Paper Menu >>
Journal Menu >>
 Pharmacology & Pharmacy, 2010, 1, 53-59 doi:10.4236/pp.2010.12008 Published Online October 2010 (http://www.SciRP.org/journal/pp) Copyright © 2010 SciRes. PP 53 Antioxidant Effect of Atorvastatin in Type 2 Diabetic Patients Najah R. Hadi1, Mohammad A. Abdelhussein2, Omran M. O. Alhamami1, Ammar R. Muhammad Rudha1, Ekhlas Sabah 1Department of Pharmacology, Faculty of Medicine, Kufa University, Najaf, Iraq; 2Department of Medicine, Faculty of Medicine, Kufa University, Najaf, Iraq. Email: drnajahhadi@yahoo.com Received August 26th, 2010; revised September 12th, 2010; accepted September 20th, 2010. ABSTRACT Evidence has long been existed regarding the relationship between oxidative stress and diabetes. The present study was conducted to assess the effect of atorvastatin on selected oxidative stress parameters and its effect on lipid profile pa- rameters in dyslipidaemic type 2 diabetic patients. Fifty nine dyslipidaemic type 2 diabetic patients were included in this study. A full history was taken and general examination was performed. The patients were taking an oral hypogly- caemic drug (glibenclamide) during the study. The patients were followed up for 60 days and divided randomly into 2 groups. Group I (n = 31) received no drug and served as dyslipidaemic diabetic control. Group II (n = 28) received atorvastatin tablets 20 mg once daily at night. Blood samples were drawn from the patients at the beginning and after 60 days of follow up between 8:30 and 10:30 am after at least 12-14 hours fasting. Fasting blood glucose, lipid profile, selective oxidative stress parameters, glutathione S reductase (GSH), malondialdehyde (MDA) levels, glutathione S transferase (GST) and catalase (CAT) activities were measured. Renal and hepatic functions were also assessed. The results showed that atorvastatin treatment produced significant increase in serum levels of GSH and High Density Lipoprotein (HDL), while serum levels of MDA, Total Cholesterol (TC), Triglyceride (TG), Low Density Lipoprotein Cholesterol (LDL-C) and Very Low Density Lipoprotein (VLDL) were significantly decreased. However, no significant effect was observed regarding CAT and GST activity. There were insignificant correlations between atorvastatin in- duced changes in the oxidation markers and the observed changes of the lipid profile. In conclusion, the antioxidant effect of atorvastatin could be unrelated to its hypolipidemic action as there was insignificant correlation between changes in lipid profile and oxidative stress in this study. Keywords: Atorvastatin, Type 2 Diabetes, Oxidative Stress, Dyslipidaemia 1. Introduction Oxidative stress is defined as tissue injury resulting from a disturbance in the equilibrium between the production of reactive oxygen species (ROS) (also known as free radicals) and antioxidant defense mechanisms [1]. Under physiologic conditions, the antioxidant defenses are able to protect against the deleterious effects of ROS, but un- der conditions where either an increase in oxidant gen- eration, a decrease in antioxidant protection or a failure to repair oxidative damage, accumulation of free radicals ensures, leading to cellular and tissue damage [2]. Excess generation of ROS in oxidative stress have pathological consequences including damage to polyunsaturated fatty acids in membrane lipids, proteins, DNA and ultimately cell death [3]. ROS have been implicated in many dis- ease state including neurodegenerative disease like Alzheimer’s and Parkinson’s disease, atherosclerosis, inflammatory conditions, certain cancers, diabetes melli- tus (DM), cataract in the eye, pulmonary, renal, heart diseases and the process of aging [4,5]. Diabetes mellitus is a group of metabolic disorders with one common manifestation: hyperglycaemia associated with defects in insulin secretion, action or both. Traditionally it has been classified into two forms Type 1 DM and Type 2 DM [6]. Type 2 DM which is known to be multifactorial, result- ing from combination of various factors such as impaired fatty acid metabolism, central fat deposition leading to insulin resistance in various tissues (liver, muscles, adi- pose) [7], beta-cell secretary defect and obesity [4]. Evi-  Antioxidant Effect of Atorvastatin in Type 2 Diabetic Patients Copyright © 2010 SciRes. PP 54 dence has long existed regarding the relationship be- tween oxidative stress and DM [8]. Eisei N. et al. postu- lated that oxidative stress is involved in the onset and progression of diabetes, initiation and exacerbation of micro- and macrovascular complications in diabetes and recently oxidative stress status markers have been asso- ciated directly with the severity and prognosis of diabetes [9]. There are multiple sources of oxidative stress in DM, including non enzymatic (glucose autoxidation, non en- zymatic glycation of proteins), enzymatic (NADPH oxi- dase, nitric oxide synthase) and mitochondrial pathway [10]. Dyslpidaemia is used to describe a group of conditions in which there are abnormal levels of lipid and lipopro- tein in the blood [11]. In type 2 diabetes, dyslipidaemia is characterized by elevated circulating levels of TG, de- creased circulating levels of HDL and usually accompa- nied by an elevation of small dense LDL-cholesterol par- ticles [12]. There is an evidence indicating that hyper- lipidaemia is associated with enhanced oxidative stress [13]. Atorvastatin belongs to 3-hydroxy-3-methylglutaryl- coenzyme A (HMG-CoA) reductase inhibitors, (or statins) which are potent inhibitors of cholesterol biosynthesis that are used extensively to treat patients with hypercho- lesterolaemia [14,15]. Atorvastatin is a synthetic lipid lowering agent [16]. It is a competitive inhibitor of HMG-CoA reductase which catalyzes the conversion of HMG-CoA to mevalonate, an early rate limiting step in cholesterol biosynthesis resulting in depletion the intra- cellular supply of cholesterol [17]. Inhibition of choles- terol biosynthesis is accompanied by an increase in he- patic LDL receptor on the cell surface which promotes uptake and clearance of circulating LDL. Thus the end result is a reduction in plasma cholesterol by both low- ered cholesterol synthesis and by increased catabolism of LDL [15]. Atorvastatin also reduces VLDL-C, TG and produces variable increase in HDL-C [18]. Atorvastatin is safe and generally well tolerated [19]. Mild gastroin- testinal side effects like dyspepsia, flatulence, abdominal pain, diarrhea and constipation may occur. Other side effects include headache, rash, pruritus and malaise. The most detrimental adverse effect of atorvastatin is hepato- toxicity and myopathy [20]. Munford [21] and Shishehbor et al. [22] stated that the overall clinical benefits observed with atorvastatin ther- apy appear to be greater than what might be expected from changes in lipid levels alone, suggesting effects beyond cholesterol lowering called pleiotropic effects. Vishal et al. indicated that some of the cholesterol-inde- pendent effects of atorvastatin involve improving endo- thelial function, enhancing the stability of atherosclerotic plaques, decreasing oxidative stress, decreasing inflam- mation, improve insulin resistance, inhibiting the throm- bogenic response in the vascular wall and impede tumor cells. Further more statin have other extrahepatic benefi- cial effects on the immune system, central nervous sys- tem and bone [23]. Atorvastatin possesses antioxidant properties by reducing lipid peroxidation and ROS pro- duction [23]. It reduces the susceptibility of lipoproteins to oxidation both in vitro and in vivo, i.e., they decrease the LDL oxidation [23]. The Aim of This Study was to clarify the effect of atorvastatin on selected oxidative stress parameters na- mely (reduced glutathione (GSH), lipid peroxidation product malondialdehyde (MDA) levels, glutathione –S- transferase (GST) and catalase (CAT) activities) and lipid profile in dyslipidaemic type 2 diabetic patients. 2. Materials and Methods 2.1. Materials Atorvastatin (Atorfit 20, Ajanta Pharma Limited, India,), EDTA & GSH (Biochemicals Co. Ltd.), DTNB (Sigma Co. Ltd.), Trichloroacetic acid (TCA) & Thiobuteric acid (TBA) (Merk Co. Ltd.) were obtained as a gift samples. CDNB, K2HPO4, KH2PO4, Na2HPO4, H2O2 (Analar company) were purchased commercially and used as received. 2.2. Patients Fifty nine patients (age: 57.16 ± 1.34 years; 32 men & 27 women) with type 2 DM and dyslipidaemia were in- cluded in this study after obtaining their written consent and formal approval of the human experimentation committee at the Faculty of Medicine, Kufa University. The mean fasting blood glucose was (7.91 ± 0.7 mmol/l), with a mean duration of diabetes of (8.4 ± 1.08 years). The mean LDL-C level was (5.48 ± 0.72 mmol/l). The patients were chosen randomly from Al- Hakeem center for researches and treatment of DM at Al-Sadr Teaching Hospital in Najaf City in the period between 5th Nov. 2006 to 24th June 2007. These patients underwent full history and complete physical examination. Patients with the following criteria were excluded from the study: 1) Patients who use any vitamin preparations, or statins in the last three months [24]; 2) Patients with renal insuffi- ciency, defined as a serum creatinine level equal to or more than 1.8 mg/dl [24]; 3) Patients with liver disease [22]; 4) Hypertensive patients, as this condition affects oxidative stress [13] and antihypertensive drugs may affect lipid profile and oxidative stress in hypertensive patients [25,26]; 5) Patients with chronic inflammatory diseases [24]; 6) Alcoholics & smokers were also ex- cluded [27]. The patients were using glibenclamide (Glibesyn, Medochemie LTD-Cyprus; Glibils, Hikma- Jordon) (an oral hypoglycaemic agent) during the study  Antioxidant Effect of Atorvastatin in Type 2 Diabetic Patients Copyright © 2010 SciRes. PP 55 as a treatment for their diabetes. According to the design of the study, type 2 diabetic patients were followed up for 60 days and divided randomly into two groups: Group I (n = 31) received no drug and served as dyslipi- daemic diabetic control. Group II (n = 28): received atorvastatin tablets 20 mg once daily at night (Atorfit 20). Only forty six patients continued to the end of the study while thirteen patients were withdrawn (eight patients from Group I and five patients from Group II) because of non compliance. The patients were put on diet control and followed up every two weeks during the time of the study in order to make sure that they were using the medication properly and to regularly checking fasting blood glucose. Values of fasting blood glucose before, during and after the study were controlled within the previously mentioned range. Blood samples were drawn from the patients at the beginning and after 60 days of follow up between 8:30 & 10:30 am after at least 12-14 hours fasting. Fasting blood glucose, lipid profile, se- lected oxidative stress parameters (GSH, MDA levels, GST, CAT activities) were measured. Renal and hepatic functions were also assessed. 2.3. Sample Preparation From each patient, 3 ml of blood was obtained without using heparin after an overnight fasting. The blood was placed in serum tube and left to stand for 30 minutes. The serum was prepared by centrifugation at 3000 rpm for 10 minute, 1.5 ml of serum was obtained for deter- mination of experimental parameters which included GSH, MDA (the byproduct of lipid peroxidation), GST enzyme, CAT enzyme and lipid profile. 2.4. Serum Reduced Glutathione (GSH) Assay Serum GSH was estimated according to a modified method of Ellman [28]. Briefly, Stock standard solution was prepared fresh daily by dissolving 0.0307 gm of GSH in a final volume of 100 ml of (0.2M) EDTA solu- tion. Dilutions were made in EDTA solution to 50, 100, 150, 200, 250, 300, 400, 500 µM. The absorbance of this series of known concentrations was determined spectro- photometricaly using Shimadzu UV-1650P (UV-visible) at 412 nm to construct the calibration curve, “Figure 1”. 2.5. Serum Lipid Peroxidation Product (MDA) Assay The level of serum MDA was determined by a modified procedure described by Guidet and Shah [29]. Add 1 ml of TCA 17.5% and 1 ml of 0.6% thiobarbituric acid (TBA) to 0.15 ml of serum sample. The solution was mixed well by vortex, incubated in boiling water bath for 15 minutes, then allowed to cool. 1 ml of 70% TCA was Figure 1. Standard curve for determination of reduced glu- tathione level. added, and the mixture was allowed to stand at room temperature for 20 minutes, centrifuged at 2000 rpm for 15 minutes, and the supernatant was taken out for scan- ning spectrophotometrically at 532 nm using Shimadzu UV-1650P (UV-visible) Spectrophotometer. Absorbance 532 LЄ at nm TheconcentrationofMDA (1) L: Light path (1 cm) : Extinction coeffoent 1.56105M 1Є.Cm 1 Total volume : Volume of the sample Ddilution factor 2.6. Serum GST Enzyme Activity Assay The absorption technique [30] includes mixing of 2.7 ml of Phosphate buffer (pH = 6.25) with 100 µL of serum and 100 µL of CDNB (1-chloro, 2, 4-dinitro benzene) and then after 3 minutes with 100 µL of GSH solution. After mixing the solutions, the absorbance was read every minute for 10 minutes at wave length 340 nm. Calculation: AVt 1000 10 Vs b U Activity ofGSTL (2) ΔA = absorbance difference between the first and tenth minute Vt = The total volume Vs = Sample volume Є = 9.6 mM-1cm-1 b = 1 cm. A31000 10 9.60.1 1 Activity ofGST (3) 2.7. Serum Catalase Activity Assay The absorption technique [31] include mixing of 2 ml of  Antioxidant Effect of Atorvastatin in Type 2 Diabetic Patients Copyright © 2010 SciRes. PP 56 diluted serum with 1ml of H2O2 The solution was mixed well and the first absorbance (A1) was read after 15 sec- onds (t1) then the second absorbance (A2) after 30 seconds (t2) at a wave length 240 nm. 1 ml of Phosphate buffer solution was used instead of H2O2 for the blank solution. Calculation: 3A1 K2. 60 t2 Vt x xlog x Vs A (4) K = rate constant of the reaction Δt = (t2 – t1) 15 seconds A1 = absorbance after 15 seconds A2 = absorbanse after 30 seconds Vt = total volume (3ml) Vs = volume of the sample 2.8. Serum Lipid Profile Assay Total cholesterol, Triglyceride and HDL were measured according to procedures supplied by Spinreact company kits, using Shimadzu UV-1650P (UV-visible)) Spectro- photometer. Serum LDL measure according to the fol- lowing equation [32] LDL= total cholesterol - HDL – VLDL (5) VLDL= TG/2.2. (6) 2.9. Statistical Methods The data expressed as mean ± SEM unless otherwise stated. Statistical analyses were done by using paired t-test. Pearson,s correlations were also performed with significant difference set at P < 0.05. 3. Results 3.1. Effect of Atorvastatin on Oxidative Parameters Atorvastatin treatment increased serum GSH and reduced MDA level significantly (P 0.05) while no significant change in serum GST and CAT activity was observed (P 0.05). There was no significant change (P 0.05) in oxida- tive stress parameters in diabetic control group apart from significant increase in MDA level “Table 1”. 3.2. Effect of Atorvastatin on Lipid Profile A significant decrease in serum TC, TG, LDL and VLDL and significant increase in HDL levels after atorvastatin treatment (P 0.05) while no significant change in lipid profile in diabetic control group was observed “Table 2”. 3.3. Correlations between Observed Changes in Oxidation Markers and Observed Changes in Lipid Parameters in Atorvastatin Group There were no significant correlations between atorvas- tatin induced changes in the oxidation markers and the observed changes in the lipid profile (P 0.05) “Table 3”. 4. Discussion 4.1. Effect of Oxidative Stress Parameters The significant increase in GSH and significant decrease in MDA levels (P 0.05) following atorvastatin treatment is supported by the findings of Save et al. [33] and Koter et al. [34], respectively. The most likely explanation for the increment of GSH and reduction of MDA by ator- vastatin was attributed to the antioxidant mediated effect of atorvastatin which results from inhibition of mevalo- nate pathway. This effect results in a reduction in the synthesis of important intermediates including isopre- noids ((farnesyl pyrophosphate & geranylgeranyl pyro- phosphate). The latter serve as lipid attachments for in- tracellular signaling molecules in particular inhibition of small GTPase binding proteins (Rho, Rac, Ras and G proteins) whose proper membrane localization and func- tion are dependent on isoprenylation. These proteins modulate a variety of cellular processes including sig- naling, differentiation and proliferation [35,36]. Atorvas- tatin attenuates endothelial reactive oxygen species (ROS) Table 1. Effect of atorvastatin (20 mg/day) on oxidative stress parameters after 60 days of treatment and changes in dyslipidaemic diabetic control (n = 23 in each group). Diabetic control Atorvastatin Parameters Before treatment After treatment Before treatment After treatment GSH(mmol/l) 0.24 ± 0.0079 0.22 ± 0.0036* 0.23±0.0034 0.40±0.0009** MDA(mol/l) 1.25 × 10–4 1.59×10–4 1.24×10–4 0.24×10–4 ± 0.0146 ±0.0210** ±0.0178 ± 0.004** GST(U/l) 13.68 ± 0.18 13.87 ± 0.2194* 13.95 ± 0.234 14.07 ± 0.212* CAT(K/ml) 0.49 ± 0.0123 0.5001 ± 0.016* 0.48 ± 0.0133 0.483 ± 0.012* *p > 0.05; **p < 0.01; Values expressed as mean ± SEM. 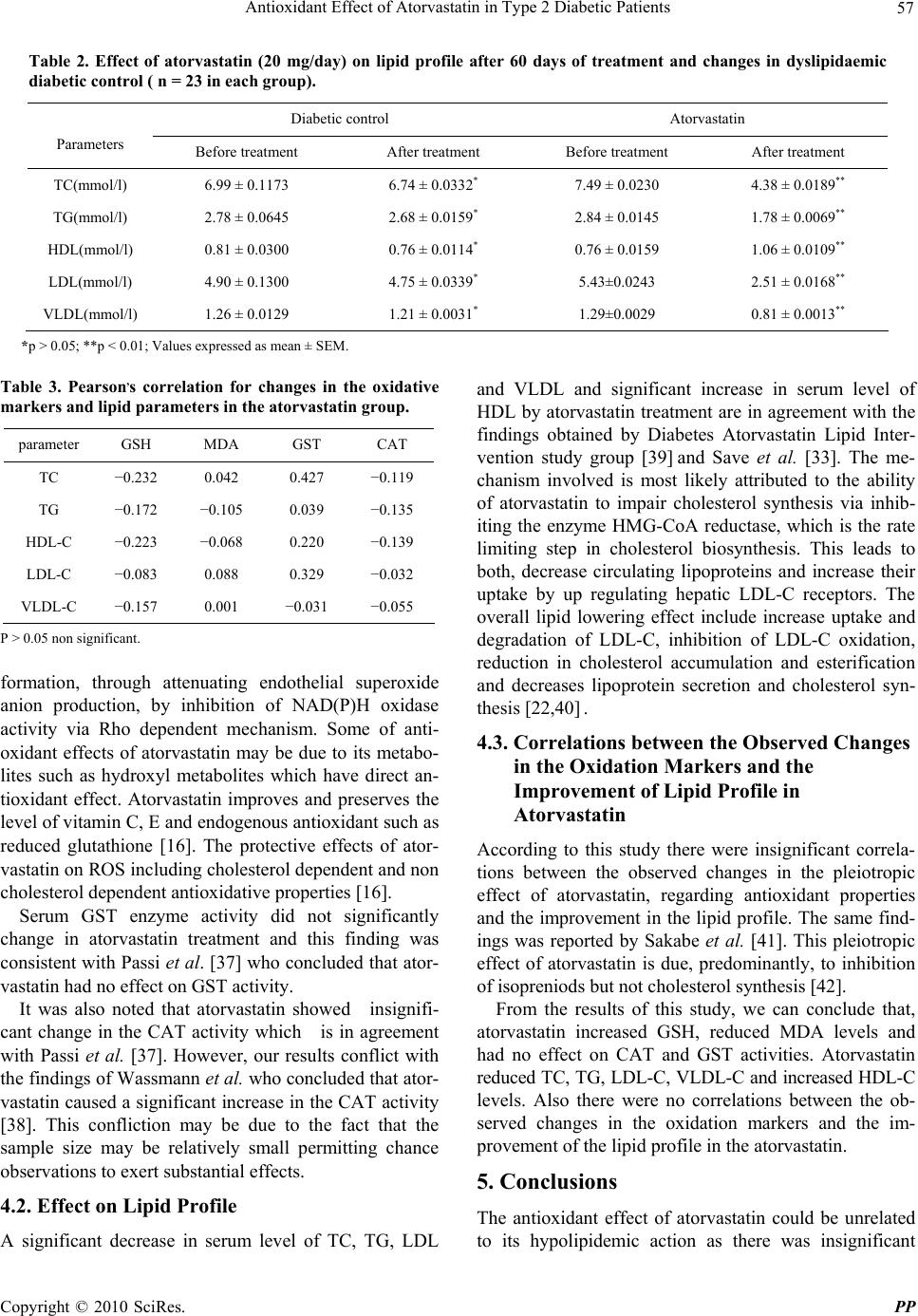 Antioxidant Effect of Atorvastatin in Type 2 Diabetic Patients Copyright © 2010 SciRes. PP 57 Table 2. Effect of atorvastatin (20 mg/day) on lipid profile after 60 days of treatment and changes in dyslipidaemic diabetic control ( n = 23 in each group). Diabetic control Atorvastatin Parameters Before treatment After treatment Before treatment After treatment TC(mmol/l) 6.99 ± 0.1173 6.74 ± 0.0332* 7.49 ± 0.0230 4.38 ± 0.0189** TG(mmol/l) 2.78 ± 0.0645 2.68 ± 0.0159* 2.84 ± 0.0145 1.78 ± 0.0069** HDL(mmol/l) 0.81 ± 0.0300 0.76 ± 0.0114* 0.76 ± 0.0159 1.06 ± 0.0109** LDL(mmol/l) 4.90 ± 0.1300 4.75 ± 0.0339* 5.43±0.0243 2.51 ± 0.0168** VLDL(mmol/l) 1.26 ± 0.0129 1.21 ± 0.0031* 1.29±0.0029 0.81 ± 0.0013** *p > 0.05; **p < 0.01; Values expressed as mean ± SEM. Table 3. Pearson,s correlation for changes in the oxidative markers and lipid parameters in the atorvastatin group. parameter GSH MDA GST CAT TC −0.232 0.042 0.427 −0.119 TG −0.172 −0.105 0.039 −0.135 HDL-C −0.223 −0.068 0.220 −0.139 LDL-C −0.083 0.088 0.329 −0.032 VLDL-C −0.157 0.001 −0.031 −0.055 P > 0.05 non significant. formation, through attenuating endothelial superoxide anion production, by inhibition of NAD(P)H oxidase activity via Rho dependent mechanism. Some of anti- oxidant effects of atorvastatin may be due to its metabo- lites such as hydroxyl metabolites which have direct an- tioxidant effect. Atorvastatin improves and preserves the level of vitamin C, E and endogenous antioxidant such as reduced glutathione [16]. The protective effects of ator- vastatin on ROS including cholesterol dependent and non cholesterol dependent antioxidative properties [16]. Serum GST enzyme activity did not significantly change in atorvastatin treatment and this finding was consistent with Passi et al. [37] who concluded that ator- vastatin had no effect on GST activity. It was also noted that atorvastatin showed insignifi- cant change in the CAT activity which is in agreement with Passi et al. [37]. However, our results conflict with the findings of Wassmann et al. who concluded that ator- vastatin caused a significant increase in the CAT activity [38]. This confliction may be due to the fact that the sample size may be relatively small permitting chance observations to exert substantial effects. 4.2. Effect on Lipid Profile A significant decrease in serum level of TC, TG, LDL and VLDL and significant increase in serum level of HDL by atorvastatin treatment are in agreement with the findings obtained by Diabetes Atorvastatin Lipid Inter- vention study group [39] and Save et al. [33]. The me- chanism involved is most likely attributed to the ability of atorvastatin to impair cholesterol synthesis via inhib- iting the enzyme HMG-CoA reductase, which is the rate limiting step in cholesterol biosynthesis. This leads to both, decrease circulating lipoproteins and increase their uptake by up regulating hepatic LDL-C receptors. The overall lipid lowering effect include increase uptake and degradation of LDL-C, inhibition of LDL-C oxidation, reduction in cholesterol accumulation and esterification and decreases lipoprotein secretion and cholesterol syn- thesis [22,40] . 4.3. Correlations between the Observed Changes in the Oxidation Markers and the Improvement of Lipid Profile in Atorvastatin According to this study there were insignificant correla- tions between the observed changes in the pleiotropic effect of atorvastatin, regarding antioxidant properties and the improvement in the lipid profile. The same find- ings was reported by Sakabe et al. [41]. This pleiotropic effect of atorvastatin is due, predominantly, to inhibition of isopreniods but not cholesterol synthesis [42]. From the results of this study, we can conclude that, atorvastatin increased GSH, reduced MDA levels and had no effect on CAT and GST activities. Atorvastatin reduced TC, TG, LDL-C, VLDL-C and increased HDL-C levels. Also there were no correlations between the ob- served changes in the oxidation markers and the im- provement of the lipid profile in the atorvastatin. 5. Conclusions The antioxidant effect of atorvastatin could be unrelated to its hypolipidemic action as there was insignificant  Antioxidant Effect of Atorvastatin in Type 2 Diabetic Patients Copyright © 2010 SciRes. PP 58 correlation between changes in lipid profile and oxidative stress in this study. 6. Acknowledgements Special thanks to everybody that help us in accomplish- ing this work. REFERENCES [1] D. J. Betteridge, “What is Oxidative Stress?” Metabolism, Vol. 49, No.2, 2000, pp. 3-8. [2] B. Halliwell, “Free radicals, Antioxidants & Human Dis- ease: Curiosity, Cause or Consequence,” Lancet, Vol. 344, No. 8924, 1994, pp. 721-724. [3] K. Kannan and S. K. Jain, “Oxidative Stress & Apop- tosis,” Pathophysiology, Vol. 7, No. 3, 2000, pp. 153-163. [4] S. Shah, M. Iqbal, J. Karam, M. Salifu and S. I. Mcfarlane, “Oxidative stress, Glucose Metabolism & the Prevention of Ttype 2 Diabetes: Pathophyiological In- sights,” Antioxidants & Rredox Signaling, Vol. 9, No. 7, 2007, pp. 911-929. [5] I. S. Young and J. V. Woodside, “Antioxidants in Health & Disease,” Journal of Clinical Pathology, Vol. 54, No. 3, 2001, pp. 176-186. [6] J. A. Florence and B. F Yeager, “Treatment of Type 2 Diabetes Mellitus,” American Family Physician, Vol. 59, 1999, p. 10. [7] J. L. Mehta, N. Rasouli, A. K. Sinha and B. Molavi, “Oxidative Stress in Diabetes: A mechanistic Overview of its Effects on Atherogenesis and Myocardial Dysfunc- tion,” The International Journal of Biochemistry & Cell Biology, Vol. 38, No. 5-6, June 2006, pp. 794-803. [8] L. W. Oberley, “Free Radicals and Diabetes,” Free Radi- cal Biology & Medicine, Vol. 5, No. 2, 1988, pp. 113- 124. [9] N. Eisei and T. Hirokazu, “Parameters for Measurement of Oxidative Stress in Diabetes Mellitus: Applicability of ELISA for Clinical Evaluation,” Jòurnal of Investigation Medicine, Vol. 53, No.4, May 2005, pp. 167-175. [10] J. S. Johansen, A. K. Harris, D. J. Rychly and A. Ergul, “Oxidative Stress & the Use of Antioxidants in Diabetes: Linking Basic Science to Clinical Practice,” Cardiovas- cular Diabetology, Vol. 4, April 2005, p. 5. [11] J. Patel, “Dyslipidaemia in Diabetes,” British Midical Journal Clinical Evidence, Vol. 8, No. 4, 2006, pp. 355- 364. [12] C. M. Florkowski, “Management of Co-Existing Diabetes Mellitus & Dyslipidaemia: Defining the Role of Thia- zolidinediones,” American Journal of Cardiovascular Drugs, Vol. 2, No. 1, 2002, pp. 15-21. [13] F. Violi, L. Loffredo, L. Musella and A. Marcoccia, “Should Antioxidant Status be Considered in Interven- tional Trials with Antioxidants?” Heart, Vol. 90, No. 6, 2004, pp. 598-602. [14] M. C. Sheffield, “Multiple Effects of Statins in Non Lipid Disease States,” US Pharmacist, Vol. 6, 2004, pp. 38-54. [15] J. K. Liao, “Isoprenoids as Mediators of the Biological Effects of Statins,” Journal Clinical Investigation, Vol. 110, No. 3, August 2002, pp. 285-288. [16] F. R. Danesh and Y. S. Kanwar, “Modulatory Effects of HMG-CoA Reductase Inhibitors in Diabetic Microan- giopathy,” The FASEB Journal, Vol. 18, No. 7, 2004, pp. 805-815. [17] J. Beltowski, “Statins & Modulation of Oxidative Stress,” Toxicology Mechanisms & Methods, Vol. 15, No. 2, 2005, pp. 61-92. [18] B. B. Hodgson and R. J. Kizior, “Saunders Nursing Drug Handbook,” Jones and Bartlett, Boston, 2003. [19] A. Heerey, M. Barry, M. Ryan and A. Kelly, “The Poten- tial for Drug Interactions with Statin Therapy in Iraland,” Irish Journal of Medical Science, Vol. 169, No. 3, 2001, pp. 176-179. [20] A. J. Ellsworth, D. M. Witt, D. C. Dugdale and L. M. Oliver, “Mosby,s Medical Drug Reference,” 2003. [21] R. S. Munford, “Statins & the Acute Phase Response,” New England Journal of Medicine, Vol. 344, No. 26, June 2001, pp. 2016-2018. [22] M. H. Shishehbor, M. L. Brennan, R. J. Aviles, X. Fu, M. S. Penn, D. L. Sprecher and S. L. Hazen, “Statins Pro- mote Potent Systemic Antioxidant Effects Through Spe- cific Inflammatory pathways,” Circulation, Vol. 108, No. 4, 2003, pp. 426-431. [23] T. Vishal, G. Bano, V. Khajuria, A. Parihar and S. Gupta, “Pleiotropic Effects of Statins,” Indian Journal Pharma- cology, Vol. 37, No. 2, 2005, pp. 77-85. [24] M. E. Marketou, E. A. Zacharis, D. Nikitovic, E. S. Ganotakis, F. I. Parthenakis and N. Maliaraki, “Early Ef- fects of Simvastatin Versus Atorvastatin on Oxidative Stress Proinflammatory Cytokines in Hyperlipidaemic Subjects,” Angiology, Vol. 57, No. 2, March 2006, pp. 211-218. [25] V. V. Uzunova, A. N. Tolekova, G. S. IIieva and A. P. Trifonova, “Renin-Angiotensin System & Lipid Peroxi- dation,” Bulgarian Journal of Veterinary Medicine, Vol. 1, 2005, pp. 69-75. [26] B. I. Kasiske, J. Z. Ma, R. S. Kalil and T. A Louis, “Ef- fects of Antihypertensive Therapy on serum lipids,” An- nals of Internal Medicine, Vol. 122, No. 2, January 1995, pp. 133-141. [27] N. Kebapci, B. Efe, F. Akyuz, E. Sunal and C. Demirustu, “Oxidative Stress & Antioxidant Therapy in Type 2 Dia- betes Mellitus,” Turkish Journal of Endocrinology & Metabolism, Vol. 4, 1999, pp. 153-162. [28] G. L. Ellman, “Tissue Sulfhydyl Groups,” Archives of Biochemistry and Biophys, Vol. 82, No. 1, May 1959, pp. 70-77. [29] B. Guidet and S. V. Shah, American Journal of Physiol- ogy, Vol. 257, No. 26, 1989, p. 440. [30] W. H. Michael, M. J. Pabst and W. B. Jakoby, “Glu- tathione-S-Transferase. The First Enzymatic Step in Mercapturic Acid Formation,” Journal of Biological  Antioxidant Effect of Atorvastatin in Type 2 Diabetic Patients Copyright © 2010 SciRes. PP 59 Chemistry, Vol. 22, No. 25, 1974, pp. 7130-7139. [31] H. Aebi, In Methods of Enzymatic Analysis, H.U. Bergme-tered, ed, New York, Academic press 1974, Vol, 2, pp. 674-678. [32] W.T. Friedewald, R.I. Levy and D.S. Fredrickson, “Esti- mation of the Concentration of LDL-C in Plasma without Use the Preparative Ultracentrifuge,” Clinical Chemistry, Vol. 18, No. 6, Jun 1972, 499-502. [33] V. Save, N. Patil, N, Moulik and G. Rajadhyaksha, “Ef- fect of Atorvastatin on Type 2 Diabetic Dyslipidaemia,” Journal of Cardiovascular Pharmacololgy & Therapeu- tics, Vol. 11, No. 4, December 2006, pp. 262-270. [34] M. Koter, M. Broncel, J. Chjnowska-Jezierska, K. Klikczynska and I. Franiak, “The Effect of Atorvastatin on Erythrocyte Membranes & Serum Lipids in Patients with Type 2 Hypercholesterolaemia,” European Journal of Clinical Pharmacology, Vol. 58, No. 8, 2002, pp. 501-506. [35] J.K. Liao and U. Laufs, “Pleiotropic Effects of Statins,” Annual Review Pharmacology & Toxicology, Vol. 45, Febreuary 2005, pp. 89-118. [36] J. C. Mason, “Statins & Their Role in Vascular Protec- tion,” Clinical Science, Vol. 105, 2003, pp. 251-266. [37] S. Passi, A. Stancato, E. Aleo, A. Dmitrieva and G.P. Littarru, “Statins Lower Plasma & Lymphocyte Ubiqui- nol Ubiquinone Without Affecting Other Antioxidants & PUFA,” Biofactors, Vol. 18, No. 1-4, 2003, 113-124. [38] S. Wassmann, U. Laufs, K. Muller, C. Konkol, K. Ahl- bory and .T. Baumer, et al, “Cellular Antioxidant Effects of Atorvastatin in Vitro & in Vivo,” Arteriosclerosis, Thrombosis and Vascular Biology, Vol. 22, 2002, pp. 300-305. [39] DALI Study Group, “The Effect of Aggressive Versus Standard Lipid Lowering by Atorvastatin on Diabetic Dyslipidaemia: the DALI Study: A double Blind Ran- domized Placeb Controlled Trial in Patients with Type 2 Diabetes & Diabetic Dyslipidaemia,” Diabetes Care,Vol. 24, No. 8, 2001, pp. 1335-1341. [40] M. Alegret and J.S. Silvestre, “Pleiotropic Effects of Statins & Related Pharmacological Experimental Ap- proaches,” Methods and Finding Experimental Clinical Pharmacology, Vol. 28, No.9, 2006, pp. 627. [41] K. Sakabe, N. Fukuda, K. Wakayama, T. Nada, H. Shi- nohara and Y. Tamura “Effects of Atorvastatin Therapy on the Low - Density Lipoprotein Subfraction, Rem- nant-Like Particles Cholesterol, and Oxidized Low-Den- sity Lipoprotein within 2 Weeks in Hypercholesterolemic Patients,” Circulation Journal, Vol. 67, No. 10, 2003, pp. 866-870. [42] U. Laufs and J.K. Liao, “Isoprenoid Metabolism & the Pleiotropic Effects of Statins,” Current Atherosclerosis Reports, Vol. 5, No.5, 2003, pp. 372-378. |

