Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2010, 3, 1073-1077 JBiSE doi:10.4236/jbise.2010.311139 Published Online November 2010 (http://www.SciRP.org/journal/jbise/). Published Online November 2010 in SciRes. http://www.scirp.org/journal/jbise Evaluation of titanium dental implants after early failure of osseointegration by means of X-ray photoelectron spectoscopy, electron microscopy and histological studies P. Lázaro1, M. Herrero1, F. J. Gil2 1Master de Implantología y Periodoncia, Universidad de Sevilla, Sevilla, Spain; 2CREB. Department Ciencia de los Materiales e Ingeniería Metalúrgica. ETSEIB. Universidad Politécnica de Cataluña, Barcelona, Spain. Email: francesc.xavier.gil@upc.edu Received 21 August 2010; revised 14 September 2010; accepted 20 September 2010. ABSTRACT In this work, we analysed 56 clinically failed and re- trieved implants by means of scanning electron mi- croscopy (SEM), X-ray photoelectron spectroscopy (XPS) and histological studies. The surface contami- nation was compared to that of unused control im- plants and with that of the same implants after cleaning in a basic medium. The surfaces of the un- used implants presented considerable contamination. In particular, high levels of carbon were detected. The nature of this C was elucidated by XPS analysis of the lubricant used in the machining process. The same contamination was observed in the retrieved implants. Histological studies were carried out by means of light microscopy. Fibrosis and granuloma- tous lesions were detected in the tissues. XPS analysis indicated the presence of traces of other elements (Na, Ca, Zn, S, F, etc.) that were not related to impurities in cpTi. We examined a cleaning process in a basic medium that eliminates the organic components of the implant surfaces. The cleaned implants were im- planted in the patients and the results were excellent. None of the implants failed in following 7 months. Keywords: Dental Implant; Titanium; Contamination; Osseointegration 1. INTRODUCTION Titanium is a highly reactive metal. In fact, its surface is always covered by a native passivating oxide layer (about 2-5 nm thick). Titanium dioxide is the material that is actually in contact with the body tissues. This allows the direct apposition of the bone to the implant surface. Control of the surface characteristics of titanium implants is essential to achieving optimal tissue response during the healing of bone and soft tissues. A lack of attention to surface cleanliness could be re- sponsible for the loss of an implant—perhaps years after surgery. The origin of surface contaminants warrants consideration. Serious contamination could arise during the production of the material. Impurities from material processing migrate to grain boundaries and surfaces; hence, a nominally pure material (> 99%) might exhibit considerable deviation from the bulk composition at the surface if these impurities are not removed. The sterili- zation procedure and the handling of the implant during surgery are other possible sources of contamination. In this study, unused and non-osseointegrated screw-type dental implants were examined by means of X-ray pho- toelectron spectroscopy (XPS), scanning electron mi- croscopy (SEM) and histological studies. We also evalu- ated the efficiency of a technique for cleaning contami- nated implant surfaces. 2. MATERIALS AND METHODS Fifty-six root-form implants were retrieved from 32 pa- tients who showed no medical or dental contraindica- tions to implant placement. All of the implants were of the same brand, bore the CE marking (European Union standard) and were made of titanium CP grade 2. The implants had an acid-etched surface with a roughness of about Ra = 2.2 m. The availability of these failed im- plants depended solely on fate and on the collaboration of the Spanish Implantology Society. The dentists indi- cated the “residence time” of each implant, which ranged from 1 week to 6 months. This “residence time” is not the same as the “failure time”. Immediately after each implant surgery (following the protocol), no complications or infections were noted. However, after a period ranging from 2 weeks to 6 months, the implants were found to be encapsulated (fi-  P. Lázaro et al. / J. Biomedical Science and Engineering 3 (2010) 1073-1077 Copyright © 2010 SciRes. JBiSE 1074 brous tissues) in the maxilla or in the jawbone and were easily withdrawn. The mobile implants were lifted out of their sites using forceps. The retrieved implants were immediately immersed in 4% formaline (in glass con- tainers) and kept there until the analyses. In the first part of the study, the 56 failed implants and 16 unused control implants were first examined by X-ray induced photoelectron spectroscopy (XPS, Physical Electronics 5500). An Al Kα radiation (1486.6 eV) sour- ce was used after monochromatization, focusing on the sample surface. Spectra were recorded from areas rang- ing in size from 300 µm to 1000 µm in diameter. The analyses were carried out in a vacuum (0.6 10-6 Pa) [1-3]. The software used was Multipack V 6.0. Due to the complex geometry of the titanium implant screw, we are unable to present depth profile information. The 16 unused control implants were mounted with- out pre-treatment. Eight packages of these implants were opened in the XPS chamber prior to evacuation in order to minimize contact time with the air. Another eight packages were opened and mounted under more clini- cally realistic conditions, with a longer contact time with the air. All specimens were observed in a JEOL 6400 scan- ning electron microscope at an electron beam voltage of 20 KV. Body tissues surrounding 6 implants were obtained from 4 patients. All samples were fixed in 10% formalin, embedded in paraffin and serially sectioned. Hematoxy- line eosine and histochemical Perls coloration were used for the histological light microscope observations. In the second part of the study, 16 failed implants were used to test a cleaning technique for removing sur- face contaminants: cleaning in ultrasonic baths contain- ing NaOH in aqueous solution with lauryl sulphate for 15 min. Forty unused implants that underwent the cleaning treatment were implanted in 22 patients with the col- laboration of dentists. The patients were monitored every day during the first week after implantation, every three days during the second week and every week for the next 7 months. 3. RESULTS AND DISCUSSION The SEM observations of the unused implants showed the roughness produced by the acid-etching process (H2SO4 and HF), as shown in Figure 1. At low magnifi- cation, the implants looked rather clean. However, at high magnification it was evident that some areas of the implant surface were covered by a dense layer of organic matter (Figure 2). This contamination was mainly on the upper part of the implant (near the prosthetic part). XPS showed the presence of carbon, oxygen, nitrogen, 700 μm Figure 1. Unused implant at low magnification. 100 μm Figure 2. Dark stains found in the thread of an unused dental implant. titanium, fluorine, sodium, and sulphur. The as-received Ti implants displayed considerable surface hydrocarbon contamination (Table 1). On the surface, 40% atom C was found. These analyses exclude hydrogen, which is not detected by XPS. From the sur- face topographies revealed in the SEM micrographs (Figure 2), it seems unlikely that hydrocarbon would form continuously in layers. Rather, we suspect that the hydrocarbon was located mainly in the pits and pores on the plate surfaces. The amount of hydrocarbon detected on the plates varied. The nature of the hydrocarbon contamination was studied in greater detail. The narrow scan of the C 1s region was fitted using 4 peaks. The main peak is at 285.0 eV, with the subsequent peaks showing shifts of + 1.4, + 2.9 and 4.2 eV. The main peak is assigned to the C-C and C-H environment (48%) and the shifts are con- 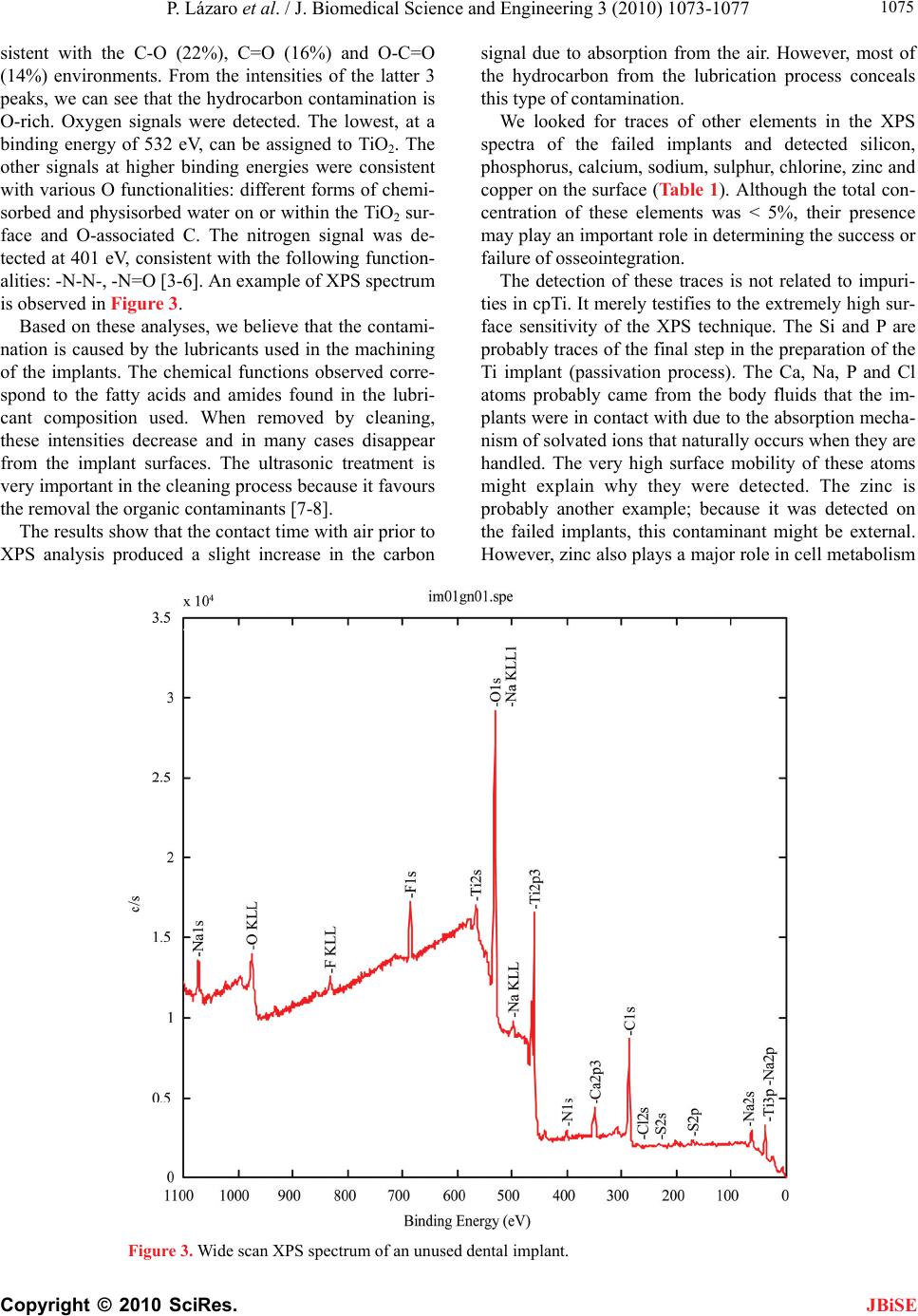 P. Lázaro et al. / J. Biomedical Science and Engineering 3 (2010) 1073-1077 Copyright © 2010 SciRes. JBiSE 1075 sistent with the C-O (22%), C=O (16%) and O-C=O (14%) environments. From the intensities of the latter 3 peaks, we can see that the hydrocarbon contamination is O-rich. Oxygen signals were detected. The lowest, at a binding energy of 532 eV, can be assigned to TiO2. The other signals at higher binding energies were consistent with various O functionalities: different forms of chemi- sorbed and physisorbed water on or within the TiO2 sur- face and O-associated C. The nitrogen signal was de- tected at 401 eV, consistent with the following function- alities: -N-N-, -N=O [3-6]. An example of XPS spectrum is observed in Figure 3. Based on these analyses, we believe that the contami- nation is caused by the lubricants used in the machining of the implants. The chemical functions observed corre- spond to the fatty acids and amides found in the lubri- cant composition used. When removed by cleaning, these intensities decrease and in many cases disappear from the implant surfaces. The ultrasonic treatment is very important in the cleaning process because it favours the removal the organic contaminants [7-8]. The results show that the contact time with air prior to XPS analysis produced a slight increase in the carbon signal due to absorption from the air. However, most of the hydrocarbon from the lubrication process conceals this type of contamination. We looked for traces of other elements in the XPS spectra of the failed implants and detected silicon, phosphorus, calcium, sodium, sulphur, chlorine, zinc and copper on the surface (Table 1). Although the total con- centration of these elements was < 5%, their presence may play an important role in determining the success or failure of osseointegration. The detection of these traces is not related to impuri- ties in cpTi. It merely testifies to the extremely high sur- face sensitivity of the XPS technique. The Si and P are probably traces of the final step in the preparation of the Ti implant (passivation process). The Ca, Na, P and Cl atoms probably came from the body fluids that the im- plants were in contact with due to the absorption mecha- nism of solvated ions that naturally occurs when they are handled. The very high surface mobility of these atoms might explain why they were detected. The zinc is probably another example; because it was detected on the failed implants, this contaminant might be external. However, zinc also plays a major role in cell metabolism Figure 3. Wide scan XPS spectrum of an unused dental implant.  P. Lázaro et al. / J. Biomedical Science and Engineering 3 (2010) 1073-1077 Copyright © 2010 SciRes. JBiSE 1076 Table 1. Surface chemical composition in atomic % determined by XPS. (Standard deviation). n Ti C Ca N O F S Cl Na Mg Si Cu Sn Zn Unused control 16 10.2 (3.1) 41.0 (8.6) 0.4 (0.1) 6.0 (1.3) 36.1 (8.8) 2.0 (0.8) 0.2 (0.1) 1.4 (0.4) 1.1 (0.4) 0.1 (0.0) 0.2 (0.1) 0.5 (0.1) 0.2 (0.1) 0.3 (0.1) Failed 52 4.0 (1.8) 40.2 (10.6) 1.6 (0.5) 6.2 (1.3) 38.5 (9.1) 2.1 (0.7) 0.3 (0.1) 1.2 (0.3) 1.2 (0.5) 0.2 (0.1) 1.8 (0.2) 1.6 (0.4) 0.6 (0.1) 1.7 (0.3) Cleaned 16 26.0 (8.7) 21.5 (5.6) 1.1 (0.2) 7.3 (1.4) 29.6 (6.9) 1.8 (0.5) 0.1 (0.0) 2.0 (0.6) 3.0 (0.2) trace 0.6 (0.3) trace trace trace and division. It is a cofactor of many metalloenzymes and binds to proteins involved in the regulation of DNA synthesis [9-10]. These major functions of zinc probably account for the fact that zinc is one of the most abundant trace elements in the human body. Zinc and Cu, which were detected by XPS in the implants, are retained in the mouth after rinsing with solutions or brushing with toothpaste containing Zn o Cu salts [10-13]. These metal ions are added to toothpastes and mouthwash solutions as antiplaque agents. Their activity is believed to be due to retention in “oral microreservoirs” such as soft oral tissues, tooth surfaces and bacterial plaque (12). Further studies should be undertaken to verify whether these cations (Zn, Cu) interfere with titanium implant surfaces, thereby preventing correct osseointegration. F and S were incorporated during the acid-etching process. The histological studies show that the implants were encapsulated in loose connective tissue, where fibro- blasts, a small number of plasma cells and lymphocytes were observed. In some cases, a small amount of osteoid tissue was found near the apical end of the implants. In some cases, in areas of denser connective tissue, colla- gen fibres ran parallel to the implant threads [12-15]. We did not find foreign bodies in the granulation tissue around the implants. In two cases, disorganized hemor- rhagic tissue with a significant degree of inflammatory cell infiltrate was observed in contact with the implant threads. Infected areas with polymorphonuclear cells were also observed. However, areas of necrosis were not seen. The cleaned implants after 7 months implanted have shown a good osseointegration in all cases [15-20]. The investigation of the metabolic conditions govern- ing the microenvironment of Ti implants will help de- termine which physical and chemical properties optimize the osseointegration process. The data found in this work is interesting in view of the considerable care taken in the surface use of Ti implants. 4. CONCLUSIONS The main contamination consists of a hydrocarbon layer rich in oxygen-carbon functionalities such as fatty acids and amides, which are usually used as lubricants for ti- tanium machining. In the XPS analysis, other elements (Ca, Na, S. P, Zn, etc.) were detected, although at lower yet significant concentrations. The contamination causes a failure of osseointegration shortly after implant place- ment. Histological studies showed an encapsulation of the implant with connective tissue. The tested cleaning process was successful and the treated implants were successfully osseointegrated. 5. ACKNOWLEDGEMENTS This work was made possible by a grant from the CICYT and the Spanish Implantolgy Society (SEI). REFERENCES [1] Albrektsson, T., Branemark, P.I., Hansson, H.A. and Lindström, J. (1981) Acta Orthopedica Scandinavia, 52, 155. [2] Arys, A., Philippart, C., Dourov, N., He, Y. and Pireaux, J.J. (1998) Journal of Biomedical Materials Research Part B: Applied Biomaterials, 43, 300-312. [3] Kasemo, B. and Lausma, J. (2006) Surface science as- pects on inorganic biomaterials. CRC Critical Reviews in Biocompatibility, 2, 335-380. [4] Kasemo, B. (1983) Journal of Prosthetic Dentistry, 49, 832-837. [5] Keller, J., Stanford, C.M., Wightman, J.P., Draughn, A.A., Raharias, R. (1994) J. Biomed. Mater. Res., 28, 939-946. [6] Rodriguez, D., Manero, J.M., Gil, F.J. and Planell, J.A. (2001) J. of Mater. Sci. Mater. in Med., 12, 935-937. [7] Muntasell, J., Tamarit, J.Ll., Guilemany, J.M., Gil, F.J. and Cesari, E. (1988) Mater. Res. Bull., 23, 1585-1590. [8] Muntasell, J., Tamarit, J.Ll., Cesari, E., Guilemany, J.M. and Gil, F.J. (1989) Mater. Res. Bull., 24, 445-452. [9] Mouhyi, J., Sennerby, L., Pireaux, J.J., Dourov, N. Nammour, S. and Van Reck, J. (1998) Clin. Oral Impl. Res., 9, 185-194. [10] Afseth, J., Helgeland, K. and Bonesvoll, P.J. (1983) J. Dent. Res., 91, 42-45. [11] Ong, J.L., Lucas, L.C., Raikar, G.N., Connaster, R. and Gregory, J.C. (1995) J. of Mater. Sci.: Mater. in Med., 6, 113-119. [12] Ameen, A.P., Short, R.D., Johns, R. and Schwach, G. (1993) Clin. Oral Impl. Res., 4, 144-150. [13] Afseth, J., Helgeland, K. and Bonesvoll, P.J. (1983) J. Dent. Res., 91, 42-45. [14] Gil, F.J., Manero, J.M., Ginebra, M.P., Planell, J.A. (2003) Mat. Sci. and Eng. A., 349, 150-155.  P. Lázaro et al. / J. Biomedical Science and Engineering 3 (2010) 1073-1077 Copyright © 2010 SciRes. JBiSE 1077 [15] Aparicio, C., Gil, F.J., Fonseca, C., Barbosa, M. and Planell, J.A. (2003) Biomater, 24(2), 263-273. [16] Harrap, G.J., Best, J.S. and Saxton, C.A. (1984) Arch. Oral Biol., 29, 87-92. [17] Gilbert, R.J. and Ingram, G.S. (1988) J. Pharm. Phar- macol., 40, 399-402. [18] Aparicio, C., Gil, F.J., Planell, J.A. and Engel, E. (2002) J. of Mater. Sci.: Mater. in Med., 13, 1105-1111. [19] Rodriguez, D., Gil, F.J., Jorge, E., Zapata, A. and Planell, J.A. (1999) J. of Mater. Sci.: Mater. in Med., 10(12) 847-852. [20] Artola, A., Goñi, I., Gil, F.J., Ginebra, M.P., Manero, J.M. and Gurruchaga, M. (2004) J. of Biomed. Mater. Res. (Appl. Biomater) Part B. 64B(1), 44-54. |

