Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2010, 3, 1069-1072 JBiSE doi:10.4236/jbise.2010.311138 Published Online November 2010 (http://www.SciRP.org/journal/jbise/). Published Online November 2010 in SciRes. http://www.scirp.org/journal/jbise Basic peptide protamine exerts antimicrobial activity against periodontopathic bacteria ——Growth inhibition of periodontopathic bacteria by protamine Tadashi Miura1, Keishi Iohara2, Tetsuo Kato3, Kazuyuki Ishihara4, Masao Yoshinari1 1Division of Oral Implants Research, Oral Health Science Center, Tokyo Dental College, Mihama-ku, Japan; 2Central Research Institute, Maruha Nichiro Holdings, Inc., Tsukuba, Japan; 3Laboratory of Chemistry, Oral Health Science Center; Tokyo Dental College, Mihama-ku, Japan; 4Department of Microbiology, Oral Health Science Center; Tokyo Dental College, Mihama-ku, Japan. Email: tamiura@tdc.ac.jp Received 28 September 2010; revised 18 October 2010; accepted 21 October 2010. ABSTRACT Protamine was investigated for its antibacterial ac- tivity against the periodontal pathogens, Porphyro- monas gingivalis, Prevotella intermedia and Aggrega- tibacter actinomycetemcomitans. We determined the minimum inhibitory concentrations of protamine and its hydrolysate and their bactericidal activity. Pro- tamine inhibited the growth of all periodontopathic bacteria tested on agar plates. Protamine, which MIC was 6.3 × 10-7 g L-1, was most effective against P. gin- givalis. The antibacterial effect of native protamine was higher than that of its hydrolysate. An ATP bio- luminescence assay revealed that protamine showed bactericidal activity against P. gingivalis in a time- dependent manner. These results indicate that pro- tamine could be candidate peptide for prevention of P. gingivalis infection. Keywords: Antimicrobial Peptide; Periodontopathic Ba- cteria; Implantitis; Protamine 1. INTRODUCTION Bacterial infection arising from an accumulation of mi- crobial plaque around dental implants is a major cause of periodontal disease including peri-implantitis. Five spe- cies of periodontal pathogen detected in cases where a titanium implant was used [1]. This indicates the impor- tance of maintaining biofilm-free surfaces on both the sub-gingival and supra-gingival portions of dental im- plants in preventing peri-implantitis. Porphyromonas gingivalis [2] and Aggregatibacter actinomycetemcomi- tans [3] are believed to be major etiologic bacteria in many cases of human periodontitis. Prevotella interme- dia [4] has also been associated with human periodontal disease. Research at our laboratory has focused on de- veloping a system of defense against infection on dental implant surfaces [5]. Reducing plaque has been empha- sized in the prevention of periodontal diseases, including peri-implantitis [6]. It is possible that using antimicrobial materials can help reduce oral bacteria. The loading of antimicrobial peptides onto the surface of a dental implant is an important candidate for achiev- ing antimicrobial activity. Antimicrobial peptides, a promising new type of antimicrobial agent, offer the advantage of not easily acting as antigens against the host [7]. One of the aims of our ongoing study is to cre- ate a defense system against peri-implantitis. We are currently exploring the antimicrobial potential of pep- tides affixed to the surfaces of dental materials [8]. We investigated the anti-periodontopathic activity of the antimicrobial peptide, protamine and its hydrolysate. Protamine, a basic peptide [9], discovered from salmon testicles by F. Miescher in 1869 was later found to be involved in the folding of nucleic acids in salmon sperm [10]. Protamine has been isolated from more than 50 kinds of fish, and is used as a natural food preservative. Protamine has several characteristics, including high stability under heat and a preservative effect in neutral or alkaline food. It does not influence the texture, smell, or taste of food to which it is added. It can also be eaten as a raw material, and has been used as a food additive for many years. An acute toxicity test of protamine in mouse [11] and a sub-long-term toxicity test in rat [12] demon- strated its safety and confirmed that it was an excellent antibacterial agent in milt. The antibacterial activity of protamine is strongest against Gram-positive bacteria such as the Bacillus species [13] and lactobacilli [14], whereas its activity against fungi and yeast [15] is weak. Our previous study revealed that hydrolyzed protamine,  T. Miura et al. / J. Biomedical Science and Engineering 3 (2010) 1069-1072 Copyright © 2010 SciRes. JBiSE 1070 was most effective against the biofilm formation of C. albicans [16]. In this study, we investigated the inhibi- tory effect of protamine and its hydrolysate on growth of periodontopathic bacteria. 2. MATERIALS AND METHODS 2.1. Bacteria and Culture Condition For liquid culture, P. gingivalis ATCC 33277, ATCC53977, W50 (ATCC, American Type Culture Collection) and P. intermedia ATCC 25611 were cultured in trypticase soy broth (Becton Dickinson and Company, Sparks, MD, USA) supplemented with hemin (5 g L-1; Sigma Chemi- cal Co., St Louis, MO) and menadione (0.5 g L-1; Wako Pure Chemical Industries, Osaka, Japan). A. actimomy- cetemcomitans 310a and Y4 were cultured in Todd Hew- itt Broth (Becton Dickinson and Company) supple- mented with Yeast Extract (10 g L-1; Becton Dickinson and Company). For plate culture, the bacteria were grown on blood agar plates consisting of Tryptic soy agar (Becton Dickinson and Company) supplemented with 10% defibrinated horse blood, hemin (5 g L-1), and menadione (0.5 g L-1). Cultures were performed at 37°C in an anaerobic chamber filled with an atmosphere of 80% N2, 10% H2 and 10% CO2. 2.2. Preparation of Protamine and its Hydrolysates Protamine (designated as Prot) was obtained from Ma- ruha Nichiro foods, Inc., Tokyo, Japan. This product is prepared from the milt of salmon (Oncorhynchus keta), living in the northern part of Japan. This milt has been reported to contain four different molecular species [17] rich in arginine, the primary structures of which were reported to be as follows: PRRRRRSSSRPIRRRRRPRASRRRRRGGRRRR, PRRRRSSRRPVRRRRRPRVSRRRRRRGGRRRR, PRRRRSSSRPVRRRRRPRVSRRRRRRGGRRRR, PRRRRASRRIRRRRRPRVSRRRRRGGRRRR. The Prot consisted of 30 or 32 amino acids in this study, and the arginine residues constituted 60-70% of the amino acid sequences. Protamine hydrolysate (des- ignated as Brom) obtained by digestion with the enzyme bromelain [18], a cysteine protease, was kindly donated by Maruha Nichiro foods, Inc. 2.3. Evaluation of Minimum Inhibitory Concentration. Agar plates containing Prot or Brom were used to de- termine minimum inhibitory concentration (MIC). Pep- tide concentration was adjusted by stepwise dilution. Using a loop, each bacterial strain was streaked onto an agar plate. The agar plates were then incubated for 3-7 days in the anaerobic chamber at 37°C. Minimum in- hibitory concentration was defined as the lowest concen- tration of peptides that would inhibit the visible growth of the microorganism after incubation. The values were expressed as the mean ± SD of four experiments. 2.4. Antibacterial Activity of Protamine against P. gingivalis ATCC 33277 P. gingivalis ATCC 33277 was anaerobically grown at 37°C to the early-stationary phase in the broth described above. The harvested cells were washed once in auto- claved water and then resuspended in freshly autoclaved water containing adequate concentrations of protamine. Cell suspensions were incubated at 37°C, and every 30 minutes the samples were examined for cell viability. Cell viability was determined by ATP-bioluminescent assay using the BacTiter-Glo Microbial Cell Viability Assay kit (Promega, Madison, USA). Briefly, a volume of BacTiter-Glo reagent equal to the volume of each suspension was added and briefly mixed. The lumines- cence of the solution was then recorded by using the AUTO-LUMICOUNTER Model 1422EX (Microtec Co., LTD, Funabashi, Japan). The value obtained was ex- pressed as the ratio to that at the start of incubation. The results were expressed as the mean ± SD of three ex- periments. 2.5. Statistical Analysis Data were analyzed for statistical significance using a two-way analysis of variance (ANOVA) followed by the Scheffe test for multiple comparisons. 3. RESULTS AND DISCUSSION As shown in Figure 1, protamine and its derivative showed an inihibitory effect on growth of all the perio- dontopathic bacteria tested, with inhibitory effect great- est on growth of the P. gingivalis strains. The inhibitory effect of native protamine was greater than that of its hydrolysate (p < 0.05). The MIC values for P. gingivalis ranged from 6.3 × 10-7 to 7.5 × 10-7 g L-1, while those for A. actinomycetemcomitans and P. intermedia required a higher concentration, of almost double or more. Recently, we reported that protamine absorbed onto PMMA or PMMA treated with oxygen (O2) plasma caused a mar- ginal decrease in initial attachment of C. albicans [16]. In the case of C. albicans, the initial amount of fungal attachment to Brom- or O2 plasma-treated PMMA di- minished slightly in comparison to that treated with na- tive peptide. These findings suggest that protamine ex- hibits selective inhibitory action against growth of oral microorganisms. 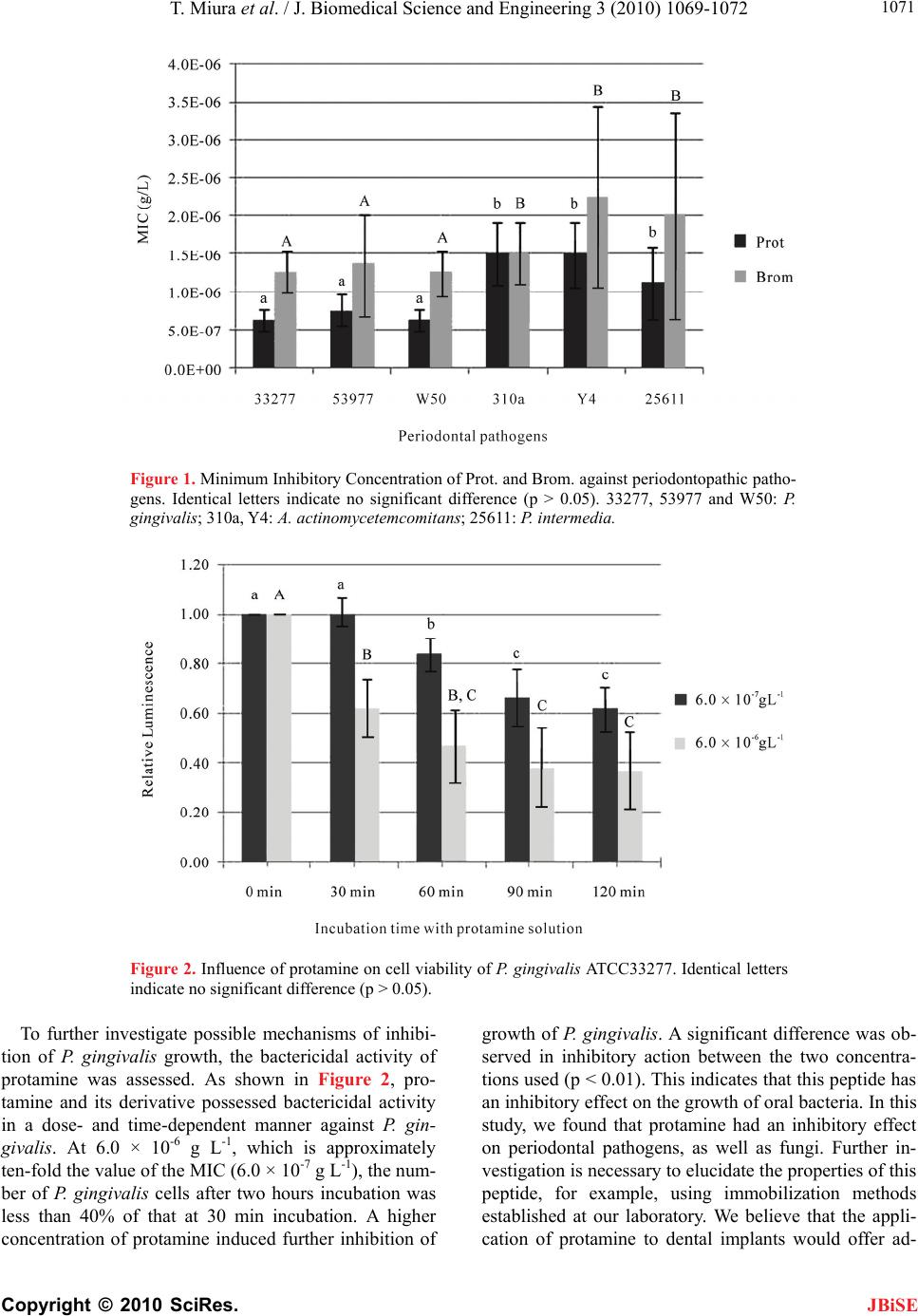 T. Miura et al. / J. Biomedical Science and Engineering 3 (2010) 1069-1072 Copyright © 2010 SciRes. JBiSE 1071 Figure 1. Minimum Inhibitory Concentration of Prot. and Brom. against periodontopathic patho- gens. Identical letters indicate no significant difference (p > 0.05). 33277, 53977 and W50: P. gingivalis; 310a, Y4: A. actinomycetemcomitans; 25611: P. intermedia. Figure 2. Influence of protamine on cell viability of P. gingivalis ATCC33277. Identical letters indicate no significant difference (p > 0.05). To further investigate possible mechanisms of inhibi- tion of P. gingivalis growth, the bactericidal activity of protamine was assessed. As shown in Figure 2, pro- tamine and its derivative possessed bactericidal activity in a dose- and time-dependent manner against P. gin- givalis. At 6.0 × 10-6 g L-1, which is approximately ten-fold the value of the MIC (6.0 × 10-7 g L-1), the num- ber of P. gingivalis cells after two hours incubation was less than 40% of that at 30 min incubation. A higher concentration of protamine induced further inhibition of growth of P. gingivalis. A significant difference was ob- served in inhibitory action between the two concentra- tions used (p < 0.01). This indicates that this peptide has an inhibitory effect on the growth of oral bacteria. In this study, we found that protamine had an inhibitory effect on periodontal pathogens, as well as fungi. Further in- vestigation is necessary to elucidate the properties of this peptide, for example, using immobilization methods established at our laboratory. We believe that the appli- cation of protamine to dental implants would offer ad-  T. Miura et al. / J. Biomedical Science and Engineering 3 (2010) 1069-1072 Copyright © 2010 SciRes. JBiSE 1072 vantages in the prevention of periodontal diseases such as peri-implantitis and oral care. 4. ACKNOWLEDGEMENTS This research was supported by Oral Health Science Center Grant hrc7 from Tokyo Dental College, and by a “High-Tech Research Center” Project for Private Universities: matching fund subsidy from MEXT (Ministry of Education, Culture, Sports, Science and Technology) of Japan, 2006-2010. The authors would like to thank Associate Professor Jeremy Wil- liams, Tokyo Dental College, for his assistance with the English of this manuscript. REFERENCES [1] Sumida, S., Ishihara, K., Kishi, M. and Okuda, K. (2002) Transmission of periodontal disease-associated bacteria from teeth to osseointegrated implant regions. Interna- tional Journal of Oral & Maxillofacial Implants, 17(5), 696-702. [2] Slots, J. and Genco, R.J. (1984) Black-pigmented Bac- teroides species, Capnocytophaga species, and Actinoba- cillus actinomycetemcomitans in human periodontal dis- ease: Virulence factors in colonization, survival, and tis- sue destruction. Journal of Dental Research, 63(3), 412- 421. [3] Dzink, J.L., Tanner, A.C., Haffajee, A.D., Socransky, S.S. (1985) Gram negative species associated with active de- structive periodontal lesions. Journal of Clinical Perio- dontology, 12(8), 648-659. [4] Sweeney, E.A., Alcoforado, G.A., Nyman, S. and Slots, J. (1987) Prevalence and microbiology of localized prepu- bertal periodontitis. Oral Microbiology and Immunology, 2(2), 65-70. [5] Yoshinari, M., Oda, Y., Kato, T. and Okuda, K. (2001) Influence of surface modifications to titanium on anti- bacterial activity in vitro. Biomaterials, 22(14), 2043- 2048 [6] Norowski, P.A. Jr. and Bumgardner, J.D. (2009) Bioma- terial and antibiotic strategies for peri-implantitis: A re- view. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 88(2), 530-543 [7] Yoshinari, M., Kato, T., Matsuzaka, K., Hayakawa, T. and Shiba, K. (2010) Prevention of biofilm formation on titanium surfaces modified with conjugated molecules comprised of antimicrobial and titanium-binding peptides. Biofouling, 26(1), 103-110. [8] Yoshinari, M., Kato, T., Matsuzaka, K., Hayakawa, T., Inoue, T., Oda, Y., Okuda, K. and Shimono, M. (2006) Adsorption behavior of antimicrobial peptide histatin 5 on PMMA. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 77(1), 47-54. [9] Evans, E.W., Beach, F.G., Moore, K.M., Jackwood, M.W., Glisson, J.R. and Harmon, B.G. (1995) Antimicrobial ac- tivity of chicken and turkey heterophil peptides CHP1, CHP2, THP1, and THP3. Veterinary Microbiology, 47(3-4), 295-303. [10] Roque, A., Orrego, M., Ponte, I. and Suau, P. (2004) The preferential binding of histone H1 to DNA scaffold-asso- ciated regions is determined by its C-terminal domain. Nucleic Acids Research, 32(20), 6111-6119. [11] Li, Y.T., Kwon, Y.M., Spangrude, G.J., Liang, J.F., Chung, H.S., Park, Y.J. and Yang, V.C. (2009) Preliminary in vivo evaluation of the protein transduction domain- modified ATTEMPTS approach in enhancing asparagi- nase therapy. Journal of Biomedical Materials Research Part A, 91(1), 209-220. [12] Sarwar, G., Kakimoto, D., Onishi, M. and Nagata, R. (1989) Potentiality of protamine sulfate as mutagen. Journal of Toxicological Sciences, 14(3), 215-225. [13] Tsukiyama, R., Katsura, H., Tokuriki, N. and Kobayashi, M. (2002) Antibacterial activity of licochalcone A against spore-forming bacteria. Antimicrob Agents Chemother, 46(5), 1226-1230. [14] Agren, G. (1954) Investigations of substances inhibiting or stimulating the growth of some lactobacilli. III. Effects of protamin and some heat-stable protein fractions from spleen and urine. Acta Pathologica Microbiologica Scandinavica, 35(1), 97-104. [15] Burton, E., Gawande, P.V., Yakandawala, N., LoVetri, K., Zhanel, G.G., Romeo, T., Friesen, A.D. and Madhyastha, S. (2006) Antibiofilm activity of GlmU enzyme inhibi- tors against catheter-associated uropathogens. Antimicrob Agents Chemother, 50(5), 1835-1840. [16] Miura, T., Hayakawa, T., Okumori, N., Iohara, K. and Yoshinari, M. (2010) Antifungal activity against Candida albicans on PMMA coated with protamine derivatives. Journal of Oral Tissue Engineering, in Press. [17] Hoffmann, J.A., Chance, R.E. and Johnson, M.G. (1990) Purification and analysis of the major components of chum salmon protamine contained in insulin formula- tions using high-performance liquid chromatography. Protein Expression and Purification, 1(2), 127-133. [18] Hunter, R.G., Henry, G.W. and Heinicke, R.M. (1957) The action of papain and bromelain on the uterus. American Journal of Obstetrics and Gynecology, 73(4), 867-874. |

