Paper Menu >>
Journal Menu >>
 Journal of Biomaterials and Nanobiotechnology, 2010, 1, 50-60 doi:10.4236/jbnb.2010.11007 Published Online October 2010 (http://www.SciRP.org/journal/jbnb) Copyright © 2010 SciRes. JBNB Synthesis and Characterization of Novel Hybrid Poly(methyl methacrylate)/Iron Nanowires for Potential Hyperthemia Therapy Huey-Wen Liou1, Hong-Ming Lin1*, Yeu-Kuang Hwu2, Wen-Chang Chen3, Wei-Jen Liou1, Li-Chung Lai4, Wei-Syuan Lin1, Wen-An Chiou4 1Department of Materials Engineering, Tatung University, DeHui St., Taipei, Taiwan; 2Institute of Physics, Academia Sinica, Academia Rd., Nankang, Taipei, Taiwan; 3Department of Chemical and Materials Engineering, National Yunlin University of Science and Technology, University Road, Section, Douliou, Yunlin, Taiwan; 4NISP Lab, Jeong H. Kim Engineering Building, NanoCenter, University of Maryland, College Park, U.S.A. Email: *hmlin@ttu.edu.tw Received July 22nd, 2010; revised August 14th, 2010; accepted September 30th, 2010. ABSTRACT Externally applied magnetic fields have been used in this study to fabricate bamboo-like iron nanowires with or without a layer of Poly(methyl methacrylate) (PMMA). The hybrid PMMA/Fe nanowires were synthesized via hard X-ray synchrotron radiation polymerization with various treatment parameters. The results of XRD show that an oxide layer formed on the surface of the iron nanowires. The Fe2O3 and Fe3O4 phases coexist in the iron nanowires without X-ray irradiation. After X-ray irradiation, the Fe 2O3 phase transform ed into Fe3O4, which stabilized th e iron nanowires. The results of XAS proved this phase transformation. TGA analysis confirmed the thermal properties and solid contents in these specimens. Their ferromagnetic behaviors were examined by magnetic hysteresis measurement, which indicated that the magnetic and structural properties of the nanowires can be manipulated by irradiation treatment. This may lead to a novel synthesis for iron nan owires that can be used in high thermal efficiency hyperthermia therapy. Keywords: Iron Nanowires, Poly(methyl methacrylate), Hybrid, X-Ray Irradiation, XAS, Magnetic Materials 1. Introduction Recently, several research groups have carried out inves- tigations of the magnetic properties of metal wires. They used different methods to prepare nanowires and mi- crowires. Menon et al. [1], Bandyopadhyay et al. [2] and Zheng et al. [3] fabricated Fe, Co and Ni nanowires, re- spectively, using electrodeposition into nanoporous an- odic alumina templates. Pan et al. synthesized NiCo mi- crowires using a hydrothermal method [4]. A template- free method that combines chemical reduction and a ma- gnetic field is applied to prepare Ni nanowires [5]. The latter method only needs one step in its preparation and no thermal treatment. Herein, iron nanowires have been prepared by an ex- ternally applied magnetic field. Hybrid Poly(methyl methacrylate) (PMMA)/iron nanowires are synthesized by combining iron nanowires with radiation polymeriza- tion of PMMA. Hard X-ray synchrotron radiation can be used to initiate polymerization of PMMA and success- fully produces PMMA beads [6]. This method has two essential characteristics. First, it requires only one step for the preparation of Fe nanowires with a polymerized PMMA coating by hard X-ray synchrotron radiation. Second, the PMMA/iron nanowires can be successfully synthesized without adding emulsifier. Compared with the usual chemical methods of preparing organic/inor- ganic hybrid materials, the method presented here is a quick, simple and ‘green’ process, which may reduce environmental pollution and increase broad, practical applications. Hybrid PMMA/Fe nanowires are intention- ally designed to manipulate their magnetic properties for application in magnetic hyperthermia. The needle-sharp nanowires enhance friction heating while an alternative magnetic field is applied that increases the heating effi- ciency in hyperthermia therapy. In this study, hybrid PMMA/Fe nanowires are investi- gated by X-ray diffraction (XRD), scanning electron mi- croscopy (SEM), high resolution transmission electron microscopy (HRTEM), X-ray absorption spectroscopy  Synthesis and Characterization of Novel Hybrid Poly (methyl methacrylate) / Iron 51 Nanowires for Potential Hyperthemia Therapy (XAS), thermogravimetry (TGA), and vibrating sample magnetometry (VSM). An XAS spectrum, which is ba- sically the measured X-ray absorption coefficient as a function of the incoming photon energy, is generally di- vided by the role of multiple scattering photoelectrons into two different regions: X-ray Absorption Near-Edge Structure (XANES) and Extended X-ray Absorption Fine Structure (EXAFS). XANES, also referred as NEXAS (Near Edge X-ray Absorption Fine Structure), can be used to investigate the electronic structure of specific elements, and EXAFS is used mainly to investigate the local atomic structure concerning the type and number of elements as well as the nearest neighbor bond length [7]. In this study, the oxidation states of iron will be exam- ined by XAS to reveal the effects of X-ray irradiation on the formation of iron oxides in iron nanowires. 2. Experimental Methyl methacrylate monomer (MMA, Fluka Chemical Reagent Co.) was purified by reduced pressure distilla- tion to remove the inhibitor before polymerization. Ferric chloride hexahydrate (FeCl3·6H2O) and sodium boro- hydride (NaBH4) were obtained from Sigma-Aldrich, Inc. Polyvinylpyrrolidone (PVP, K-30, average molecular weight: ca. 40,000 g/mol) was obtained from Tokyo Ka- sei Kogyo Chemical Reagent Co. Nitric acid (reagent ACS 99.5%), methanol and ethyl alcohol were bought from Wako Pure Chemical Industries. All solutions were prepared with deionized water (> 18 MΩ). 2.1. Preparation of Iron Wires and PMMA/Iron Nanowires Five specimens of iron nanowires and PMMA/Fe nano- wires with various parameters were prepared in this stu- dy. The first specimen was fabricated using the standard synthesis procedure for magnetic wires. Iron nanowires, denoted as specimen (I), were prepared using an exter- nally applied magnetic field with an intensity of 0.3 T. Normally, Fe nanowires are produced in a 0.7 M aqueous solution of FeCl3·6H2O at room temperature in a high purity nitrogen atmosphere with an externally applied magnetic field after adding 0.8 M NaBH4 aqueous solu- tion. After synthesis, this notated specimen I is washed with anhydrous ethanol and dried in air. The chemical reaction mechanism (Equation 1) is shown as follows: 23 243 216)(62 1862 HNaClOHBFe OHNaBHFeCl (1) The second specimen is the same as specimen I after it undergoes hard X-ray synchrotron radiation for 10 min and is notated as specimen (II). The irradiation is carried out at the National Synchrotron Radiation Research Center (NSRRC) in Taiwan, with the hard X-ray beam line (BL01A). This NSRRC synchrotron light provides a 300 mA, 1.5 GeV electron beam to generate a photon source for scientific research. The hard X-ray beam line is a semi-white-light beam line. The photon flux is 1 1012 photons/sec, and the radiation is incident onto the sample with a beam footprint of 18.6 9.3 mm2. The distribution range of photon energy is between 10 and 15 keV, and the dose rate is 5.1 ± 0.9 kGy/s. After exposure, the specimen was washed with anhydrous ethanol and then dried. For the third specimen, a 0.7 M solution of iron ion was prepared by dissolving FeCl3·6H2O in methanol and then mixing it with PVP and MMA monomer in a high purity nitrogen atmosphere. Then, a 0.8 M NaBH4 aque- ous solution was dropped into the previously prepared solution to synthesize iron nanowires in an externally applied magnetic field. After forming the iron nanowires, the sample was exposed to hard X-ray synchrotron radia- tion for 10 min. The obtained PMMA/Fe nanowires are denoted as specimen (III). For the fourth specimen, a 0.7 M FeCl3·6H2O metha- nol solution was mixed with PVP and MMA monomers in a high purity nitrogen atmosphere and then exposed to hard X-rays for 10 min to polymerize before a 0.8M NaBH4 aqueous solution was added under an externally applied magnetic field. The obtained sample underwent polymerization once again by hard X-ray exposure for another 10 min. For the fifth specimen, PMMA polymer was synthe- sized before iron nanowires formation. MMA stock solu- tion was prepared by dissolving PVP (5 vol.%) in methanol (35 vol.%) with de-ionized water (45 vol.%) and mixing it with MMA monomer (15 vol.%). The stock solution was stirred in high purity nitrogen gas to eliminate oxygen. In addition, the stock solution of iron precursor was prepared in deionized water with concen- trations of 0.7 M FeCl3·6H2O. Ten milliliter quantities of MMA stock solution were prepared in sealed bottles, ready for irradiation-induced polymerization for 10 min. After that, we prepared the volume ratios of iron stock solution to PMMA emulsion solution at 1:1. Next, 0.8 M NaBH4 was added to the mixture solution for self-as- sembled hybrid PMMA/Fe nanowires under high-purity nitrogen gas atmosphere and a magnetic field. These are called specimen (V). The obtained sample was separated from the solution and washed with anhydrous ethanol and deionized water and then dried in air. Fe nanowires (I, II) and PMMA/Fe nanowires (III, IV, and V) were then dispersed on a carbon adhesive tape and observed by SEM. The structural properties of these specimens Copyright © 2010 SciRes. JBNB 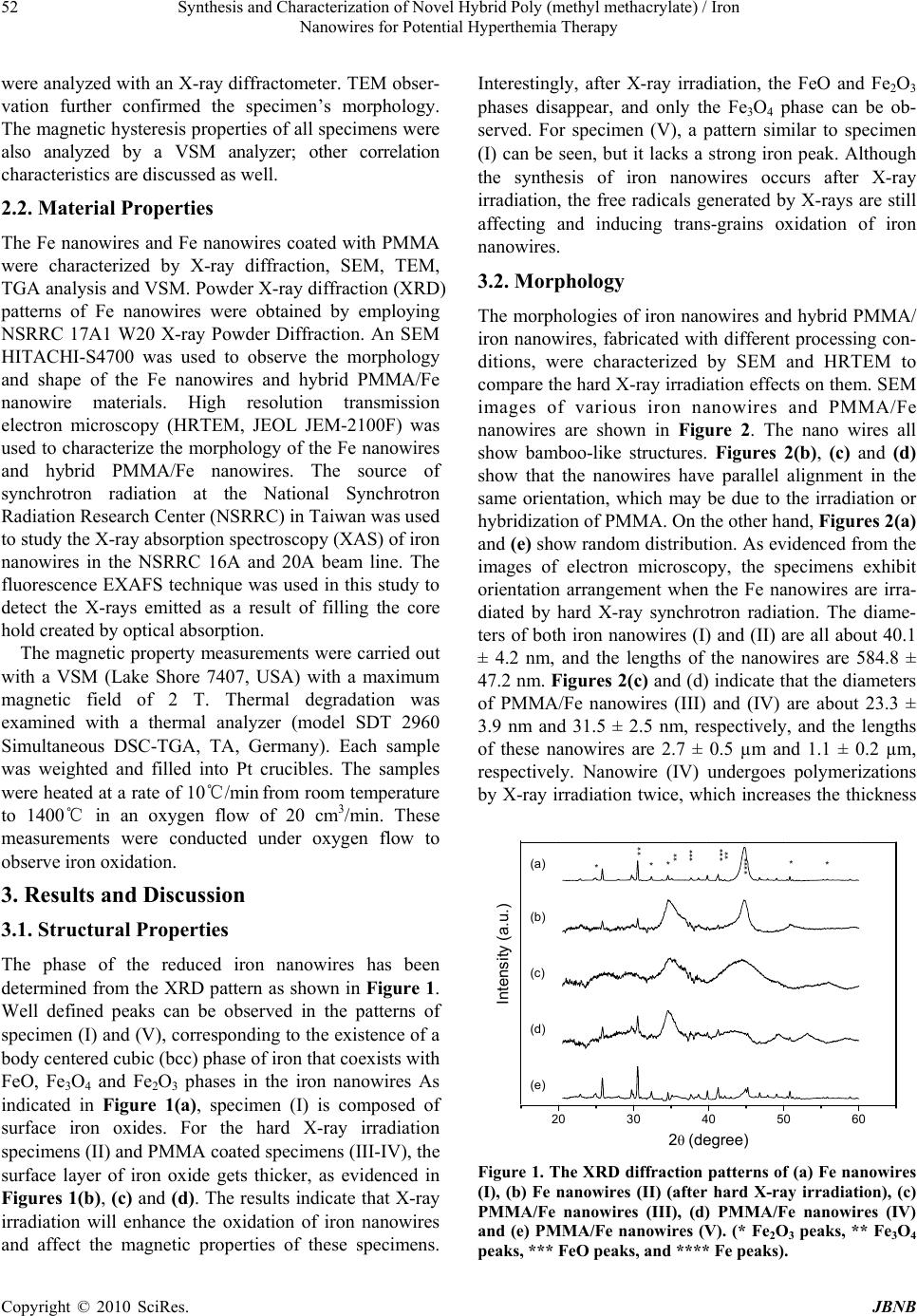 Synthesis and Characterization of Novel Hybrid Poly (methyl methacrylate) / Iron 52 Nanowires for Potential Hyperthemia Therapy were analyzed with an X-ray diffractometer. TEM obser- vation further confirmed the specimen’s morphology. The magnetic hysteresis properties of all specimens were also analyzed by a VSM analyzer; other correlation characteristics are discussed as well. 2.2. Material Properties The Fe nanowires and Fe nanowires coated with PMMA were characterized by X-ray diffraction, SEM, TEM, TGA analysis and VSM. Powder X-ray diffraction (XRD) patterns of Fe nanowires were obtained by employing NSRRC 17A1 W20 X-ray Powder Diffraction. An SEM HITACHI-S4700 was used to observe the morphology and shape of the Fe nanowires and hybrid PMMA/Fe nanowire materials. High resolution transmission electron microscopy (HRTEM, JEOL JEM-2100F) was used to characterize the morphology of the Fe nanowires and hybrid PMMA/Fe nanowires. The source of synchrotron radiation at the National Synchrotron Radiation Research Center (NSRRC) in Taiwan was used to study the X-ray absorption spectroscopy (XAS) of iron nanowires in the NSRRC 16A and 20A beam line. The fluorescence EXAFS technique was used in this study to detect the X-rays emitted as a result of filling the core hold created by optical absorption. The magnetic property measurements were carried out with a VSM (Lake Shore 7407, USA) with a maximum magnetic field of 2 T. Thermal degradation was examined with a thermal analyzer (model SDT 2960 Simultaneous DSC-TGA, TA, Germany). Each sample was weighted and filled into Pt crucibles. The samples were heated at a rate of 10℃/min from room temperature to 1400℃ in an oxygen flow of 20 cm3/min. These measurements were conducted under oxygen flow to observe iron oxidation. 3. Results and Discussion 3.1. Structural Properties The phase of the reduced iron nanowires has been determined from the XRD pattern as shown in Figure 1. Well defined peaks can be observed in the patterns of specimen (I) and (V), corresponding to the existence of a body centered cubic (bcc) phase of iron that coexists with FeO, Fe3O4 and Fe2O3 phases in the iron nanowires As indicated in Figure 1(a), specimen (I) is composed of surface iron oxides. For the hard X-ray irradiation specimens (II) and PMMA coated specimens (III-IV), the surface layer of iron oxide gets thicker, as evidenced in Figures 1(b), (c) and (d). The results indicate that X-ray irradiation will enhance the oxidation of iron nanowires and affect the magnetic properties of these specimens. Interestingly, after X-ray irradiation, the FeO and Fe2O3 phases disappear, and only the Fe3O4 phase can be ob- served. For specimen (V), a pattern similar to specimen (I) can be seen, but it lacks a strong iron peak. Although the synthesis of iron nanowires occurs after X-ray irradiation, the free radicals generated by X-rays are still affecting and inducing trans-grains oxidation of iron nanowires. 3.2. Morphology The morphologies of iron nanowires and hybrid PMMA/ iron nanowires, fabricated with different processing con- ditions, were characterized by SEM and HRTEM to compare the hard X-ray irradiation effects on them. SEM images of various iron nanowires and PMMA/Fe nanowires are shown in Figure 2. The nano wires all show bamboo-like structures. Figures 2(b), (c) and (d) show that the nanowires have parallel alignment in the same orientation, which may be due to the irradiation or hybridization of PMMA. On the other hand, Figures 2(a) and (e) show random distribution. As evidenced from the images of electron microscopy, the specimens exhibit orientation arrangement when the Fe nanowires are irra- diated by hard X-ray synchrotron radiation. The diame- ters of both iron nanowires (I) and (II) are all about 40.1 ± 4.2 nm, and the lengths of the nanowires are 584.8 ± 47.2 nm. Figures 2(c) and (d) indicate that the diameters of PMMA/Fe nanowires (III) and (IV) are about 23.3 ± 3.9 nm and 31.5 ± 2.5 nm, respectively, and the lengths of these nanowires are 2.7 ± 0.5 µm and 1.1 ± 0.2 µm, respectively. Nanowire (IV) undergoes polymerizations by X-ray irradiation twice, which increases the thickness 20 30 40 50 60 2(degree) (e) (d) Intensity (a.u.) (c) (b) **** *** *** ** ** ** ** (a) *** Figure 1. The XRD diffraction patterns of (a) Fe nanowires (I), (b) Fe nanowires (II) (after hard X-ray irradiation), (c) PMMA/Fe nanowires (III), (d) PMMA/Fe nanowires (IV) and (e) PMMA/Fe nanowires (V). (* Fe2O3 peaks, ** Fe3O4 peaks, *** FeO peaks, and **** Fe peaks). Copyright © 2010 SciRes. JBNB 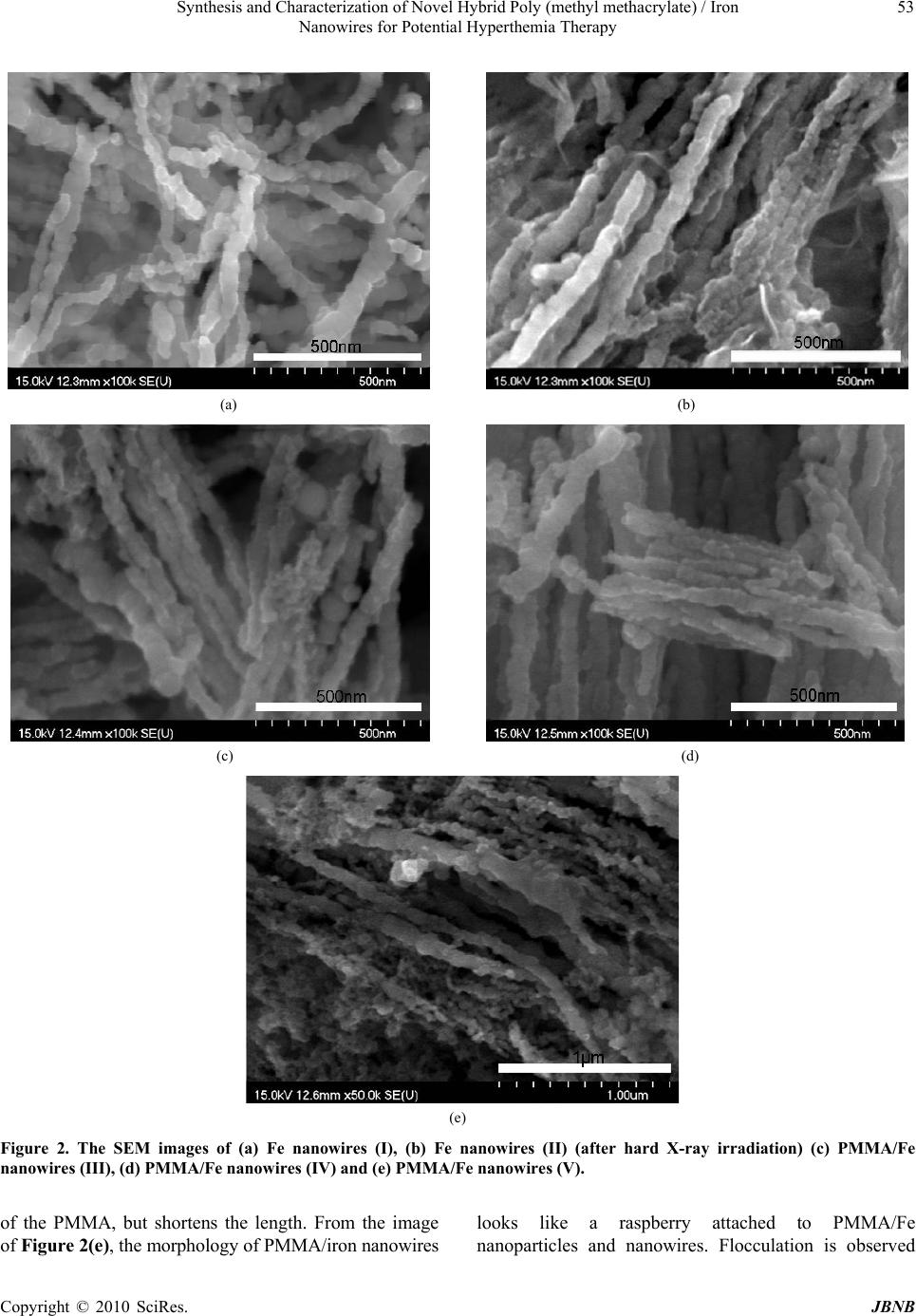 Synthesis and Characterization of Novel Hybrid Poly (methyl methacrylate) / Iron Nanowires for Potential Hyperthemia Therapy Copyright © 2010 SciRes. JBNB 53 (a) (b) (c) (d) (e) Figure 2. The SEM images of (a) Fe nanowires (I), (b) Fe nanowires (II) (after hard X-ray irradiation) (c) PMMA/Fe nanowires (III), (d) PMMA/Fe nanow ires (IV) and (e) PMMA/Fe nanowires (V). of the PMMA, but shortens the length. From the image of Figure 2(e), the morphology of PMMA/iron nanowires looks like a raspberry attached to PMMA/Fe nanoparticles and nanowires. Flocculation is observed 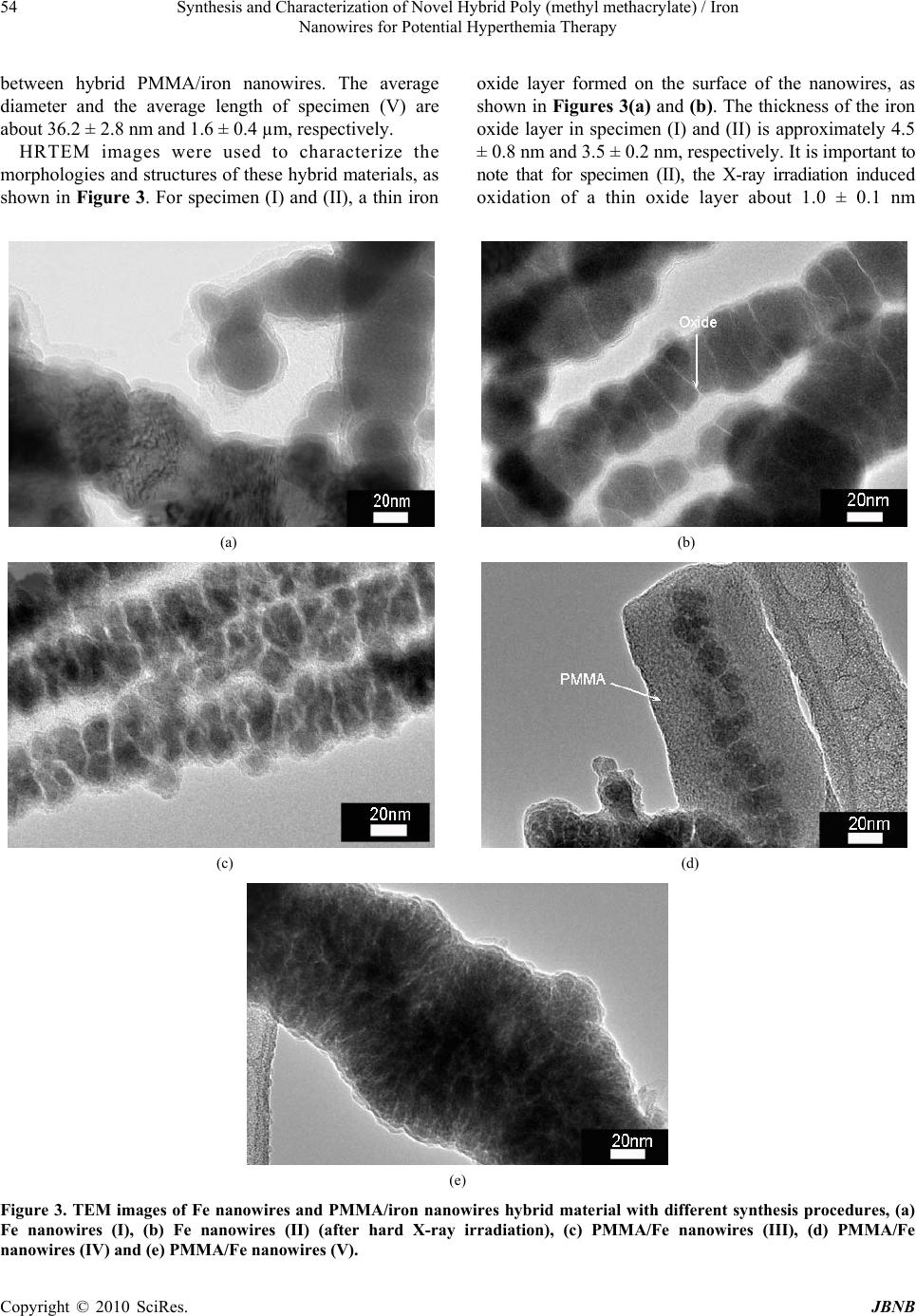 Synthesis and Characterization of Novel Hybrid Poly (methyl methacrylate) / Iron 54 Nanowires for Potential Hyperthemia Therapy between hybrid PMMA/iron nanowires. The average diameter and the average length of specimen (V) are about 36.2 ± 2.8 nm and 1.6 ± 0.4 µm, respectively. HRTEM images were used to characterize the morphologies and structures of these hybrid materials, as shown in Figure 3. For specimen (I) and (II), a thin iron oxide layer formed on the surface of the nanowires, as shown in Figures 3(a) and (b). The thickness of the iron oxide layer in specimen (I) and (II) is approximately 4.5 ± 0.8 nm and 3.5 ± 0.2 nm, respectively. It is important to note that for specimen (II), the X-ray irradiation induced oxidation of a thin oxide layer about 1.0 ± 0.1 nm (a) (b) (c) (d) (e) Figure 3. TEM images of Fe nanowires and PMMA/iron nanowires hybrid material with different synthesis procedures, (a) Fe nanowires (I), (b) Fe nanowires (II) (after hard X-ray irradiation), (c) PMMA/Fe nanowires (III), (d) PMMA/Fe nanowires (IV) and (e) PMMA/F e nanowires (V). Copyright © 2010 SciRes. JBNB 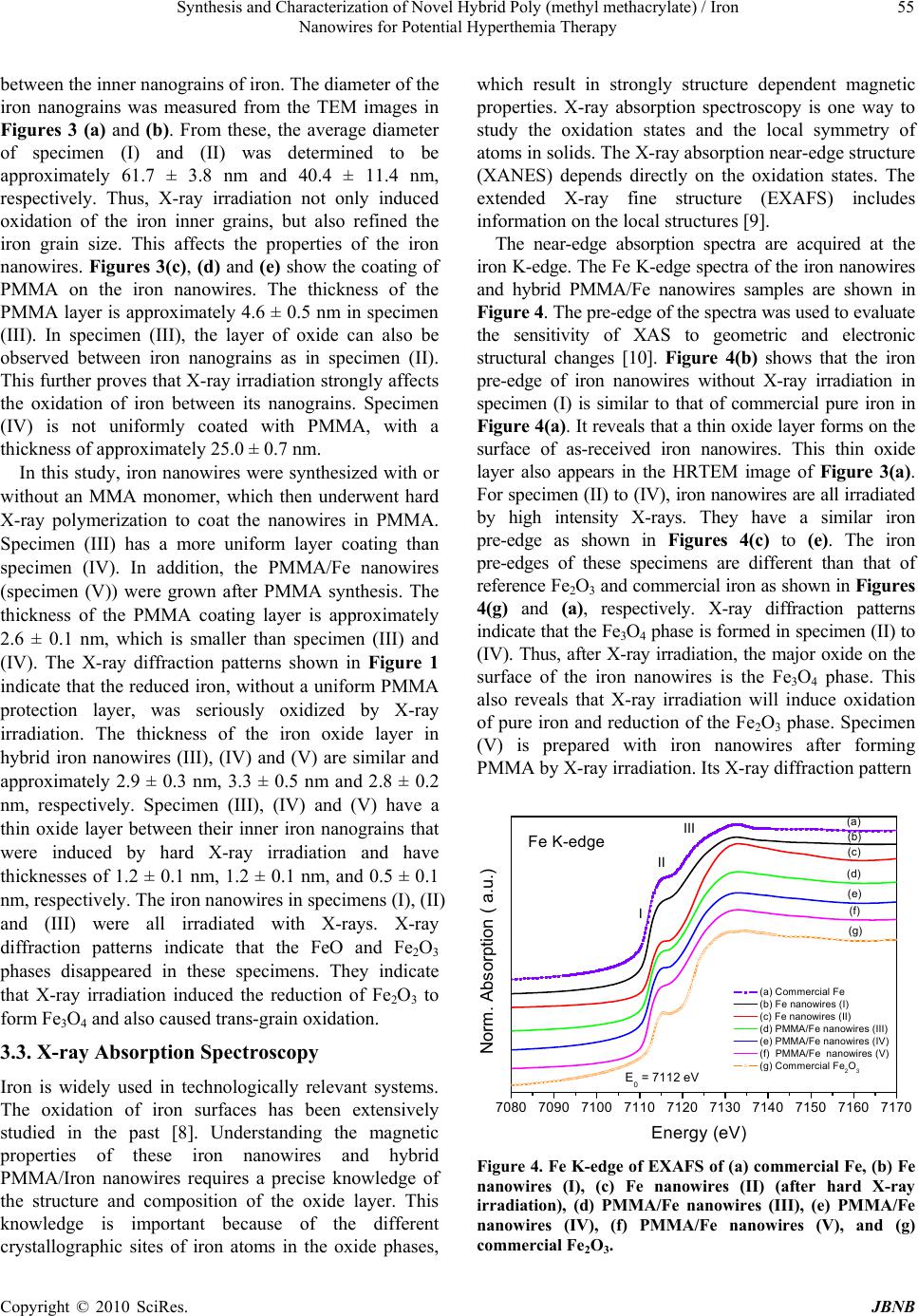 Synthesis and Characterization of Novel Hybrid Poly (methyl methacrylate) / Iron 55 Nanowires for Potential Hyperthemia Therapy between the inner nanograins of iron. The diameter of the iron nanograins was measured from the TEM images in Figures 3 (a) and (b). From these, the average diameter of specimen (I) and (II) was determined to be approximately 61.7 ± 3.8 nm and 40.4 ± 11.4 nm, respectively. Thus, X-ray irradiation not only induced oxidation of the iron inner grains, but also refined the iron grain size. This affects the properties of the iron nanowires. Figures 3(c), (d) and (e) show the coating of PMMA on the iron nanowires. The thickness of the PMMA layer is approximately 4.6 ± 0.5 nm in specimen (III). In specimen (III), the layer of oxide can also be observed between iron nanograins as in specimen (II). This further proves that X-ray irradiation strongly affects the oxidation of iron between its nanograins. Specimen (IV) is not uniformly coated with PMMA, with a thickness of approximately 25.0 ± 0.7 nm. In this study, iron nanowires were synthesized with or without an MMA monomer, which then underwent hard X-ray polymerization to coat the nanowires in PMMA. Specimen (III) has a more uniform layer coating than specimen (IV). In addition, the PMMA/Fe nanowires (specimen (V)) were grown after PMMA synthesis. The thickness of the PMMA coating layer is approximately 2.6 ± 0.1 nm, which is smaller than specimen (III) and (IV). The X-ray diffraction patterns shown in Figure 1 indicate that the reduced iron, without a uniform PMMA protection layer, was seriously oxidized by X-ray irradiation. The thickness of the iron oxide layer in hybrid iron nanowires (III), (IV) and (V) are similar and approximately 2.9 ± 0.3 nm, 3.3 ± 0.5 nm and 2.8 ± 0.2 nm, respectively. Specimen (III), (IV) and (V) have a thin oxide layer between their inner iron nanograins that were induced by hard X-ray irradiation and have thicknesses of 1.2 ± 0.1 nm, 1.2 ± 0.1 nm, and 0.5 ± 0.1 nm, respectively. The iron nanowires in specimens (I), (II) and (III) were all irradiated with X-rays. X-ray diffraction patterns indicate that the FeO and Fe2O3 phases disappeared in these specimens. They indicate that X-ray irradiation induced the reduction of Fe2O3 to form Fe3O4 and also caused trans-grain oxidation. 3.3. X-ray Absorption Spectroscopy Iron is widely used in technologically relevant systems. The oxidation of iron surfaces has been extensively studied in the past [8]. Understanding the magnetic properties of these iron nanowires and hybrid PMMA/Iron nanowires requires a precise knowledge of the structure and composition of the oxide layer. This knowledge is important because of the different crystallographic sites of iron atoms in the oxide phases, which result in strongly structure dependent magnetic properties. X-ray absorption spectroscopy is one way to study the oxidation states and the local symmetry of atoms in solids. The X-ray absorption near-edge structure (XANES) depends directly on the oxidation states. The extended X-ray fine structure (EXAFS) includes information on the local structures [9]. The near-edge absorption spectra are acquired at the iron K-edge. The Fe K-edge spectra of the iron nanowires and hybrid PMMA/Fe nanowires samples are shown in Figure 4. The pre-edge of the spectra was used to evaluate the sensitivity of XAS to geometric and electronic structural changes [10]. Figure 4(b) shows that the iron pre-edge of iron nanowires without X-ray irradiation in specimen (I) is similar to that of commercial pure iron in Figure 4(a). It reveals that a thin oxide layer forms on the surface of as-received iron nanowires. This thin oxide layer also appears in the HRTEM image of Figure 3(a). For specimen (II) to (IV), iron nanowires are all irradiated by high intensity X-rays. They have a similar iron pre-edge as shown in Figures 4(c) to (e). The iron pre-edges of these specimens are different than that of reference Fe2O3 and commercial iron as shown in Figures 4(g) and (a), respectively. X-ray diffraction patterns indicate that the Fe3O4 phase is formed in specimen (II) to (IV). Thus, after X-ray irradiation, the major oxide on the surface of the iron nanowires is the Fe3O4 phase. This also reveals that X-ray irradiation will induce oxidation of pure iron and reduction of the Fe2O3 phase. Specimen (V) is prepared with iron nanowires after forming PMMA by X-ray irradiation. Its X-ray diffraction pattern 7080 7090 71007110 7120 71307140 7150 71607170 (a) Com mercial Fe (b) Fe nanowires (I) (c) Fe nanowires (II) (d) PMMA/Fe nanowires ( III) (e) PMMA/Fe nanowires (IV) (f) PMMA/Fe nanowires (V) (g) Com mercial Fe2O3 Energy (eV) Fe K-edge E0 = 7112 eV Norm. Absorption ( a.u.) (g) (f) (b) (a) (d) (c) (e) I II III Figure 4. Fe K-edge of EXAFS of (a) commercial Fe, (b) Fe nanowires (I), (c) Fe nanowires (II) (after hard X-ray irradiation), (d) PMMA/Fe nanowires (III), (e) PMMA/Fe nanowires (IV), (f) PMMA/Fe nanowires (V), and (g) commercial Fe2O3. Copyright © 2010 SciRes. JBNB  Synthesis and Characterization of Novel Hybrid Poly (methyl methacrylate) / Iron 56 Nanowires for Potential Hyperthemia Therapy (Figure 1(e)) is similar to the iron nanowires without X-ray irradiation in Figure 1(a), only with a lower intensity in the iron peak at around 2θ = 45o. The shape of the iron pre-edge of specimen (V) is similar to that of specimen (I) and specimen (II)-(IV). X-ray irradiation effects on the iron oxidephases are enhanced by the formation of Fe3O4 and the trans-grain oxidation, as indicated in Figures 3(b) and (c). To further examine these results, we differentiated the data of Figure 4 to obtain the differential pre-edge of the Fe K-edge, as shown in Figure 5. The absorption edge differences in these samples are obvious. The first differential pre-edge peak represents the electron transition from the 1s to 4d orbital that directly relates to the oxidation state and geometry of the iron atom [11]. Also, the total intensity of this transition has been shown to in- crease with decreasing coordination number for iron due to the loss of inversion symmetry at the iron site [12]. The differential pre-edge peaks of Fe nanowires are listed in Table 1. The differential peak of Fe2O3 with Fe3+ is at 7133.3 eV, which is larger than the 7111.8 eV of com mercial pure iron with Fe. The differential pre-edges of Iron nanowires with or without X-ray irradiation and hybrid PMMA/iron nanowires are all located between Fe2O3 and pure iron. For pure Fe2O3, there is a second differential pre-edge peak at 7117.4 eV that is not observed in other specimens, including pure iron. It has been proven that synthesized iron nanowires have an iron oxidation state between Fe2+ and Fe3+ that forms a surface oxide with an iron core. The position of third differential pre-edge peaks is only observed as the differences between pure iron and iron nanowires (I) and the rest of the specimen. The second 7100 7110 7120 7130 7140 (a) Commercial Fe (b) Fe nanowires (I) (c) Fe nanowires (II) (d) PMMA/Fe nanowires (III) (e) PMMA/Fe nanowires (IV) (f) PMMA/Fe nanowires(V) (g) Commercial Fe2O3 (a) (a) (g) (g) (f) (f) (d) (d) (c) (e) (c) (e) (b) Deriv. norm. absorption Energy (eV) 1s-4d transition Fe K-edge 1s-4p transition (b) Figure 5. The differential pre-edge (NEXAFS) of the Fe- K-edge of (a) commercial Fe, (b) Fe nanowires (I), (c) Fe nanowires (II) (after hard X-ray irradiation), (d) PMMA/Fe nanowires (III), (e) PMMA/Fe nanowires (IV), (f) PMMA/ Fe nanowires (V), and (g) commercial Fe2O3. and third peaks may have been caused by the 1s-4p transition [13]. It is not sensitive to different oxidation states but is between pure iron and iron oxides. The Fourier-transformed Fe EXAFS data in Figure 6 indicate that the nearest Fe-O bond appears at about 1.4- 1.6 Å for all specimens, corresponding to a tetrahedrally coordinated cation. For commercial iron and iron nanowires without X-ray irradiation, the Fe-Fe bond length of metallic iron is about 2.1Å and can be observed, but not in other specimens. XAS, in this study, was measured in fluorescence mode. Specimens (II) to (V) have a thicker oxide layer compared to specimen (I). Thus, the Fe-Fe bond is not observed in these specimens. The radiation distribution of specimen (II) to (V) shows major differences compared to commercial Fe2O3. A previous study [14] indicated that the oxide layer in these nanowires is Fe3O4. XANES of the O K edge spectra in Figure 7 also shows dramatic differences between Fe2O3 and the rest of specimens. In the O K-edge, split absorption peaks are observed only in Fe2O3. This shows that the iron nanowires can be stabilized by the Fe3O4 surface oxide layer. As seen in the HRTEM images of Figure 3, transgrain oxidation occurs when X-rays irradiate iron nanowires. XAS and XRD results indicate that the oxide phase after X-ray irradiation is mainly Fe3O4. The oxide layer also got larger after irradiation. The mechanism of X-ray induced oxidation may be the following; FeOOFeh core 22 2 (2) surface h surface OFeOFeFeO 43 )( 32 (3) The iron atoms diffuse out from the core of the nanoparticles that form the iron nanowires. Under X-ray irradiation, iron atoms first oxidize into FeO (Equation 2) and then react with Fe2O3 to form Fe 3O4 (Equation 3). Thus, the trace Fe2O3 phase in specimen (I) is transferred into the Fe3O4 phase, a fact that is proven by XRD and XAS results. Table 1. The differential pre-edge peaks of Fe nanowires and PMMA/Fe nanowires. Specimens First (eV) Second (eV) Third (eV) Fe nanowires (I) 7112.1 - 7120.3 Fe nanowires (II) 7112.4 - 7122.7 PMMA/Fe nanowires (III) 7112.2 - 7122.7 PMMA/Fe nanowires (IV) 7112.2 - 7122.7 PMMA/Fe nanowires (V) 7112.1 - 7122.7 Commercial Fe2O3 7113.3 7117.4 7122.7 Commercial Fe 7111.8 - 7120.3 Copyright © 2010 SciRes. JBNB  Synthesis and Characterization of Novel Hybrid Poly (methyl methacrylate) / Iron 57 Nanowires for Potential Hyperthemia Therapy 0246810 (g) (f ) (e) (d) (c) (a) Commercial Fe (b) Fe nanowires I (c) Fe nanowires II (d) PMMA/Fe nano wires III (e) PMMA/Fe nanowires IV (f ) PMMA/Fe nanowires V (g) Commercial Fe2O3 FT, Ix(R)I (a.u.) (a) (b) R (A) 0 Figure 6. Fourier-transformed Fe EXAFS data of (a) commercial Fe, (b) Fe nanowires (I), (c) Fe nanowires (II) (after hard X-ray irradiation), (d) PMMA/Fe nanowires (III), (e) PMMA/Fe nanowires (IV), (f) PMMA/Fe nanowires (V), and (g) commercial Fe2O3. 510 515 520 525 530 535 540 545 550 555 560 Energy (eV) Norm. intensity (a.u.) (a) (b) (c) (d) (e) (f) (g) O K-edge Figure 7. XANES normalized intensity of the O K edge spec tra of (a) commercial Fe, (b) Fe nanowires (I), (c) Fe nanowires (II) (after hard X-ray irradiation), (d) PMMA/Fe nanowires (III), (e) PMMA/Fe nanowires (IV), (f) PMMA/Fe nanowires (V), and (g) commercial Fe2O3. 3.4. TGA Analysis To measure the mass content of the specimens, the iron nanowires and the hybrid PMMA/Fe nanowires were examined by TGA analysis with an SDT2960 Simultaneous DSC-TGA (TA Instruments) system. The results are shown in Figure 8. The curves show mass decreases in specimen (I), (II), (III), (IV) and (V) prior to 166, 303, 216, 254 and 324℃, respectively, under oxygen atmosphere. Specimen (I), (II), (III), (IV) and (V), had around 0200 400 600 800100012001400 65 70 75 80 85 90 95 100 105 110 115 Weight loss (wt%) Temperature (oC) (a)Fe nanowires (I) (b)Fe nanowires (II) (c)PMMA/Fe nanowires (III) (d)PMMA/Fe nanowires (IV) (e)PMMA/Fe nanowires (V) (a) (b) (c) (d) (e) Figure 8. The TGA spectrums of (a) Fe nanowires (I), (b) Fe nanowires (II) (after hard X-ray irradiation), (c) PMMA/Fe nanowires (III), (d) PMMA/Fe nanowires (IV) and (e) PMMA/Fe nanowires (V). 7.18, 11.29, 8.48, 9.56, and 13.31 wt% mass loss, respectively, including water, methanol and thermal decomposition of hydroxide materials. Then, the iron nanowires started to oxidize and PMMA began to decompose. After PMMA completely decomposed, the weights of the specimens were greater than 100%, due to iron oxide formation. Specimens (III), (IV) and (V) have a peak at around 500℃ that may have been cause by the carbonization of PMMA. The Curve (a) for specimen (I) in Figure 8 shows that the oxidation temperature of iron nanowires is lower than that of other specimens. This indicates that the iron nanowires without X-ray or PMMA treatments are oxidized more easily than other specimens. This shows that the inner nanograins of specimen (II) to (V) are protected by thin layers of ox- ides that resist oxidation at lower temperatures. The weight gain of specimen (I) due to oxidation is great larger than that of specimen (II). This indicates that the iron nanowires treated by X-ray irradiation are highly oxidized. This phenomenon is also proven by the X-ray dif- fraction pattern in Figure 1. The major diffraction peak of pure iron in specimen (II) is from dramatic reduction. Specimens (III) to (V) show the same phenomenon as specimen (II). Pure PMMA usually decomposes before 430℃, but the weight gain peak decreased in specimens (III), (IV) and (V) when the temperature was higher than 600℃. This may be due to further decomposition of the residues of carbonized PMMA. The iron oxides were reduced at temperatures higher than 1100℃, which causes weight loss in the TGA thermogram. At temperatures > 1350℃, the Copyright © 2010 SciRes. JBNB  Synthesis and Characterization of Novel Hybrid Poly (methyl methacrylate) / Iron 58 Nanowires for Potential Hyperthemia Therapy residual weight of the specimens indicates their iron content. The residue weight of specimen (II) at 1350℃ is 77.0wt%, less than that of specimen (I), which is about 85.8wt%, as shown in Figure 8. This confirms that X-ray irradiation induced the oxidation of specimen (II). Thus, the oxide content in specimen (II) is larger than in specimen (I). According to residual weight in the TGA thermogram, the iron contents of specimens (I) to (V) are about 85.8, 77.0, 81.2, 76.7 and 70.1wt%, respectively. 3.5. Magnetic Properties It was recently shown that nanometer thick native-oxide layers can be used as building blocks in new magnetic structures [15]. For multilayers consisting of alternating Fe and Fe-oxide layers, the magnetic moments of the Fe layers are arranged in a non-collinear fashion [16]. The structure of nanowires after X-ray irradiation can build a chain of iron nanoparticle/iron nano oxide layer structures. This may lead to novel magnetic materials for application in the electronics or medical fields. The magnetic properties of the various specimens are examined at room temperature by VSM. Figure 9 and Table 2 show magnetic hysteresis curves and data of these specimens, respectively. The hysteresis loops of specimens (I), (II), (III), (IV) and (V) reveal their ferromagnetic behavior. Specimen (II), with X-ray irradiation, has a saturation magnetization (Ms) that is 173 emu/g larger than that of specimen (I) (138 emu/g). Their remanences (Mr) are 64 and 51 emu/g, respectively. TEM images of Figure 3 (a) and (b) show that the diameter of iron nanograins is approximately 40.4 ± 11.4 nm for specimen (II) and 61.7 ± 3.8 nm for specimen (I). These results demonstrate that hard X-ray irradiation causes the refinement of grain size and also induces oxidation reactions in iron nanograins. For specimens (I) and (II), the coercivity (Hc) is 789 Oe (reduced remanence ratio 0.37) and 324 Oe (reduced remanence ratio 0.37), respectively. The saturation magnetization of PMMA/Fe nanowires (III, IV and V) is 101, 82 and 71 emu/g; the coercivity is 171, 116 and 60 Oe; and remanence is 39, 31 and 13 emu/g, respectively. The magnetic properties of these specimens are attributed to the size and structure of the iron and iron oxides. For specimens (I) and (II), it is clear that X-ray irradiation induces size refinement and reduction of iron oxide. The Fe2O3 phase is reduced to Fe3O4. This is not only the case in specimen (II). Specimens (III) and (IV) are also only reduced to Fe3O4 as observed in X-ray diffraction patterns. These nanowires are synthesized under strong magnetic fields, which arranged the nanograins in magnetically preferred orientation. -20000 -1000001000020000 -200 -150 -100 -50 0 50 100 150 200 (a)F e wire (I ) (b)F e wire (I I) (c)P MMA/F e wire (III ) (d)PM MA/F e wire (I V ) (e)PM MA/F e (V ) H (Oe) M (emu/g) (e) (d) (c) (a) (b) Figure 9. The VSM measurements of (a) Fe nanowires (I), (b) Fe nanowires (II) (after hard X-ray irradiation), (c) PMMA/Fe nanowires (III), (d) PMMA/Fe nanowires (IV) and (e) PMMA/Fe nanowires (V). Table 2. The VSM measured data of Fe nanowires and PMMA/Fe nanowires. Specimens Hc (Oe) Mr (emu/g) Ms (emu/g) Sr = Mr/Ms Fe nanowires (I) 789 51 138 0.37 Fe nanowires (II) 324 64 173 0.37 PMMA/Fe nanowires (III) 171 39 101 0.39 PMMA/Fe nanowires (IV) 116 31 82 0.38 PMMA/Fe nanowires (V) 60 13 71 0.18 The magnetic saturation of iron nanowires and PMMA/iron nanowires is higher than that of the sphere- cal Fe colloids produced by sonochemical synthesis (101 emu/g) [17] or iron nanoparticles produced by metal vapor deposition (55 emu/g) [18]. Figure 1 shows the effect of the remaining Fe2O3 and Fe3O4 phases. Both remanence and coercivity are affected by the volume fraction of the Fe3O4 phase. The magnetic properties of iron nanowires are similar to nanocrystalline soft materials where the magnetization averages over several grains [19]. The exchange interactions between iron nanograins in nanowires due to agglomeration account for the remanence enhancement and the drop of the coercive field as shown in specimen (II) with respect to the Stoner–Wohlfarth value [20]. 4. Conclusions In summary, iron nanowires and hybrid PMMA/Fe nanowires were successfully synthesized with the Copyright © 2010 SciRes. JBNB  Synthesis and Characterization of Novel Hybrid Poly (methyl methacrylate) / Iron 59 Nanowires for Potential Hyperthemia Therapy oo-like structure of the iron nanowires could be ements reveal that the na ledge the National Science REFERENCES [1] L. Menon, M. dyopadhyay and D ndyopadhyay, L. Menon, N. Kouklin, H. Zeng and Y. Liu, S. Bandyop- Z. An, J. Zhang, and G. Song, “Facile Synthesis . Yadian, B. and Y. Zhang, . Lin, Y. Hwu and W. Chen, Ray Absorption and F. H. P. M. er, nd and E. Paris, “Iron . Eisenberger and W. E. l, L. Shu, Y. M. Chiou, K. S. Hagen, M. Ito, pohl, J. G. DeWitt, B. Hedman, K. G. Harris and M. E. reducing agent NaBH4 under an externally applied magnetic field with radiation polymerization. The externally applied magnetic field played an important role in the formation of uniform and parallel iron nanowires. The bamb adh observed by SEM and HRTEM. An oxide layer formed on the surface of the iron nanowires. XRD results showed that the Fe2O3 and Fe3O4 phases coexisted in the iron nanowires without X-ray irradiation. When these samples are irradiated by hard X-ray from NSRRC, Fe3O4 is the only phase that can be observed. The Fe and O K-edges of XAS were used to distinguish the oxide structures on the surface of the iron nanowires and PMMA/iron nanowires. The results of XAS show the phase transformation of Fe2O3 to Fe3O4. Thus, X-ray irradiation will induce Fe2O3 phase transformation to Fe3O4 and stabilize these iron nanowires. TGA analysis is used here to determine the thermal properties and solid content of these specimens. Magnetic hysteresis measur iron Habraken, “Oxidation of Iron: The Relation between Oxi- dation Kinetics and Oxide Electronic Structure,” Physical Review Letters, Vol. 84, No. 15, 2000, pp. 3366-3369. S. Couet, T. H. Diederich, K. Schlage and R. Röhlsberg nowires displayed ferromagnetic behavior. For the iron nanowires (specimen (II)) prepared after X-ray irradiation, the saturation magnetization is 173 (emu/g) larger than that of the initial iron nanowires (specimen (I)). The saturation magnetization of the iron nanowires and PMMA/iron nanowires is higher than that of spherical Fe colloids produced by sonochemical synthesis or iron nanoparticles produced by metal vapor deposition. Iron nanowires-coated PMMA hybrid materials could be designed to manipulate the magnetic and biocompatibility properties for application in magnetic hyperthermia. This study indicates that X-ray irradiation treatment of these iron nanowires will provide a way to manipulate the structural and magnetic properties of novel hybrid nano-materials. 5. Acknowledgements [9] The authors gratefully acknow Blum Council, Taiwan, for financial support, and the TLS (01A) team of the National Synchrotron Radiation Research Center (NSRRC) for technical support. Zheng, H. Zeng, S. Ban. O. H J. Sellmyer, “Size Dependence of the Magnetic Properties of Electrochemically Self-Assembled Fe Quantum Dots,” Journal of Electronic Materials, Vol. 29, No. 5, pp. 510- 515. [2] S. Ba McHe D. J. Sellmyer, “Electrochemically Self-Assembled Quantum Dot Arrays,” Journal of Electronic Materials, Vol. 28, No. 5, 2000, pp. 515-519. [3] M. Zheng, L. Menon, H. Zeng, yay, R. D. Kirby and D. J. Sellmyer, “Magnetic Properties of Ni Nanowires in Self-Assembled Arrays,” Physical Review B, Vol. 62, No. 18, 2000, pp. 12282- 12286. [4] S. Pan, of Prickly CoNi Microwires,” Materials Letters, Vol. 64, No. 3, 2010, pp. 453-456. [5] P. Liu, Z. Li, B. Zhao, B “Template–Free Synthesis of Nickel Nanowires by Magnetic Field,” Materials Letters, Vol. 63, No. 20, 2009, pp. 1650-1652. [6] H. Liou, W. Liou, H “Characterization of Gold/PMMA Hybrid Nanomaterials Synthesized by Hard X-Ray Synchrotron Radiation,” Particuology, Vol. 8, No. 3, pp. 234-239. [7] H. M. Lin, C. K. Lin and Y. K. Hwu, “X- Techniques in Examination the Structural Properties of Nanocrystalline Materials,” Fu Jen Studies, Science and Engineering, Vol. 38, 2004, pp. 1-26. [8] S. J. Roosendaal, A. M. Vredenberg “A Compact UHV Deposition System for in situ Study of Ultrathin Films via Hard X-Ray Scattering and Spec- troscopy,” Review of Scientific Instruments, Vol. 79, No. 9, 2008, pp. 093908-1-093908-9. [10] G. Giuli, G. Pratesi, C. Cipriania Local Structure in Tektites and Impact Glasses by Ex- tended X-Ray Absorption Fine Structure and High- Resolution X-Ray Absorption Near-Edge Structure Spec- troscopy,” Geochimica et Cosmochimica Acta, Vol. 66, No. 24, 2002, pp. 4347-4353. [11] R. G. Shulman, Y. Yafet, P berg, “Observation and Interpretation of X-Ray Absorption Edges in Iron Compounds and Proteins,” Pro- ceedings of the National Academy of Sciences of the United States of America, Vol. 73, No. 5, 1976, pp. 1384-1388. [12] C. R. Randal N. Kitajima, R. J. Lachicotte, Y. Zang and L. Jr. Que, “X-Ray Absorption Pre-Edge Studies of High-Spin Iron (II) Complexes,” Inorganic Chemistry, Vol. 34, No. 5, 1995, pp. 1036-1039. [13] T. E. Westre, P. Kenne odgson and E. I. Solomon, “A Multiplet Analysis of Fe K-Edge 1s → 3d Pre-Edge Features of Iron Com- plexes,” Journal of The America Chemical Society, Vol. 119, No. 27, 1997, pp. 6297-6314. [14] S. Son, M. Taheri, E. Carpenter, V. nry, “Synthesis of Ferrite and Nickel Ferrite Nano- particles Using Radio-Frequency Thermal Plasma Torch,” Journal of Applied Physics, Vol. 91, No. 10, 2002, pp. Copyright © 2010 SciRes. JBNB  Synthesis and Characterization of Novel Hybrid Poly (methyl methacrylate) / Iron Nanowires for Potential Hyperthemia Therapy Copyright © 2010 SciRes. JBNB 60 ch, F. T. Parker, D. J. Smith, P. A. Crozier erger, “Non- and T. Hyeon, “Sonochemica 7589-7591. [15] G. S. D. Bea Hadj and A. E. Berkowitz, “New Magnetic Order in Buried Native Iron Oxide Layers,” Physical Review Letters, Vol. 91, No. 26, 2003, pp. 267201-1-267201-4. [16] T. H. Diederich, S. Couet and R. Röhlsb collinear Coupling of Iron Layers through Native Iron Oxide Spacers,” Physical Review B, Vol. 76, No. 5, 2007, pp. 054401-1-054401-5. [17] K. S. Suslick, M. Fang l [2 Synthesis of Iron Colloids,” Journal of the America Che- mical Society, Vol. 118, No. 47, 1996, pp. 11960-11961. [18] C. F. Kernizan, K. J. Klabunde, C. M. Sorensen and G. C. ipanayis, “Magnetic Properties of Nanometer-Scale Iron Particles Generated by Iron Atom Clustering in Cold Pentane,” Chemistry of Materials, Vol. 2, No. 1, 1990, pp. 70-74. [19] W. J. M. Mulder, G. J. Strijkers, G. A. F. van Tilborg, A, W. Griffioen and K. Nicolay, “Lipid-Based Nanoparticles for Contrast-Enhanced MRI and Molecular Imaging,” NMR in Biomedicine, Vol. 19, No. 1, 2006, pp. 142-164. 0] E. V. Shtykova, X. Huang, N. Remmes, D. Baxter, B. Stein, B. Dragnea, D. I. Svergun and L. M. Bronstein, “Structure and Properties of Iron Oxide Nanoparticles Encapsulated by Phospholipids with Poly(ethylene glycol) Tails,” The Journal of Physical Chemistry C, Vol. 111, No. 49, 2007, pp. 18078-18086. |

