical Biomedicine, 22(1), 23-37. [4] Thomas, W.R., Smith, W.A. and Hales, B.J. (2004) The allergenic specificities of the house dust mite. Chang Gung Medical Journal, 27(8), 563-569. [5] Williams, P., Sewell, W.A., Bunn, C., Pumphrey, R., Read, G. and Jolles, S. (2008) Clinical immunology review se- ries: an approach to the use of the immunology labora- tory in the diagnosis of clinical allergy. Clinical & Ex- perimental Immunology, 153(1), 10-18. [6] Bush, R.K., Wood, R.A. and Eggleston, P.A. (1998) La- boratory animal allergy. Journal of Allergy and Clinical Immunology, 102(1), 99-112.  R. Phiphatchaipaisarn et al. / HEALTH 2 (2010) 1280-1286 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 1286 [7] Chapman, M.D., Heymann, P.W., Wilkins, S.R., Brown, M.J. and Platts-Mills, T.A. (1987) Monoclonal immuno- assays for major dust mite (Dermatophagoides) allergens, Der p I and Der f I, and quantitative analysis of the aller- gen content of mite and house dust extracts. Journal of Allergy and Clinical Immunology, 80(2), 184-194. [8] Ferrandiz, R., Casas, R. and Dreborg, S. (1997) Purifica- tion and IgE binding capacity of Der s 3, a major allergen from Dermatophagoides siboney. Clinical & Experimen- tal Allergy, 27(6), 700-704. [9] Suzuki, M., Tanaka, Y., Korematsu, S., Mikami, B. and Minato, N. (2006) Crystal structure and some properties of a major house dust mite allergen, Derf 2. Biochemical and Biophysical Research Communications, 339(2), 679- 686. [10] Sheng, S.L., Bao, S.H., Huang, G. and Wang, L.M. (2008) Development of time-resolved immunofluorometric as- says for vascular endothelial growth factor and applica- tion on plasma of patients with gastric tumours. Clinical & Experimental Immunology, 151(3), 459-466. [11] Piboonpocanun, S., Malainual, N., Jirapongsananuruk, O., Vichyanond, P. and Thomas, W.R. (2006) Genetic poly- morphisms of major house dust mite allergens. Clinical & Experimental Allergy, 36(4), 510-516. [12] Tanyaratsrisakul, S., Malainual, N., Jirapongsananuruk, O., Smith, W.A., Thomas, W.R. and Piboonpocanun, S. (2009) Structural and IgE binding analyses of recombi- nant der p 2 expressed from the hosts escherichia coli and pichia pastoris. International Archives of Allergy and Immunology, 29(3), 190-198. [13] Hales, B.J., Hazell, L.A., Smith, W. and Thomas, W.R. (2002) Genetic variation of Der p 2 allergens: effects on T cell responses and immunoglobulin E binding. Clinical & Experimental Allergy, 32(10), 1461-1467. [14] Koyanagi, S., Maeda, T., Murakami, T., Kawatsu, K., Sugawara, K., Miyatsu, Y. and Mizokami, H. (2008) Large-scale production of major house dust mite allergen der f 2 mutant (C8/119S) in escherichia coli. Journal of Bioscience and Bioengineering, 106(4), 387-392. [15] Pittner, G., Vrtala, S., Thomas, W.R., Weghofer, M., Kundi M., Horak, F., Kraft, D. and Valenta, R. (2004) Compo- nent-resolved diagnosis of house-dust mite allergy with purified natural and recombinant mite allergens. Clinical & Experimental Allergy, 34(4), 597-603. [16] Yasueda, H., Mita, H., Yui, Y. and Shida, T. (1989) Com- parative analysis of physicochemical and immunoche- mical properties of the two major allergens from Der- matophagoides pteronyssinus and the corresponding al- lergens from Dermatophagoides farinae. International Archives of Allergy and Applied Immunology, 88(4), 402- 407. [17] Parra, M.D., Tuomola, M., Cabezas-Herrera, J. and Ceron, J.J. (2006) Analytical and clinical validation of a time- resolved immunofluorometric assay (TR-IFMA) for canine C-reactive protein in serum. Veterinary Research Com- munications, 30(2), 113-126. [18] Yuan, A.S. and Gilbert, J.D. (1996) Time-resolved fluo- roimmunoassay for the determination of lisinopril and enalaprilat in human serum. Journal of Pharmaceutical and Biomedical Analysis, 14(7), 773-781. [19] Ferrandiz, R., Casas, R., Dreborg, S., Einarsson, R. and Fernandez, B. (1995) Crossreactivity between Dermato- phagoides siboney and other house dust mite allergens in sensitized asthmatic patients. Clinical & Experimental Allergy, 25(10), 929-934. [20] Wu, F.B., Ouyan, H.Q., Tang, X.Y. and Zhou, Z.X. (2008) “Double-antigen sandwich time-resolved immunofluoro- metric assay for the detection of anti-hepatitis C virus total antibodies with improved specificity and sensitiv- ity,” Journal of Medical Microbiology, 57, 947-953. [21] Sheng, S.L., Wang, Q. and Huang, G. (2007) Develop- ment of time-resolved immunofluorometric assays for CA 72-4 and application in sera of patients with gastric tumors. Clinica Chimica Acta, 380(1-2), 106-111.
|