Paper Menu >>
Journal Menu >>
 Journal of Biomaterials and Nanobiotechnology, 2010, 1, 37-41 doi:10.4236/jbnb.2010.11005 Published Online October 2010 (http://www.SciRP.org/journal/jbnb) Copyright © 2010 SciRes. JBNB 37 Effect of Nano - Titanium Dioxide with Different Antibiotics against Methicillin-Resistant Staphylococcus Aureus Aashis S. Roy1, Ameena Parveen2, Anil R. Koppalkar3, M. V. N. Ambika Prasad1* 1Department of Materials Science, Gulbarga University, Gulbarga, Karnataka, India; 2Department of Physics, Gurmithkal, Yadgir, Karnataka, India; 3Materials Science Lab, S. S. Margol College, Shahabad, Gulbarga, Karnataka, India. Email: *amb1_prasad@rediffmail.com Received June 14th, 2010; revised June 25th, 2010; accepted June 30th, 2010. ABSTRACT The different investigation has been carried out on the biological activities of titanium dioxide nanoparticle but the effect of this nano product on the antibacterial activity of different antibiotics has not been yet demonstrated. In this study the nano size TiO2 is synthesized using citric acid and alpha dextrose and the enhancement effect of TiO2 nanoparticle on the antibacterial activity of different antibiotics was evaluated against Methicillin-resistant Staphylococcus aureus (MRSA). During the present study, different concentrations of nano-scale TiO2 were tested to find out the best concentration that can have the most effective antibacterial property against the MRSA culture. Disk diffusion method was used to determine the antiba cterial activity of these an tibiotics in th e absence and presence of su b inhibitory concentration of TiO2 nano particle. A clinical isolate of MRSA, isolated from Intens ive Care Unit (ICU) was used as test strain. In the presence of sub-inhibitory concentration of TiO2 nanoparticle (20 µg/disc) the antibacterial activities of all antibio tics have been increased against test strain with minimum 2 mm to maximum 10mm. The highest increase in inhibitory zone for MRSA was observed against pencillin G and amikacin (each 10 mm). Conversely, in case of nalidixic acid, TiO2 nanopa rticle showed a Synergic effect on the antibacteria l activity of this antib iotic against test strain. These results signify that the TiO2 nanoparticle potentate the antimicrobial action of beta lactums, cephalosporins, aminog lycosides, glyco peptides, ma crolids and lin cosamides, tetra cycline a possible utilizatio n of nano compound in combination effect against MRSA. Keywords: Nano - Titanium Oxide, S. Aureus, Drug Resistance, Antibacterial Activity 1. Introduction Methicillin-resistant Staphylococcus aureus (MRSA) is one of the major nosocomial pathogens responsible for a wide spectrum of infections, including skin and soft tissue infections, pneumonia, bacteraemia, surgical site infec- tions (SSI), catheter related infections [1]. Intensive care unit characteristically has higher rates of infections and in- creased transmission rates, high antibiotic use and large numbers of vulnerable patients [2]. The emergence of bac- terial resistance to antibiotics and its dissemination, how- ever, are major health problems, leading to treatment draw- backs for a large number of drugs [3,4]. Consequently there has been increasing interest in the use of inhibitors of antibiotic resistance for combination therapy [5,6]. Nanostructured materials are attracting a great deal of attention because of their potential of achieving specific processes and selectivity, especially in biological and pharmaceutical applications [7-9]. Gold, silver and cop- per have been used mostly for the synthesis of stable dispersions of nanoparticles [10,11]. A unique charac- teristic of these synthesized metal particles is that a change in the absorbance or wave length gives a measure of the particle size, shape and interparticle properties [12]. Nanomaterials are called “a wonder of modern medicine”. It is stated that antibiotics kill perhaps a half dozen different disease-causing organisms but nanomaterials can kill some 650 cells [13]. Resistant strains fail to develop if we apply nanoparticles based formulations in their culture media. The antibacterial activity of TiO2 has been found to be due to a reaction of the TiO2 surface with water. On exposure to ultraviolet (UV) irradiation, TiO2 releases free radicals such as OH, O2 -, HO2-, and H2O2. This  Effect of Nano - Titanium Dioxide with Different Antibiotics Against Methicillin-Resistant Staphylococcus Aureus Copyright © 2010 SciRes. JBNB 38 potent oxidizing power characteristically results in case of bacteria and other organic substances [14-16]. The small nanometer-scale TiO2 particles impose several effects that govern its antibacterial action we examined the antimicrobial activity of nanostructured titanium dioxide with different antibiotics against MRSA. The different investigation has been carried out on the bio- logical activities of titanium dioxide nanoparticle but the combination effects of this product with different anti- biotics have not been demonstrated. The nanocrystal- line particles of TiO2 are synthesized using ultrasonic irradiation, and the particle sizes are controlled using different solvents during the sonication process. Objectives of the present study are (i) synthesis of nano size titanium dioxide using citric acid and alpha dextrose (ii) analyse the effect of Titanium nanoparticles on the antibacterial activity of different antibiotics against MRSA (iii) estimation of MRSA growth in the presence of TiO2 nanoparticles have been reported having an extremely good safety profile and no toxicity observed when taken at different nanosize. Taken together, this compound as a highly safe compound may be considered for combination therapy against MRSA, due its potential synergetic effect with important antibiotics such as beta lactums, cephalosporins, aminoglycosides. 2. Materials and Methods Titanium dioxide particles preparation: In the following, the two step sol-gel preparation method used is described detail. Nanocrystalline titanium dioxide was prepared by employing citric acid route were saturated solution of α-Dextrose used as a surfactant. Two separate solutions were prepared. In first step: titanium nitrate and citric acid are taken in 1:3 and are thoroughly stirred using magnetic stirrer with ammonia solution at 80˚C about 5-6 hrs. Ammonia solution is used to maintain the pH 4 of solution. Finally a gel is formed. In second step, saturated solution of alpha dextrose is added and stirred for 1hr at 120˚C to the spongy type gel of nanoscaled TiO2 formed. This spongy gel is ignited at a temperature of about 300˚C for 1 hr. At this tem- perature a combustion process takes place in the spongy gel containing citric acid a result of it we have nano- structured titanium dioxide. 3. X-Ray Diffraction (XRD) X-ray diffraction Phase identification was carried out by X-Ray powder diffraction at ambient temperature. A Shintag X1 diffractometer with Cu Kα (1.54 A) radiation in θ – θ configuration was used. The patterns were recorded in the 2-70 range at 0.05 step size using 3-s acquisition time per step. The mean particle size was calculated using the Debye–Scherrer Equation 1 in which K is a constant equal to 0.9, λ is the wavelength of the Cu Kα radiation, β is the half peak width of the diffraction peak in radiant and θ is the Braggs angle of (311) plane. τ = Kλ/βcosθ (1) Staphylococcus aureus was isolated from Clinical specimens collected from ICU of Durgabai Deshmukh Hospital and Research Center and Osmania Hospital, Hyderabad, South India. Oxacillin-disc diffusion method was done for identification of methicillin-resistance. This MRSA was used as test strain. Antibiotic susceptibility test was performed for the test strain (MRSA) against 23 antibiotics by disc agar diffusion method (DAD) on Muller-Hinton agar (Himedia, India), according to the guidelines recommended by National Committee for Clinical Laboratory Standards (NCCLS) [17]. 4. Disk Diffusion Assay to Evaluate Combined Effects To determine combined effects, each standard paper disc was further impregnated with sub-inhibitory concen- tration of titanium dioxide nanoparticle (10 µg/disc). A single colony of test strains were grown overnight in Muller-Hinton broth medium on a rotary shaker (200 rpm) at 35˚C. The inoculums were prepared by diluting the overnight cultures with 0.9% NaCl to a 0.5 McFarland standard and were applied to the plates along with the standard and prepared disks containing of titanium dioxide nanoparticle (10 µg/disc). Clinical isolates of MRSA from our culture collection were used as test strains. After incubation at 37˚C for 24 hrs, the zones of inhibition were measured. The assays were performed in triplicate. 5. Estimation of MRSA Growth in the Presence of Nanocrystalline TiO2 The 2 mL of the overnight-cultured MRSA was added to 100 mL nutrient broth, containing 0.12% glucose with and without 0.01, 0.5 and 1% nano-TiO2 and incubate at 30˚C for 24 hrs. Optical density measurements were taken at 600 nm to monitor the bacterial concentration. 6. Results and Discussions The nano size titanium dioxide is synthesized using citric acid and alpha dextrose. The small nanometer scale TiO2 particles as seen in the Figure 1 will impose several effects that govern its antibacterial action. The X-ray diffraction pattern shows cubic peaks of TiO2, which indicates the nanocrystalline nature of pure nanostructured titanium dioxide and is shown in the Figure 2. By comparing the XRD pattern standard JCPS data (432-161) of TiO2, indicating the prominent peaks corresponding to 2θ = 27˚, 39˚, 48˚, 55˚ and 63˚ are due to (110), (200), (112), 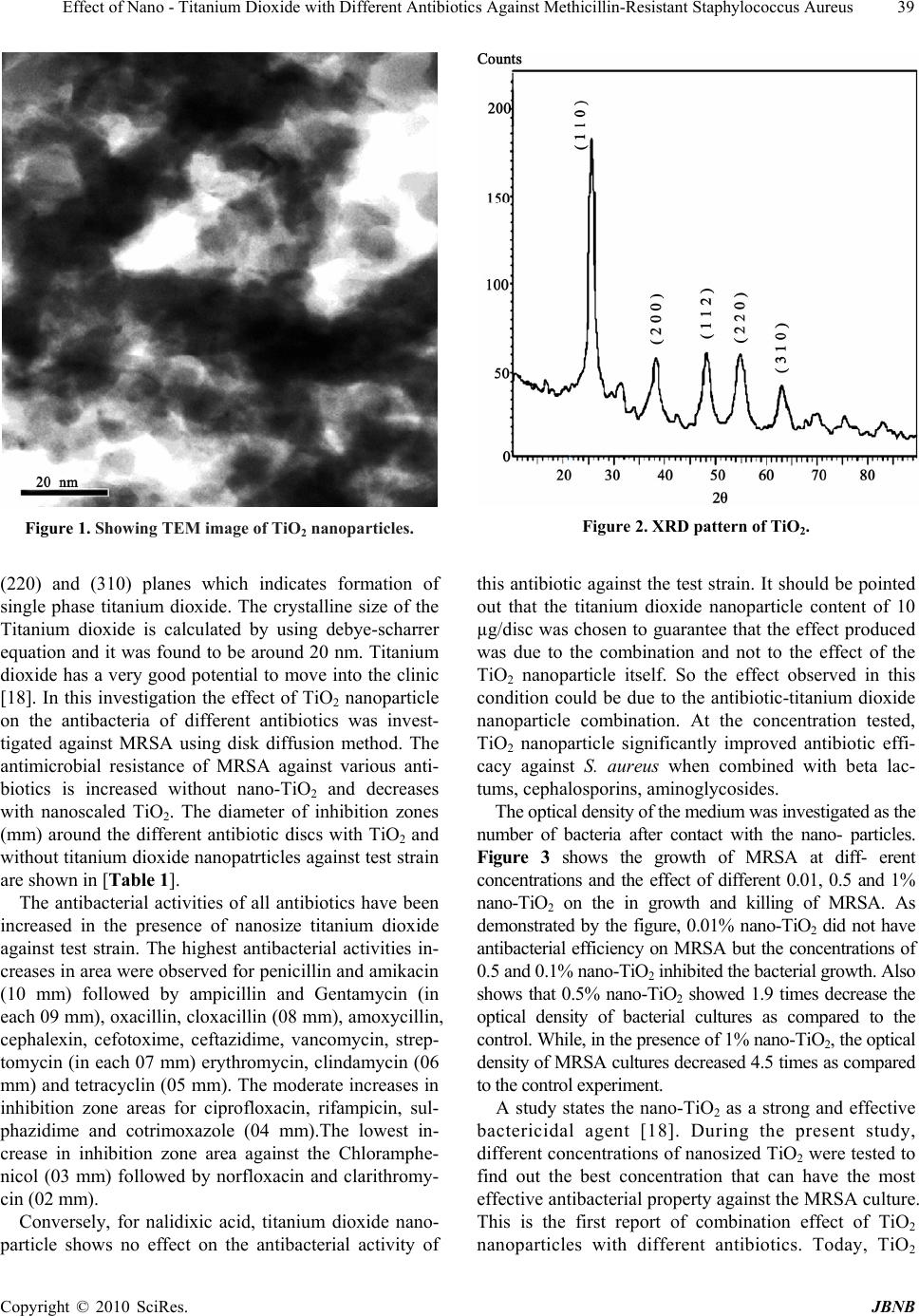 Effect of Nano - Titanium Dioxide with Different Antibiotics Against Methicillin-Resistant Staphylococcus Aureus Copyright © 2010 SciRes. JBNB 39 Figure 1. Showing TEM image of TiO2 nanoparticles. Figure 2. XRD pattern of TiO2. (220) and (310) planes which indicates formation of single phase titanium dioxide. The crystalline size of the Titanium dioxide is calculated by using debye-scharrer equation and it was found to be around 20 nm. Titanium dioxide has a very good potential to move into the clinic [18]. In this investigation the effect of TiO2 nanoparticle on the antibacteria of different antibiotics was invest- tigated against MRSA using disk diffusion method. The antimicrobial resistance of MRSA against various anti- biotics is increased without nano-TiO2 and decreases with nanoscaled TiO2. The diameter of inhibition zones (mm) around the different antibiotic discs with TiO2 and without titanium dioxide nanopatrticles against test strain are shown in [Table 1]. The antibacterial activities of all antibiotics have been increased in the presence of nanosize titanium dioxide against test strain. The highest antibacterial activities in- creases in area were observed for penicillin and amikacin (10 mm) followed by ampicillin and Gentamycin (in each 09 mm), oxacillin, cloxacillin (08 mm), amoxycillin, cephalexin, cefotoxime, ceftazidime, vancomycin, strep- tomycin (in each 07 mm) erythromycin, clindamycin (06 mm) and tetracyclin (05 mm). The moderate increases in inhibition zone areas for ciprofloxacin, rifampicin, sul- phazidime and cotrimoxazole (04 mm).The lowest in- crease in inhibition zone area against the Chloramphe- nicol (03 mm) followed by norfloxacin and clarithromy- cin (02 mm). Conversely, for nalidixic acid, titanium dioxide nano- particle shows no effect on the antibacterial activity of this antibiotic against the test strain. It should be pointed out that the titanium dioxide nanoparticle content of 10 µg/disc was chosen to guarantee that the effect produced was due to the combination and not to the effect of the TiO2 nanoparticle itself. So the effect observed in this condition could be due to the antibiotic-titanium dioxide nanoparticle combination. At the concentration tested, TiO2 nanoparticle significantly improved antibiotic effi- cacy against S. aureus when combined with beta lac- tums, cephalosporins, aminoglycosides. The optical density of the medium was investigated as the number of bacteria after contact with the nano- particles. Figure 3 shows the growth of MRSA at diff- erent concentrations and the effect of different 0.01, 0.5 and 1% nano-TiO2 on the in growth and killing of MRSA. As demonstrated by the figure, 0.01% nano-TiO2 did not have antibacterial efficiency on MRSA but the concentrations of 0.5 and 0.1% nano-TiO2 inhibited the bacterial growth. Also shows that 0.5% nano-TiO2 showed 1.9 times decrease the optical density of bacterial cultures as compared to the control. While, in the presence of 1% nano-TiO2, the optical density of MRSA cultures decreased 4.5 times as compared to the control experiment. A study states the nano-TiO2 as a strong and effective bactericidal agent [18]. During the present study, different concentrations of nanosized TiO2 were tested to find out the best concentration that can have the most effective antibacterial property against the MRSA culture. This is the first report of combination effect of TiO2 nanoparticles with different antibiotics. Today, TiO2  Effect of Nano - Titanium Dioxide with Different Antibiotics Against Methicillin-Resistant Staphylococcus Aureus Copyright © 2010 SciRes. JBNB 40 Table 1. the comparative activities of various antibiotics and antibiotic with NanosizedTiO2 against MRSA. Sl. No. Antibiotics Symbol Inhibition Zone of Antibiotic (mm) Inhibition Zone of Antibiotic with TiO2 (20nm) in (mm) Increased zone size (mm) 1 B-lactams 01. Penicillin G P (10U) 34 44 10 02. Oxacillin Wx (1 μg) 11 19 08 03. Cloxacillin Cx (30 μg) 19 27 08 04. Ampicillin A (10 μg) 29 38 09 05. Amoxycillin Am (25 μg) 20 26 07 2 Cephalosporins 06. Cephalexin Cp (30 μg) 25 32 07 07. Cefotoxime CX (30 μg) 24 33 07 08. Ceftazidime Ca (30 μg) 16 23 07 3 Glycopeptides 09. Vancomycin V (30 μg) 15 22 07 4 Aminoglycosides 10. Amikacin Ak (10 μg) 15 25 10 11. Gentamycin G (50 μg) 14 24 09 12. Streptomycin S (25 μg) 13 19 07 5 Flouroquinolones 13. Ciprofloxacin Cf (5 μg) 20 24 04 14. Norfloxacin No (10 μg) 15 17 02 6 Azlides 15. Clarithromycin Cw (15 μg) 17 19 02 7 Macrolides 16. Erythromycin E (15 μg) 15 21 06 8 Lincosamides 17. Clindamycin Cl (10 μg) 20 26 06 9 Sulphonamides 18. Cotrimoxazole Co (25 μg) 17 21 04 19. Nalidixicacid Na (30 μg) 16 16 00 20. Rifampicin R (15 μg) 25 29 04 21. Tetracyclin T (30 μg) 21 26 05 22. Sulphazidime Sz (25 μg) 12 17 04 23. Chloramphenicol C (30 μg) 18 21 03 Figure 3. MRSA growth at different concentrations of TiO2. nanoparticle are cosmetic ingredient has drawn the atten- tion of scientists because of its extensive pharmaceutical properties. In different phases, clinical trials, no toxicity except mild dehydration was observed when taken at doses as high as g/day and it is reported as an attractive choice for many disease therapies. Recently some metal nanoparticles have been eva- luated for increasing the antibacterial activities of dif- ferent antibiotics. Several investigations have suggested the possible mechanisms involving the interaction of na- nomaterials with the biological molecules. It is believed that microorganisms carry a negative charge while metal oxides carry a positive charge. This creates an “elec- tromagnetic” attraction between the microbe and treated surface. Once the contact is made, the microbe is oxi- dized and dead instantly. Generally, it is believed that nanomaterials release ions, which react with the thiol group (-SH) of the proteins present on the bacterial sur- face. Such proteins protrude through the bacterial cell membrane, allowing the transport of nutrients through the cell wall. Nanomaterials inactivate the proteins, de- creasing the membrane permeability and eventually caus- ing the cellular death [19]. In this study using disk diffu- sion assay we showed that the antibacterial activity of  Effect of Nano - Titanium Dioxide with Different Antibiotics Against Methicillin-Resistant Staphylococcus Aureus Copyright © 2010 SciRes. JBNB 41 beta lactums, cephalosporins, aminoglycosides, glycol- peptides, erythromycin, clindamycin and tetracycline can be increased by TiO2 nanoparticles. Therefore, this com- pound or its future derivatives have a good potential for combination effect against MRSA. 7. Conclusions The synthesis of nanosize titanium dioxide of 20nm was carried out successfully using citric acid and alpha dex- trose as double surfactants. The small nanometer scale TiO2 particles which impose several effects that govern its antibacterial action. The antibacterial activities at different concentrations of nano-TiO2 were investigated. The antimicrobial resistance of MRSA against various antibiotics is increased without nano-TiO2 and decreases with nano-TiO2. Need the further work to find out the exact reason to for enhancement of activity of antibiotics in presence of TiO2 nanoparticles. REFERENCES [1] N. De San, O. Denis, M. F. Gasasira , R. De Mendonca , C. Nonhoff and M. J. Struelens, “Controlled evaluation Of The IDI-MRSA Assay for Detection of Colonization by Methicillin-Resistant, Staphylococcus aureus in Di- verse Mucocutaneous Specimens,” A.J Clin Microbiol, 2007, Vol. 45, No. 4, pp. 1098-1101. [2] N. Safdar and D. G. Maki, “The Community of Risk Factors for Nosocomial Colonization and Infection with Anti-Microbial-Resistant Staphylococcus aureus, Entero- Coccus, Gramnegative Bacilli, Clostridium Difficile and Candida,” Ann Intern Med, Vol. 136, 2002, pp. 834-844. [3] L. C. Braga, A. A. Leite, K. G. Xavier , J. A. Takahashi, M. P. Bemquerer, E. Chartone-Souza, et al. “Synergic interaction between pomegranate extract and antibiotics against Staphylococcus aureus,” Canadian Journal of Microbiology, Vol. 51, No. 7, 2005, pp. 541-547. [4] G. C. Schito, “The Importance of the Development of Antibiotic Resistance in Staphylococcus aureus,” Clin Microbiol Infect, Vol. 12, Suppl. 1, 2006, pp. 3-8. [5] S. Gibbons, “Phytochemicals for Bacterial Resistance. Strengths, Weaknesses and Opportunities,” Planta Med, Vol. 74, No. 6, 2008, pp. 594-602. [6] G. D. Wright, “Resisting Resistance: New Chemical Strategies for Battling Superbugs,” Chemistry and Biology, Vol. 7, No. 6, 2000, pp. R127-R132. [7] X. Wu, H. Liu, J. Liu, K. N. Haley, J. A. Treadway, J. P. Larson, et al., “Immunofluorescent Labeling of Cancer Marker Her2 and Other Cellular Targets with Semicon- ductor Quantum Dots,” Nat Biotechnol, Vol. 21, No. 1, 2003, pp. 41-46. [8] J. D. Fortner, D. Y. Lyon, C. M. Sayes, A. M. Boyd, J. C. Falkner, E. M. Hotze, Alemany et al., “C-60 in Water: Nanocrystal Formation and Microbial Response,” Envi- ron Sci Technol, Vol. 39, No. 11, 2005, pp. 4307-4316. [9] P. Li, J. Li, Q. Wu and J. Li, “Synergistic Antibacterial Effects of Lactum Antibiotic Combined with Silver Nanoparticles,” J. Nanotechnol, Vol. 16, No. 9, 2005, pp. 1912-1917. [10] A. M. Smith, H. Duan, M. N. Rhyner, G. Ruan and S. A. Nie, “Synthesis of Gold Nanoparticles Bearing the Bioconjugation,” Phys Chem Chem Phys, Vol. 8, 2006, p. 3895. [11] G. J. Kearns, E. W. Foster and J. E. Hutchison, “Substrates for Direct Imaging of Chemically Functionalized SiO2 Surfaces by Transmission Electron Microscopy,” Anal Chem, Vol. 78, 2006, p. 298. [12] P. Mulvaney, “Surface Plasmon Spectroscopy of Nanosized Metal Particles Langmuir,” Vol. 12, 1996, p. 788. [13] T. Sungkaworn, W. Triampo, P. Nalakarn, D. Triampo, I. M. Tang, Y. Lenbury, et al. “The Effects of TiO2 Nano- particles on Tumor Cell Colonies: Fractal Dimension and Morphological Properties,” Int J Biomed Sci, Vol. 2, No. 1, 2007, pp. 67-74. [14] M. Cho, H. Chung, W. Choi, et al., “Different Inac- tivation Behaviors of MS-2 Phage and Escherichia coli in TiO2 Photocatalytic Disinfection,” Appl. Eniron. Mi- crobiol., Vol. 71, 2005, pp. 270-275. [15] A. Fujishima, N. R. Tata and A. T. Donald, “Titanium Dioxide Photocatalysis,” J. Photochem. Photobiol. C. Photochem. Rev., Vol. 1, 2000, pp. 1-21. [16] K. Shiraishi, H. Koscki, T. Tsurumoto, et al., “Anti- microbial Metal Implant with a TiO2-Conferred Photo- catalytic Bactericidal Effect against Staphylococcus aureus,” Surf. Inter. Anal., Vol. 41, 2008, pp. 17-21. [17] NCCLS, “Performance Standards for Antimicrobial Sus- ceptibility Testing,” 12th Informational Supplement M100-S12, National Committee for Clinical Laboratory Standards, Villanova, PA. 2002. [18] B. Shopsin, M. Gomez, S. O. Montgomery, D. H. Smith, M. Waddington, D. E. Dodge, D. A. Bost, M. Riehman, S. Naidich and B. N. Krieswirth, “Evalution of Protein A Gene Polymorphic Region DNA Sequencing for Typing of Staphylococcus aureus Strains,” J. Clin. Microbiol, Vol. 37, 1999, pp. 3556-3563. [19] H. Zhang and G. Chen, “Potent Antibacterial Activities of Ag/TiO2 Nanocomposite Powders Synthesized by a One-Pot Sol-Gel Method,” Environ Sci Technol, Vol. 43, No. 8, 2009, pp. 2905-2910. |

