Paper Menu >>
Journal Menu >>
 Journal of Biomaterials and Nanobiotechnology, 2010, 1, 17-30 doi:10.4236/jbnb.2010.11003 Published Online October 2010 (http://www.SciRP.org/journal/jbnb) Copyright © 2010 SciRes. JBNB 17 Water—A Key Substance to Comprehension of Stimuli-Responsive Hydrated Reticular Systems* Miloslav Milichovsky Department of Wood, Pulp and Paper, University of Pardubice, Faculty of Chemical Technology, Pardubice, Czech Republic. Email: miloslav.milichovsky@upce.cz Received August 3rd, 2010; revised August 24th, 2010; accepted September 20th, 2010. ABSTRACT Thermo-responsive hydrated macro-, micro- and submicro-reticular systems (TRHRS), particularly polymers forming hydrogels or similar networks, have attracted extensive interest because comprise biomaterials, smart or intelligent materials. Phase transition temperature (LCST or UCST, i.e. low or upper critical solution temperature, respectively) at about the TRHRS exhibiting a unique hydration-dehydration change is a typical characteristic. The characterization and division of the TRHRS are described followed by explanation of their behaviour. The presented original explana- tion is based on merely combination of basic thermodynamical state of individual useful macromolecule chains (long-chain or coil) with inter- and intra-mutual action of attractive and repulsive intramolecular hydration forces among them being strongly dependent upon temperature. Acquainted with this piece of knowledge, a theoretical con- cept of really biological systems movement, e.g. muscle tissues or artificial muscle etc., can be formulated. Keywords: Thermally Responsive Materials, Hydrogels, Hydration Forces, Volume Phase Transition 1. Introduction Stimuli-responsive polymers – so-called smart polymers – have attracted great interest in academic and applied science recently. Most commonly, approaches take ad- vantage of thermally induced, reversible phase transitions. In this context, polymers forming hydrated reticular sys- tems found great interest. Hydrated reticular systems, i.e. networks in water environment, feature all of bio-objects and the products of their existence. We can identify these structures in nano- (submicro-), micro- and macro-scale as submicro-, micro- and macro-reticular hydrated sys- tems, respectively. Supramolecular and hypermolecular structures are typical, e.g. the hydrogels on peptide basis and fibre-networks on cellulosic basis. Hydrogels consist of elastic networks that can uptake as much as 90–99% w/w of water in their interstitial space. Hydrogels have high water content and a soft and rubbery consistency. Such systems have been especially focused in the biomedical area as they provide adequate semiwet three-dimensional environment for cells and tissue interaction and they can be combined with bio- logical or therapeutic molecules. They can be also chemi- cally controlled and designed to tailor their mechanical and functional properties [1-3]. Therefore hydrogels have been proposed for a series of biomedical and biological applications, including tissue engineering [3-4], drug release systems [5-9], biological sensors [12-14], tem- perature and light-responsive films [15] or tuneable hy- drogel photonic crystals as optical sensors [16]. The most common hydrogels are the ones obtained by chemical crosslinking of hydrophilic macromolecules. Such link- ages prevent the dissolution of the material but water can penetrate within the structure, causing the swelling of the structure without disrupting the mechanical and geomet- rical integrity of the structure. If the macromolecules composing the network react with some external variable, e.g. temperature, switching between a stretched to a squeezed states then the corresponding hydrogel could reversible swell and deswell in response to this stimulus. Such smart hydrogels have been proposed for a series of biomedical applications [17-18], not only in the delivery of therapeutic agents [5-9], but also in tissue engineering [10-11], intelligent microfluidic switching [19-21], sen- sors/diagnostic devices [22-23] and actuators [24-25]. For these purposes predominantly the recent new sur- face techniques are utilised. These smart designs are mostly based on stimuli-responsive materials forming self-assembled monolayers and polymer films. Methods such as spin coating, chemical vapour deposition, laser *This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic under the Research Project MSM0021627501.  Water—A Key Substance to Comprehension of Stimuli-Responsive Hydrated Reticular Systems 18 ablation, plasma deposition and chemical or electro- chemical reactions have been widely applied to the fab- rication of thin polymer films [26]. Further utilisation of the effect of external temperature stimuli was already demonstrated in several applications of nanometre-thick poly(N-isopropylacrylamide) (PNIPAAm)-grafted sur- faces for separation processes [27-38] including gel per- meation chromatography [33], size exclusion chroma- tography [36] and aqueous chromatography [27-30,37] inclusive high performance liquid chromatography (HPLC) [31,33-36]. In most of these applications, the packing material is modified with PNIPAAm to change the property of the stationary of the column in response to alteration of temperature. The most peculiar property of these systems, however, is probably their stimuli-responsive behaviour. The thermo- responsive behaviour is typical but only for hydrogels because fibre-networks are composed of high consis- tency hydrogel fibres distributed in macro-space of water environment. Thermo-responsive hydrogels undergo a phase transition in response to temperature changes. Up to now, almost all of the thermo-responsive hydrogels have been featured with negatively thermo- responsive volume phase transition, i.e. with the existence of a lower-critical solution temperature, LCST. Below LCST, the un-crosslinked polymer chains are soluble in water whereas above LCST the polymer chains form submicro- and micro-aggregates, which separate from solution. Thermo-responsive hydrogels composed of cross-linked polymer chains undergo fast [38-39], reversible structural changes from a swollen to a collapsed state by expulsing water. However, also another kind of thermo-responsive hydrogels exist which is opposite to that LCST-hydrogels, i.e. the hydrogels with an upper-critical solution tem- perature, UCST. These hydrogels shrink at lower tem- perature and swell at higher temperature. Obviously, due to short history of this family and the fact that these materials are not commercially available, a great deal of fundamental knowledge regarding their properties is still lacking. Polymer interactions are very complex and no complete molecular-level understanding exists to date. Mostly, the absence of water molecule interaction is typical for theoretical interpretation of this specifically behavior. 2. Classification of TRHRS According to behavior of thermo-responsive hydrated reticular systems (TRHRS) during dilution we can divide them onto water dilute-able and non dilute-able, the crosslinked 3D networks (see Figure 1) or crosslinked 2D networks – films. Additionally, it is possible to divide the dilute-able TRHRS onto fully dilute-able polymer solutions at T< LCST (or T > UCST) and coacervated [51-53] submicro- or micro-TRHRS or flocculated macro-TRHRS. The dilute-able TRHRS coacervate or flocculate in water environment due to weak bonds among of polymer chains, micro-particles and hydrogel particles or fibers and micro-fibers, respectively. It is typical of the submi- cro-, micro- and macro-networks that are disrupting dur- ing dilution process, i.e. the quasi-hydrogels are coacer- vating and the fiber networks are flocculating, respec- tively. As a temperature changes, the sol-gel reversible hydrogels transition occurs due to non-chemical cross- links being formed among grafted and branched elements of copolymers. The crosslinked structures created by strong particu- larly chemical bonds among polymer chains like micro- and macro-sponges have been then swelled or shrunk in response to the temperature change over the LCST. 2.1. LCST Hydrogels PNIPAAm, has been the most used macromolecule in thermo-responsive hydrogels. The changing in properties with temperature in PNIPAAm is based on a phenome- non that is thermodynamically similar to that causing temperature-induced protein folding [17]. Above the LCTS a reversible structural transition occurs from ex- panded coil (soluble chains) to compact globule (insolu- ble state), at around 32°C in pure water [3,13-14,40-42]. Below the LCST, the hydrogel is swollen and absorbs a significant amount of water, while above LCST, the hy- drogel dramatically releases free water and begins to shrink. Mostly opinion is prevailing that the solubility is affected because the amphiphilic PNIPAAm chains hide the hydrophilic amide groups and expose the hydropho- bic isopropyl groups in the compact globule structure. The most common LCST hydrogels are the ones ob- tained by chemical crosslinking of hydrophilic macro- molecules. Such linkages prevent the dissolution of the material but water can penetrate within the micro-re- ticular structure, causing the swelling of the structure without disrupting the mechanical and geometrical integ- rity of the structure. During a volume transition the hydrogel anti-bonding system formed between water molecules and the poly- meric chains is disturbed, being this thermodynamically favourable increasing in entropy the main driving force for the occurrence of the transition. For the case of crosslinking systems this transition can be seen through an abrupt shrinkage of the hydrogel above the LCST as- sociated with the change in the swelling capability of the hydrogel. In addition, DSC analysis indicates that this process is accompanied especially for cross-linked hy- drogel with heat consumption, i.e. an endothermic proc- ess [1-2,39]. The LCST, the characteristic temperature Copyright © 2010 SciRes. JBNB 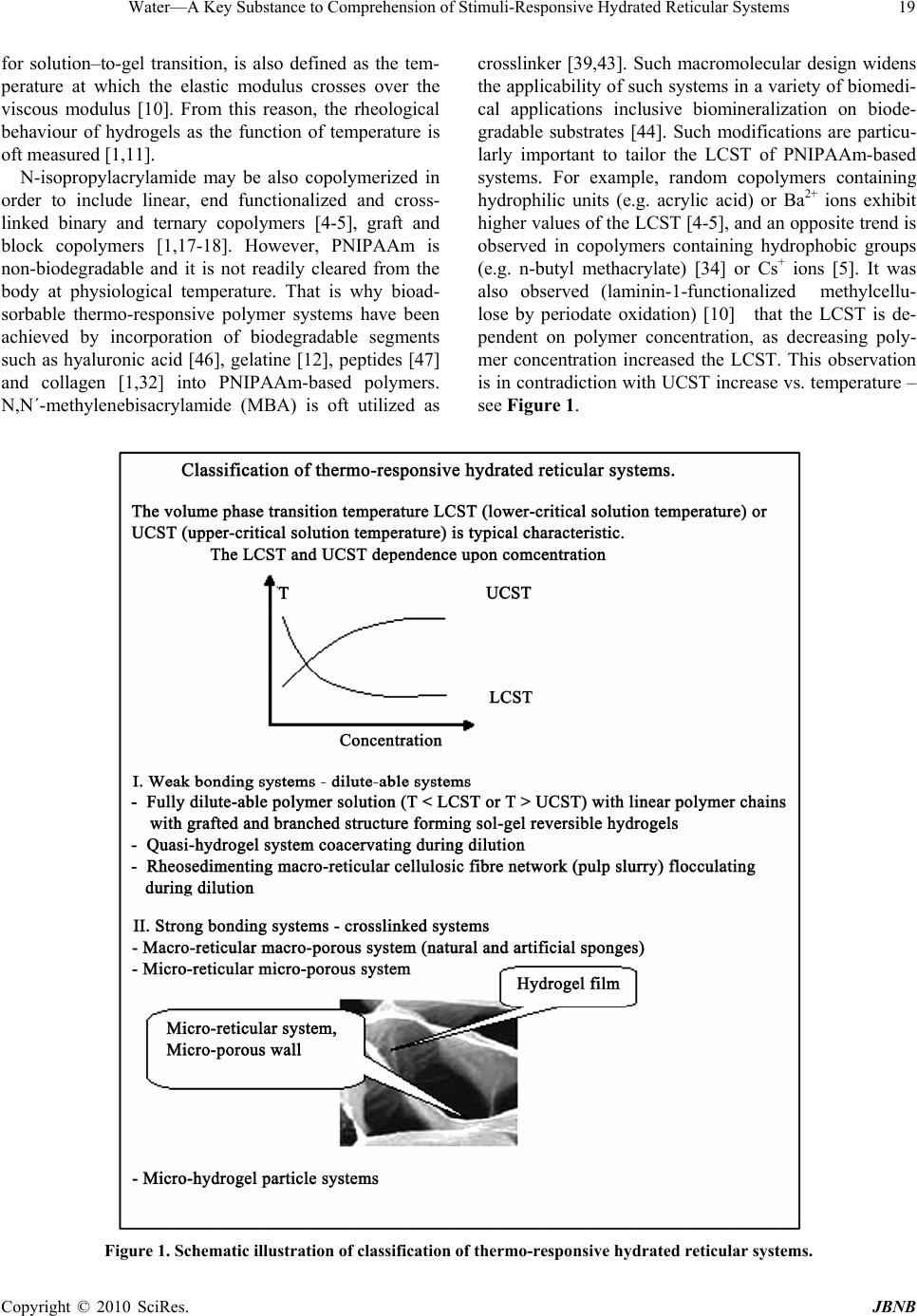 Water—A Key Substance to Comprehension of Stimuli-Responsive Hydrated Reticular Systems Copyright © 2010 SciRes. JBNB 19 for solution–to-gel transition, is also defined as the tem- perature at which the elastic modulus crosses over the viscous modulus [10]. From this reason, the rheological behaviour of hydrogels as the function of temperature is oft measured [1,11]. N-isopropylacrylamide may be also copolymerized in order to include linear, end functionalized and cross- linked binary and ternary copolymers [4-5], graft and block copolymers [1,17-18]. However, PNIPAAm is non-biodegradable and it is not readily cleared from the body at physiological temperature. That is why bioad- sorbable thermo-responsive polymer systems have been achieved by incorporation of biodegradable segments such as hyaluronic acid [46], gelatine [12], peptides [47] and collagen [1,32] into PNIPAAm-based polymers. N,N´-methylenebisacrylamide (MBA) is oft utilized as crosslinker [39,43]. Such macromolecular design widens the applicability of such systems in a variety of biomedi- cal applications inclusive biomineralization on biode- gradable substrates [44]. Such modifications are particu- larly important to tailor the LCST of PNIPAAm-based systems. For example, random copolymers containing hydrophilic units (e.g. acrylic acid) or Ba2+ ions exhibit higher values of the LCST [4-5], and an opposite trend is observed in copolymers containing hydrophobic groups (e.g. n-butyl methacrylate) [34] or Cs+ ions [5]. It was also observed (laminin-1-functionalized methylcellu- lose by periodate oxidation) [10] that the LCST is de- pendent on polymer concentration, as decreasing poly- mer concentration increased the LCST. This observation is in contradiction with UCST increase vs. temperature – see Figure 1. Figure 1. Schematic illustration of classification of thermo-responsive hydrated reticular systems.  Water—A Key Substance to Comprehension of Stimuli-Responsive Hydrated Reticular Systems 20 Instead of PNIPAAm-based polymers also other polymer bases exist, e.g. poly(N,N-diethylacrylamide) (pDEAAm) [45], poly(N-cyclopropylacrylamide) [15], a “dual” nanocomposite based on poly(vinyl acetate) (PVAc) and cellulose whiskers [12], poly-D-lysine- functionalised chitosan [11], the hydrogel photoresist, which was formulated by mixing poly(HEMA-co-MMA) synthesized by radical copolymerization of 2-hy- droxyethyl methacrylate (HEMA) and methyl methacry- late (MMA) with a crosslinker, tetramethoxymethyl gly- coluril [16]. Most promising family of protein polymers is elastine-like polypeptides (ELP) [1,32]. ELPs have shown an outstanding biocompatibility. In addition, the ELPs have an acute “smart” nature. Below the transition temperature, the uncrosslinked polymer chains are soluble in water but above LCST, the polymer starts a complex self-assembling process that leads to an aggregation of polymer chains, initially forming nano- and micro-particles, which segregates from the solution [32]. The copolymers with branched structure,(e.g.poly-(NIPAAm-co-AAc-co-HEMAPTMC, i.e. prepared by copolymerization of NIPAAm, acrylic acid (AAc) and biodegradable monomer hydroxyethyl methacrylate-poly(trimethylenecarbonate)(HEMAPTMC)) are accompanied above LCST with sol-gel transition occurred immediately when the clear solution is im- mersed into the water bath. After incubation, a highly flexible gum-like material is then formed and de-swelling is further observed during continued warming in water bath [4]. For the case of crosslinking three-dimensional systems this transition can be seen through an abrupt shrinkage of the hydrogel above the LCST associated with the change in the swelling capability of the hydro-reticular material. The volume phase transition process upon heating con- nected with water molecules expulsion from hydro-re- ticular spaces - known as hydrogel syneresis [50] - is reversible with thermal and stimuli responsivity [13,39, 48-49] but with different response dynamic and volume changes. It was proved that the reversible transition dur- ing the heat cycle is due to the elasticity of crosslinked hydrogels [16]. Since the rapid response dynamic and large volume changes due to temperature variation is the essential function for intelligent hydrogels applications, the thermo-responsive hydrogels with improved response rate and large volume changes to an external temperature stimulus are preferred [31,39]. The improved response dynamic of the hydrogels are obtained through incorpo- rating siloxane linkage [31], cold polymerization and the pore-forming agent, etc. [39]. Though a lot of information exists describing the be- haviour of TRHRS some of the mechanisms involved in the volume transition critical solution temperature are still not well understood. The smart surface designs mostly based on stimuli-responsive materials forming self-assembled monolayers (SAMs) and surface-tethered polymers, known as polymer brushes, suggested that the polymer chains of PNIPAAm and its copolymers have two structures in aqueous solution [26]. Below its LCST, PNIPAAm polymer is in an extended, solvent-swelled structure, but when heated up above LCST, the polymer undergoes a phase transition to yield a collapsed mor- phology that excludes water [17,44]. For example, these widespread structural changes enable the multiresponsive surfaces reversible change on silicon substrate to be real- ised between superhydrophilicity and superhydrophobic- ity [13]. It is important that the silicon surface roughness becomes the main factor in intensifying this behaviour. In contrast to magnitude of the contact angel changes on flat film, a remarkably large change in this one was in- duced on rough substrate. Logically, for a rough surface with a high surface free energy, the film is more hydro- philic or more hydrophobic. Nevertheless, PNIPAAm surfaces cannot be described only in terms of surface wettability, because above the LCST the surfaces are only partially dehydrated [33]. Usually, this behaviour is based on confusing explana- tion that a hydrogen bond network between the amide groups and water molecules are formed at lower tem- perature, whereas at higher temperatures the stabilizing H-bonds break up and the hydrophobic interactions be- come predominant [26]. Thus the hydrophobic interac- tions among the hydrophobic groups become stronger which subsequently induce the freeing of the entrapped water molecules from the hydrogel network [13,26,39]. However, indication confirming an important role of water molecules in behaviour of TRHRS was observed. Temperature- and light-responsive polyacrylamide co- polymers featuring salicylideneanilin as a photochromic group is reported which structure by irradiation and turning off the UV light is changed, but the respective LCSTs values remained higher than before irradiation. The LCST shift after irradiation can be explained by an intramolecular stabilization of the exited keto form in high polar media such as water because after evaporation of the samples solutions and redissolving in water, the values for the LCST were the same as before irradiation [15]. A similar mechanism of structural transition from ex- panded coil to squeezed proper thermodynamically ad- vantageous structure is possible to expect at crosslinked TRHRS as the temperature is raised above the LCST, because macroporous hydrogels, i.e. hydrated macro- reticular systems, are consisted from walls which are formed by micro- and submicro-porous sections of the hydrogel character as well as. These facts ensue from the Copyright © 2010 SciRes. JBNB 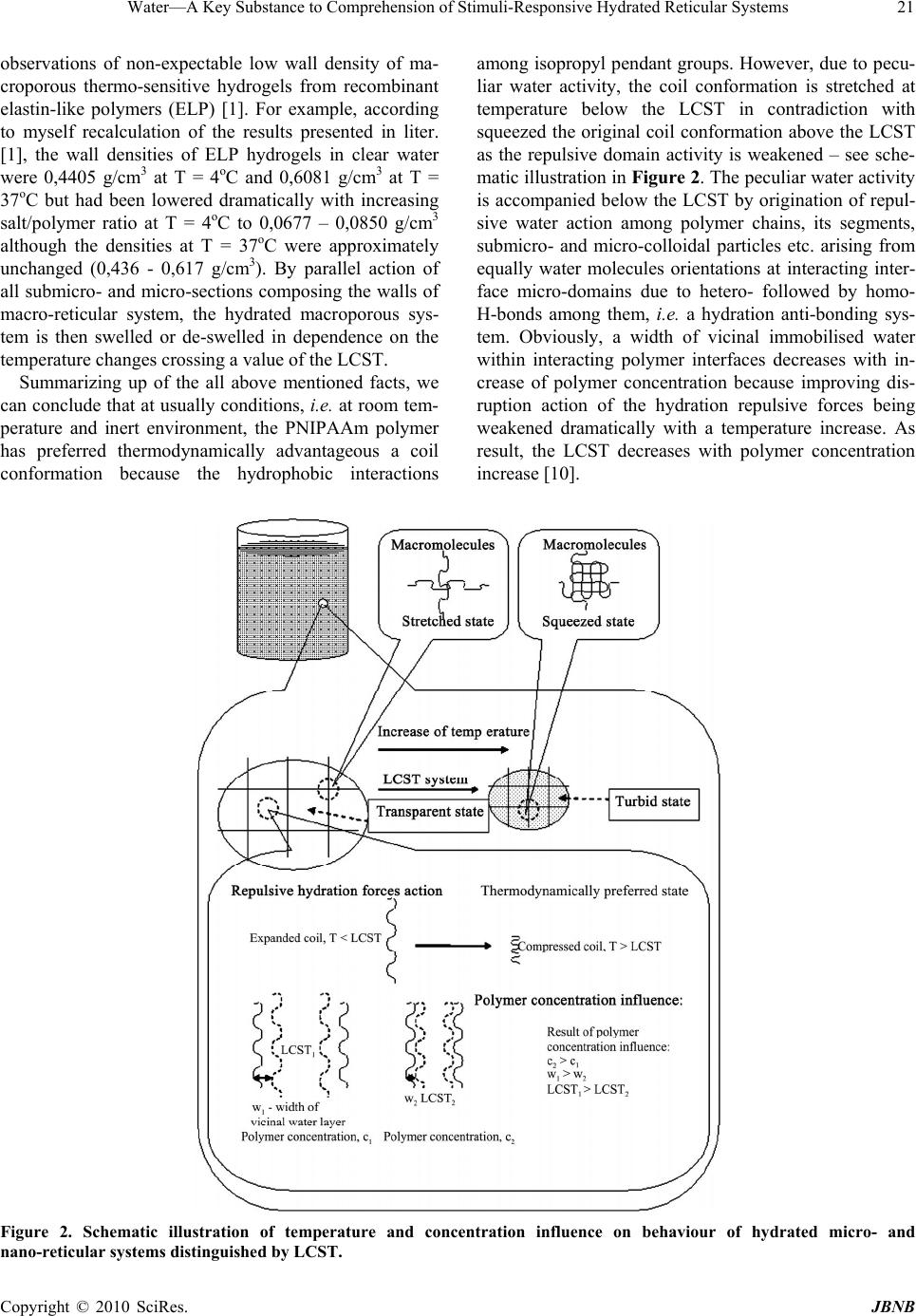 Water—A Key Substance to Comprehension of Stimuli-Responsive Hydrated Reticular Systems21 observations of non-expectable low wall density of ma- croporous thermo-sensitive hydrogels from recombinant elastin-like polymers (ELP) [1]. For example, according to myself recalculation of the results presented in liter. [1], the wall densities of ELP hydrogels in clear water were 0,4405 g/cm3 at T = 4oC and 0,6081 g/cm3 at T = 37oC but had been lowered dramatically with increasing salt/polymer ratio at T = 4oC to 0,0677 – 0,0850 g/cm3 although the densities at T = 37oC were approximately unchanged (0,436 - 0,617 g/cm3). By parallel action of all submicro- and micro-sections composing the walls of macro-reticular system, the hydrated macroporous sys- tem is then swelled or de-swelled in dependence on the temperature changes crossing a value of the LCST. Summarizing up of the all above mentioned facts, we can conclude that at usually conditions, i.e. at room tem- perature and inert environment, the PNIPAAm polymer has preferred thermodynamically advantageous a coil conformation because the hydrophobic interactions among isopropyl pendant groups. However, due to pecu- liar water activity, the coil conformation is stretched at temperature below the LCST in contradiction with squeezed the original coil conformation above the LCST as the repulsive domain activity is weakened – see sche- matic illustration in Figure 2. The peculiar water activity is accompanied below the LCST by origination of repul- sive water action among polymer chains, its segments, submicro- and micro-colloidal particles etc. arising from equally water molecules orientations at interacting inter- face micro-domains due to hetero- followed by homo- H-bonds among them, i.e. a hydration anti-bonding sys- tem. Obviously, a width of vicinal immobilised water within interacting polymer interfaces decreases with in- crease of polymer concentration because improving dis- ruption action of the hydration repulsive forces being weakened dramatically with a temperature increase. As result, the LCST decreases with polymer concentration increase [10]. Figure 2. Schematic illustration of temperature and concentration influence on behaviour of hydrated micro- and nano-reticular systems distinguished by LCST. Copyright © 2010 SciRes. JBNB 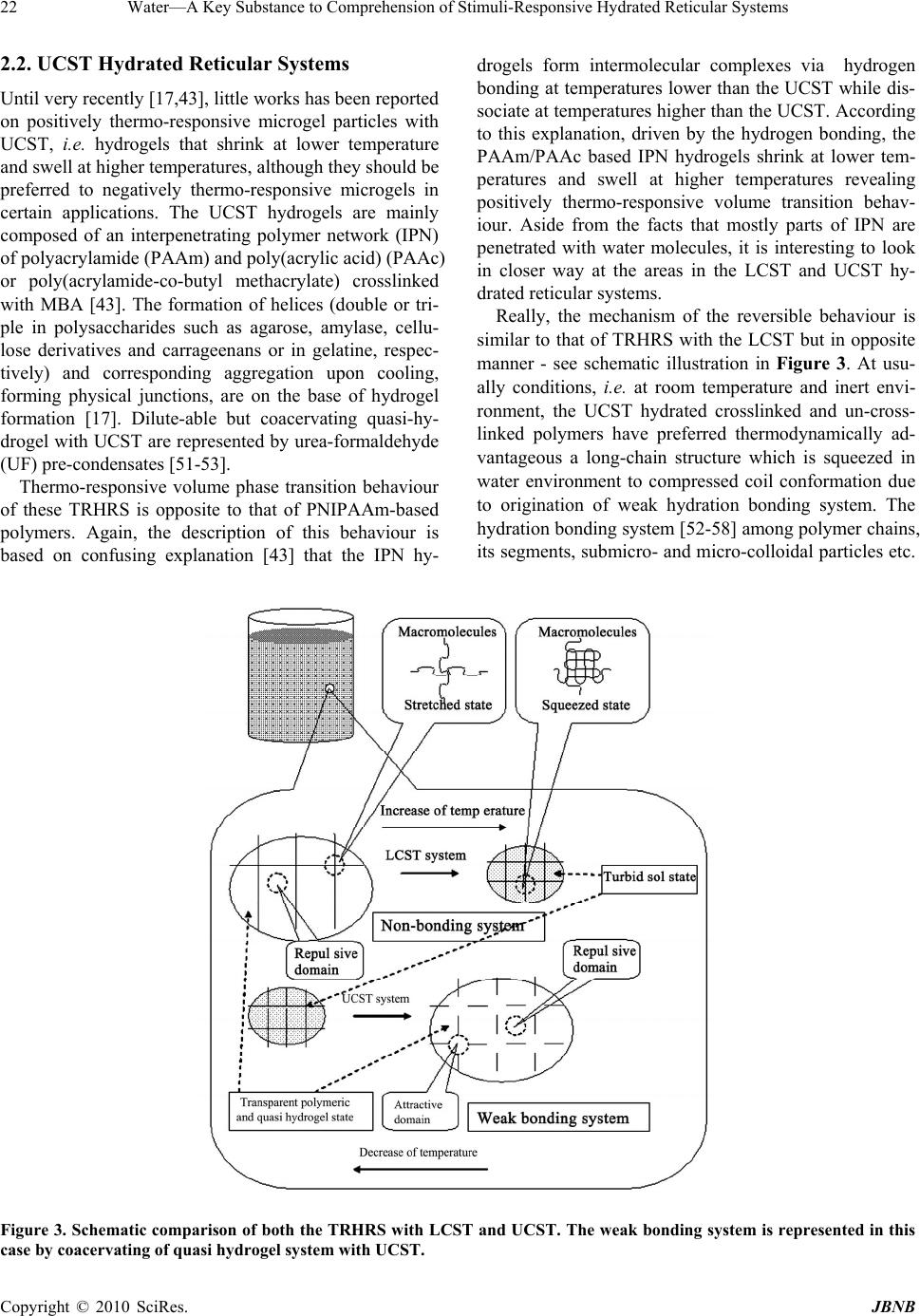 Water—A Key Substance to Comprehension of Stimuli-Responsive Hydrated Reticular Systems 22 2.2. UCST Hydrated Reticular Systems Until very recently [17,43], little works has been reported on positively thermo-responsive microgel particles with UCST, i.e. hydrogels that shrink at lower temperature and swell at higher temperatures, although they should be preferred to negatively thermo-responsive microgels in certain applications. The UCST hydrogels are mainly composed of an interpenetrating polymer network (IPN) of polyacrylamide (PAAm) and poly(acrylic acid) (PAAc) or poly(acrylamide-co-butyl methacrylate) crosslinked with MBA [43]. The formation of helices (double or tri- ple in polysaccharides such as agarose, amylase, cellu- lose derivatives and carrageenans or in gelatine, respec- tively) and corresponding aggregation upon cooling, forming physical junctions, are on the base of hydrogel formation [17]. Dilute-able but coacervating quasi-hy- drogel with UCST are represented by urea-formaldehyde (UF) pre-condensates [51-53]. Thermo-responsive volume phase transition behaviour of these TRHRS is opposite to that of PNIPAAm-based polymers. Again, the description of this behaviour is based on confusing explanation [43] that the IPN hy- drogels form intermolecular complexes via hydrogen bonding at temperatures lower than the UCST while dis- sociate at temperatures higher than the UCST. According to this explanation, driven by the hydrogen bonding, the PAAm/PAAc based IPN hydrogels shrink at lower tem- peratures and swell at higher temperatures revealing positively thermo-responsive volume transition behav- iour. Aside from the facts that mostly parts of IPN are penetrated with water molecules, it is interesting to look in closer way at the areas in the LCST and UCST hy- drated reticular systems. Really, the mechanism of the reversible behaviour is similar to that of TRHRS with the LCST but in opposite manner - see schematic illustration in Figure 3. At usu- ally conditions, i.e. at room temperature and inert envi- ronment, the UCST hydrated crosslinked and un-cross- linked polymers have preferred thermodynamically ad- vantageous a long-chain structure which is squeezed in water environment to compressed coil conformation due to origination of weak hydration bonding system. The hydration bonding system [52-58] among polymer chains, its segments, submicro- and micro-colloidal particles etc. Figure 3. Schematic comparison of both the TRHRS with LCST and UCST. The weak bonding system is represented in this case by coacervating of quasi hydrogel system with UCST. Copyright © 2010 SciRes. JBNB 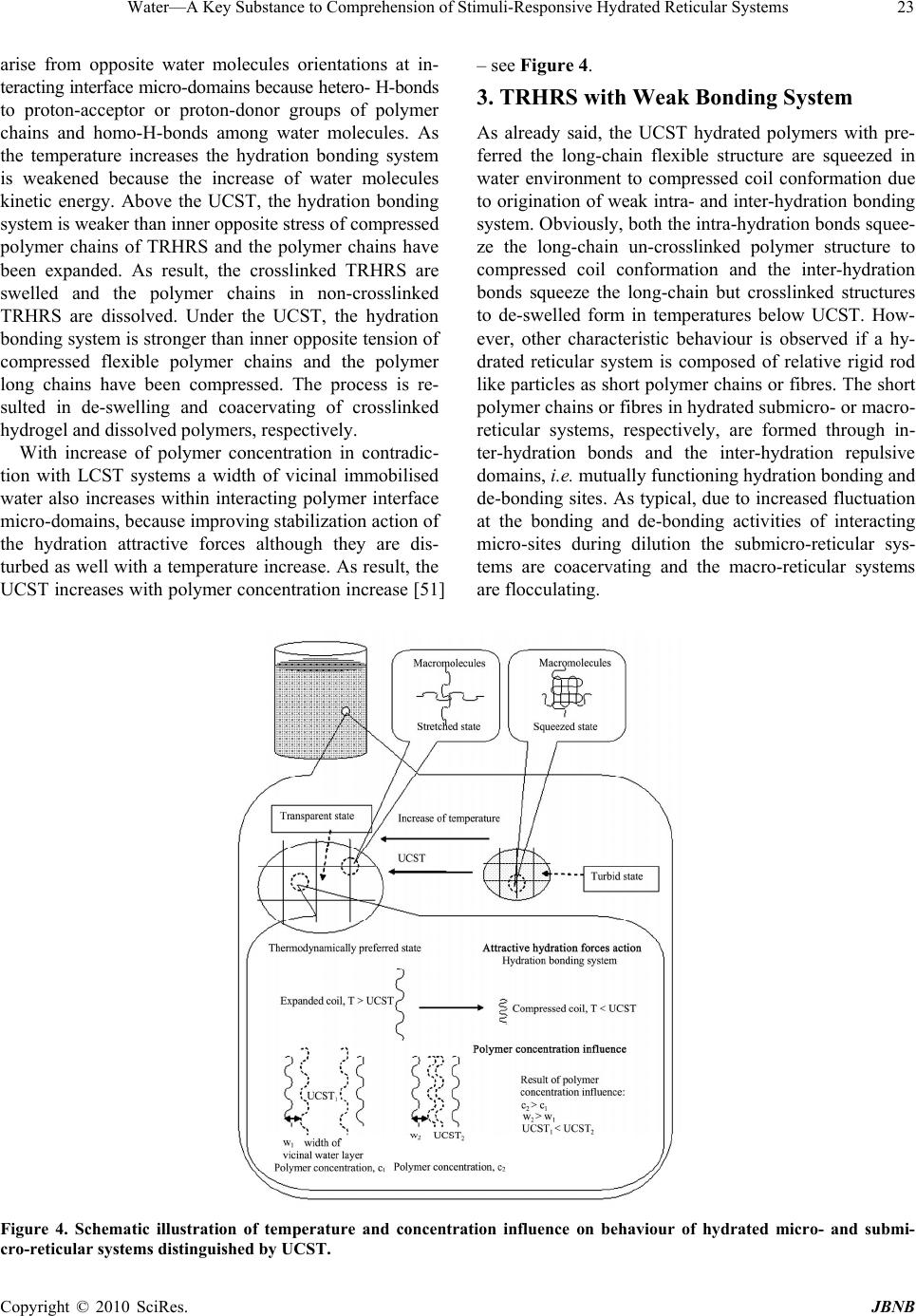 Water—A Key Substance to Comprehension of Stimuli-Responsive Hydrated Reticular Systems23 arise from opposite water molecules orientations at in- teracting interface micro-domains because hetero- H-bonds to proton-acceptor or proton-donor groups of polymer chains and homo-H-bonds among water molecules. As the temperature increases the hydration bonding system is weakened because the increase of water molecules kinetic energy. Above the UCST, the hydration bonding system is weaker than inner opposite stress of compressed polymer chains of TRHRS and the polymer chains have been expanded. As result, the crosslinked TRHRS are swelled and the polymer chains in non-crosslinked TRHRS are dissolved. Under the UCST, the hydration bonding system is stronger than inner opposite tension of compressed flexible polymer chains and the polymer long chains have been compressed. The process is re- sulted in de-swelling and coacervating of crosslinked hydrogel and dissolved polymers, respectively. With increase of polymer concentration in contradic- tion with LCST systems a width of vicinal immobilised water also increases within interacting polymer interface micro-domains, because improving stabilization action of the hydration attractive forces although they are dis- turbed as well with a temperature increase. As result, the UCST increases with polymer concentration increase [51] – see Figure 4. 3. TRHRS with Weak Bonding System As already said, the UCST hydrated polymers with pre- ferred the long-chain flexible structure are squeezed in water environment to compressed coil conformation due to origination of weak intra- and inter-hydration bonding system. Obviously, both the intra-hydration bonds squee- ze the long-chain un-crosslinked polymer structure to compressed coil conformation and the inter-hydration bonds squeeze the long-chain but crosslinked structures to de-swelled form in temperatures below UCST. How- ever, other characteristic behaviour is observed if a hy- drated reticular system is composed of relative rigid rod like particles as short polymer chains or fibres. The short polymer chains or fibres in hydrated submicro- or macro- reticular systems, respectively, are formed through in- ter-hydration bonds and the inter-hydration repulsive domains, i.e. mutually functioning hydration bonding and de-bonding sites. As typical, due to increased fluctuation at the bonding and de-bonding activities of interacting micro-sites during dilution the submicro-reticular sys- tems are coacervating and the macro-reticular systems are flocculating. Figure 4. Schematic illustration of temperature and concentration influence on behaviour of hydrated micro- and submi- cro-reticular systems distinguished by UCST. Copyright © 2010 SciRes. JBNB 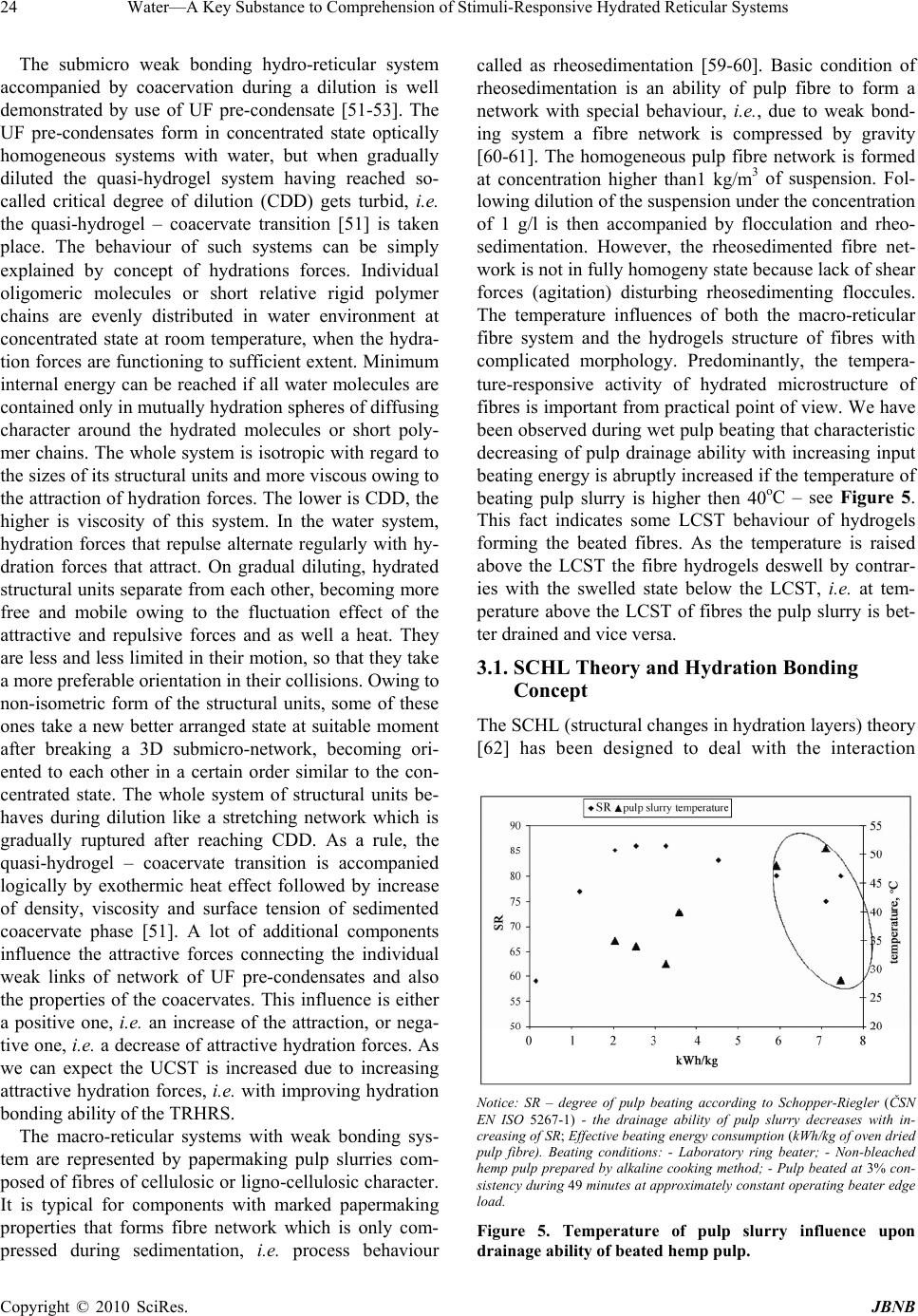 Water—A Key Substance to Comprehension of Stimuli-Responsive Hydrated Reticular Systems 24 The submicro weak bonding hydro-reticular system accompanied by coacervation during a dilution is well demonstrated by use of UF pre-condensate [51-53]. The UF pre-condensates form in concentrated state optically homogeneous systems with water, but when gradually diluted the quasi-hydrogel system having reached so- called critical degree of dilution (CDD) gets turbid, i.e. the quasi-hydrogel – coacervate transition [51] is taken place. The behaviour of such systems can be simply explained by concept of hydrations forces. Individual oligomeric molecules or short relative rigid polymer chains are evenly distributed in water environment at concentrated state at room temperature, when the hydra- tion forces are functioning to sufficient extent. Minimum internal energy can be reached if all water molecules are contained only in mutually hydration spheres of diffusing character around the hydrated molecules or short poly- mer chains. The whole system is isotropic with regard to the sizes of its structural units and more viscous owing to the attraction of hydration forces. The lower is CDD, the higher is viscosity of this system. In the water system, hydration forces that repulse alternate regularly with hy- dration forces that attract. On gradual diluting, hydrated structural units separate from each other, becoming more free and mobile owing to the fluctuation effect of the attractive and repulsive forces and as well a heat. They are less and less limited in their motion, so that they take a more preferable orientation in their collisions. Owing to non-isometric form of the structural units, some of these ones take a new better arranged state at suitable moment after breaking a 3D submicro-network, becoming ori- ented to each other in a certain order similar to the con- centrated state. The whole system of structural units be- haves during dilution like a stretching network which is gradually ruptured after reaching CDD. As a rule, the quasi-hydrogel – coacervate transition is accompanied logically by exothermic heat effect followed by increase of density, viscosity and surface tension of sedimented coacervate phase [51]. A lot of additional components influence the attractive forces connecting the individual weak links of network of UF pre-condensates and also the properties of the coacervates. This influence is either a positive one, i.e. an increase of the attraction, or nega- tive one, i.e. a decrease of attractive hydration forces. As we can expect the UCST is increased due to increasing attractive hydration forces, i.e. with improving hydration bonding ability of the TRHRS. The macro-reticular systems with weak bonding sys- tem are represented by papermaking pulp slurries com- posed of fibres of cellulosic or ligno-cellulosic character. It is typical for components with marked papermaking properties that forms fibre network which is only com- pressed during sedimentation, i.e. process behaviour called as rheosedimentation [59-60]. Basic condition of rheosedimentation is an ability of pulp fibre to form a network with special behaviour, i.e., due to weak bond- ing system a fibre network is compressed by gravity [60-61]. The homogeneous pulp fibre network is formed at concentration higher than1 kg/m3 of suspension. Fol- lowing dilution of the suspension under the concentration of 1 g/l is then accompanied by flocculation and rheo- sedimentation. However, the rheosedimented fibre net- work is not in fully homogeny state because lack of shear forces (agitation) disturbing rheosedimenting floccules. The temperature influences of both the macro-reticular fibre system and the hydrogels structure of fibres with complicated morphology. Predominantly, the tempera- ture-responsive activity of hydrated microstructure of fibres is important from practical point of view. We have been observed during wet pulp beating that characteristic decreasing of pulp drainage ability with increasing input beating energy is abruptly increased if the temperature of beating pulp slurry is higher then 40oC – see Figure 5. This fact indicates some LCST behaviour of hydrogels forming the beated fibres. As the temperature is raised above the LCST the fibre hydrogels deswell by contrar- ies with the swelled state below the LCST, i.e. at tem- perature above the LCST of fibres the pulp slurry is bet- ter drained and vice versa. 3.1. SCHL Theory and Hydration Bonding Concept The SCHL (structural changes in hydration layers) theory [62] has been designed to deal with the interaction Notice: SR – degree of pulp beating according to Schopper-Riegler (ČSN EN ISO 5267-1) - the drainage ability of pulp slurry decreases with in- creasing of SR; Effective beating energy consumption (kWh/kg of oven dried pulp fibre). Beating conditions: - Laboratory ring beater; - Non-bleached hemp pulp prepared by alkaline cooking method; - Pulp beated at 3% con- sistency during 49 minutes at approximately constant operating beater edge load. Figure 5. Temperature of pulp slurry influence upon drainage ability of beated hemp pulp. Copyright © 2010 SciRes. JBNB 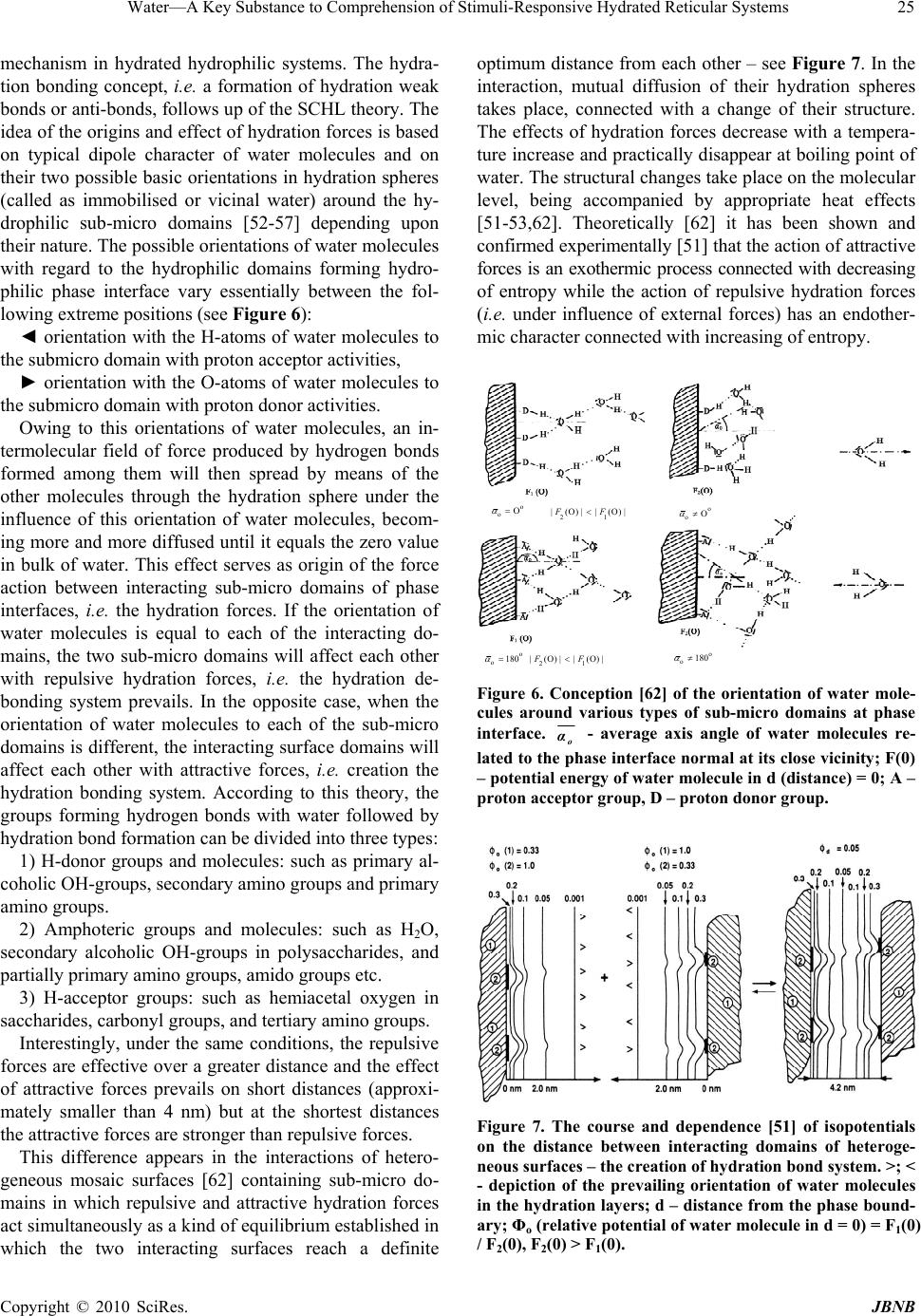 Water—A Key Substance to Comprehension of Stimuli-Responsive Hydrated Reticular Systems25 mechanism in hydrated hydrophilic systems. The hydra- tion bonding concept, i.e. a formation of hydration weak bonds or anti-bonds, follows up of the SCHL theory. The idea of the origins and effect of hydration forces is based on typical dipole character of water molecules and on their two possible basic orientations in hydration spheres (called as immobilised or vicinal water) around the hy- drophilic sub-micro domains [52-57] depending upon their nature. The possible orientations of water molecules with regard to the hydrophilic domains forming hydro- philic phase interface vary essentially between the fol- lowing extreme positions (see Figure 6): ◄ orientation with the H-atoms of water molecules to the submicro domain with proton acceptor activities, ► orientation with the O-atoms of water molecules to the submicro domain with proton donor activities. Owing to this orientations of water molecules, an in- termolecular field of force produced by hydrogen bonds formed among them will then spread by means of the other molecules through the hydration sphere under the influence of this orientation of water molecules, becom- ing more and more diffused until it equals the zero value in bulk of water. This effect serves as origin of the force action between interacting sub-micro domains of phase interfaces, i.e. the hydration forces. If the orientation of water molecules is equal to each of the interacting do- mains, the two sub-micro domains will affect each other with repulsive hydration forces, i.e. the hydration de- bonding system prevails. In the opposite case, when the orientation of water molecules to each of the sub-micro domains is different, the interacting surface domains will affect each other with attractive forces, i.e. creation the hydration bonding system. According to this theory, the groups forming hydrogen bonds with water followed by hydration bond formation can be divided into three types: 1) H-donor groups and molecules: such as primary al- coholic OH-groups, secondary amino groups and primary amino groups. 2) Amphoteric groups and molecules: such as H2O, secondary alcoholic OH-groups in polysaccharides, and partially primary amino groups, amido groups etc. 3) H-acceptor groups: such as hemiacetal oxygen in saccharides, carbonyl groups, and tertiary amino groups. Interestingly, under the same conditions, the repulsive forces are effective over a greater distance and the effect of attractive forces prevails on short distances (approxi- mately smaller than 4 nm) but at the shortest distances the attractive forces are stronger than repulsive forces. This difference appears in the interactions of hetero- geneous mosaic surfaces [62] containing sub-micro do- mains in which repulsive and attractive hydration forces act simultaneously as a kind of equilibrium established in which the two interacting surfaces reach a definite optimum distance from each other – see Figure 7. In the interaction, mutual diffusion of their hydration spheres takes place, connected with a change of their structure. The effects of hydration forces decrease with a tempera- ture increase and practically disappear at boiling point of water. The structural changes take place on the molecular level, being accompanied by appropriate heat effects [51-53,62]. Theoretically [62] it has been shown and confirmed experimentally [51] that the action of attractive forces is an exothermic process connected with decreasing of entropy while the action of repulsive hydration forces (i.e. under influence of external forces) has an endother- mic character connected with increasing of entropy. o oO 21 |(O)||(O)|FF o oO o o21 180|(O) ||(O) |FF o o180 Figure 6. Conception [62] of the orientation of water mole- cules around various types of sub-micro domains at phase interface. o α - average axis angle of water molecules re- lated to the phase interface normal at its close vicinity; F(0) – potential energy of water molecule in d (distance) = 0; A – proton acceptor group, D – proton donor group. Figure 7. The course and dependence [51] of isopotentials on the distance between interacting domains of heteroge- neous surfaces – the creation of hydration bond system. >; < - depiction of the prevailing orientation of water molecules in the hydration layers; d – distance from the phase bound- ary; Φo (relative potential of water molecule in d = 0) = F1(0) / F2(0), F2(0) > F1(0). Copyright © 2010 SciRes. JBNB  Water—A Key Substance to Comprehension of Stimuli-Responsive Hydrated Reticular Systems 26 Moreover, the SCHL theory predicts and experiments support the fact that density of immobilised water is higher than the density of bulk water in dependence on its vicinity to the hydrophilic domain interface. With increasing distance from the domain interface the density of water decreases. The difference [63] between vicinal and bulk water is changed in the range of 2 to 40 %. Be- side of this, an important implication is possible to derive that density of water, comparatively to the bulk of water, located between interacting hydrophilic surfaces is higher in the case of attractive hydration forces and lower in the case of repulsive ones. 3.2. Biomimetic Systems Especially Artificial Muscles Recently, discovery of cellulose as a smart material was described that can be used for biomimetic sensor/actuator devices and micro-electromechanical systems [25]. This smart cellulose is termed electroactive paper (EAPap) because it can produce a large bending displacement with low actuation voltage and low power consumption. The authors in [25] are proposed that electroactive paper is advantageous for many applications such as micro-insect robots, micro-flying objects, micro-electromechanical systems, biosensors, and flexible electrical displays. By use of this phenomenon it is possible also to explain and simulate muscles movement. EAPap is made with a cellulose film (cellophane) on which gold electrodes are deposited on both sides. An EAPap actuator was supported vertically in environment chamber that can be controlled the humidity and tem- perature. By excitation of voltage application to the ac- tuator a bending deformation is evoked. The authors [25] believe that the actuation is due to a combination of two mechanisms: ion migration (diffusion of sodium ions to anode?) and dipolar orientation. Again, in spite of their confusing and irrational explanation of the EAPap movement the received results have high inspiring poten- tial and challenge. The tip displacement of the EAPap actuator is dependent on applied electric field, its fre- quency, EAPap sample thickness and temperature but predominantly on humidity. The humidity affects the displacement, where a high relative humidity leads to a large displacement. It is no problem to explain this behaviour by use of SCHL theory. An orientation of water molecules in immobilized lay- ers around cellulose macromolecules in stratified struc- ture of EAPap actuator is determined by presence of proton donor groups or proton acceptor groups at their interacted surfaces. The overall film structure and its shape are formed among structural cellulosic units due to both the hydrogen-bonding bridging in dry state and the hydration-bonding bridging in wet state. Extent and in- tensity of this bonding system is determined by size, concentration and distribution of nano-domains either with the attractive or the repulsive force action, i.e. among interacting opposite nano-surfaces with reversal or identical basic orientation of water molecules, respec- tively. The basic orientation of water molecule is given by presence of surface proton donor groups or proton acceptor groups of cellulose. Whilst hemiacetal and gly- cosidic oxygen in cellulose is typical proton-acceptor groups the hydroxyl groups can behave as proton-donor and proton-acceptor groups. Nevertheless, one is sup- posed that mostly behaviour of hydroxyl groups in cellu- losic materials has more a proton-donor character. In consequence of this preposition, the domains of pre- vailing hydration-bonding bridging are regularly distrib- uted within cellulosic material with flat formation. By any disturbing this distribution, the paper strip curling is evoked because the inner tension equilibrium is disturbed. As schematically presented in Figure 8, by application of oriented electric field on cellulosic material in wet state the water molecules in bonding nano-domains contained nearest the electrodes are reoriented. However, reorienta- tion at cathode is different of the reorientation at anode – at anode are reoriented only all the water molecules hav- ing been oriented to this pole with hydrogen atoms and at cathode only these ones having been oriented to this pole with oxygen atoms at basic origin state. Moreover, the distribution of attractive forces formed around both the A and D and the D and A nano-centres is not the same – it is supposed a prevailing A - D structure orientation in bonding domains. At this situation, an application of dc electric field is evoked a weaker bond system in layers laying near anode and vice-versa a stronger bond system in layers near cathode. Due to this effect the paper strip gets to bend to anode. Logically, the effect is strongly dependent upon relative humidity, the reorientation of water molecules is independent on diffusion process and it is relatively quickly. Obviously, by similar effect, but in microscale, a mus- cles movement is possible to explain. The main preposi- tion – the non-symmetrical distribution of attractive forces formed around both the A and D and the D and A nano-centres. The enhancement of the protein folding owing to the physical properties and microstructure of the host organic- inorganic nanoporous silica matrix induced by the nature of the functional groups and the siloxane network is probably a further similar effect of the hydration forces system activity [64]. 4. Conclusions During last decade a lot of information was collected Copyright © 2010 SciRes. JBNB  Water—A Key Substance to Comprehension of Stimuli-Responsive Hydrated Reticular Systems Copyright © 2010 SciRes. JBNB 27 describing the behaviour of hydrated reticular systems (e.g. hydrogels, quasi-hydrogels, pulp fibre network) with a temperature response, i.e. the hydrogels with LCST and the hydrogels with UCST. Particularly, the TRHRS with LCST were studied because their biomedical importance. It seems that the behaviour is given by specific thermo- dynamically base conformation state of a main polymer forming the TRHRS at room temperature, i.e.: - squeezed state of polymer coil conformation above LCST being stretched by an influence of inner repulsive intermediary forces under LCST and, - more stretched state of polymer coil conformation above UCST being contrary compressed by an influence of the intermediary attractive forces under UCST. Figure 8. Schematically representation of water molecules reorientation in vicinity of electric input field inside nano-localities of cellulosic materials.  Water—A Key Substance to Comprehension of Stimuli-Responsive Hydrated Reticular Systems 28 It was shown that both sorts of the intermediary forces are the repulsive and attractive intramolecular hydration forces theoretically explained by SCHL theory. However, more challenging attention is evoking by swelling or de-swelling activity of elastic crosslinked polymers forming hydrogels with rapid thermoresponsive dynamics, because their similarity to biomimetic dy- namic of muscle tissues of animals. At present, the aim to better understanding to movement of biological sys- tems prompting the TRHRS to offer the crosslinked hy- drogels serve as artificial objects of experimental studies. Acceptable moveable hydrogel system, e.g. with strati- fied structure composed of useful crosslinked both the LCST and the UCST hydrogels, is possible to create and to evoke its movement by changing temperature around LCST and UCST but only in a connection with mutually water transport. The recently described biomimetic sys- tems not behave as a really artificial muscle but only as the hydrogel with rapid syneresis. Obviously, a muscle movement represented by mutual movement of different muscle tissue like myosin and actins take place on an- other principle than that one connected with water expul- sion and retention. Moreover, the dynamic of real muscle response is incomparable higher because it is not con- nected with water transport. As a matter of fact, actually how is it possible to real- ise this basic bio-property? As theoretically follows of the hydration bonding - de- bonding concept being applied upon micro- and submit- cro-reticular systems, the stratified muscle tissue is squeezed by prevailing of hydrated bond formation and stretched by prevailing of hydrated anti-bonds. The all of changes are evoked only by merely overturning the ori- entation of interacting water molecules in hydration lay- ers around interacting hydrophilic interface micro-do- mains. This overturning is relative very quickly and it is connected with heat evolving - the formation of attrac- tive hydration forces is prevailing - or heat consumption - the formation of repulsive hydration forces is prevailing. A real mechanism evoking this water molecule orienta- tion changing by inner or outer stimuli in muscle tissue is still not known. Probably, the evocation of amphoteric hydroxyl or amino interface groups is responsible for this alternating orientation. REFERENCES [1] L. Martín, M. Alonso, A. Girotti, F. J. Arias and J. C. Rodríguez-Cabello, “Synthesis and Characterization of Macroporous Thermosensitive Hydrogels from Recom- binan Elastin-Like Polymers,” Biomacromolecules, Vol. 10, No. 11, 2009, pp. 3015-3022. [2] A. Girotti, J. C. Reguera, F. J. Rodríguez-Cabello, M. Arias, A. Alonso and J. MaTestera, “Design and Biopro- duction of a Recombinanat Multi(Bio)Functional Elastin-Like Protein Polymer Containing Cell Adhesion Sequences for Tissue Engineering Purposes,” Journal of Matierals Science, Materials in Medicine, Vol. 15, 2004, pp. 479-484. [3] J. E. Wong, A. K. Gaharvar, D. Müller-Schulte, D. Ba- hadur and W. Richtering, “Dual-stimuli Responsive PNiPAM Microgel Achieved Via Layer-by Layer As- sembly: Magnetic and Thermoresponsive,” Journal of Colloid and Interface Science, Vol. 324, 2008, pp. 47-54. [4] K. L. Fujimoto, M. Zuwei, D. M. Nelson, R. Hashizume, J. Guan, K. Tobita and W. R.Wagner, “Synthesis, Char- acterization and Therapeutic Efficacy of a Biodegradable, Thermoresponsive Hydrogel Designed for Application in Chronic Infarcted Myocardum,” Biomaterials, Vol. 30, 2009, pp. 4357-4368. [5] J. Xiao-Jie, C. Liang-Yin, L. Li, M. Peng and M. L. Young, “A Novel Thermoresponsive Hydrogel with Ion-Recognition Property through Supramolecular Host-Guest Complexation,” Journal of Physical Chemis- try B, Vol. 112, 2008, pp. 1112-1118. [6] J. J. Kang Derwent and W. F. Mieler, “Thermoresponsive Hydrogels as a New Ocular Drug Delivery Platform to the Posterior Segment of the Eye,” Transactions of the American Ophthalmological Society, Vol. 106, 2008, pp. 206-214. [7] Y. H. Bae, R. Okano, S. Hsu, W. Kim, “Thermo-Sensitive Polymers as on-off Switches for Drug Release,” Makromolekulare Chemie Rapid Communica- tions, No. 8, 1987, pp. 481-485. [8] D. Ghate and H. F. Edelhause, “Ocular Drug Delivery,” Expert Opinion on Drug Delivery, No. 3, 2006, pp. 275-287. [9] T. Yasukawa, Y. Ogura, H. Kimura, E. Sakurai and Y. Tabata, “Drug Delivery from Ocular Implants,” Expert Opinion on Drug Delivery, No. 3, 2006, pp. 261-273. [10] S. E. Stabenfeldt, A. J. García and M. C. LaPlaca, “Thermoreversible Laminin-Functionalized Hydrogel for Tissue Engineering,” Journal of Biomedical Materials Research Part A, 2006, pp. 718-725. [11] K. E. Crompton, J. D. Goud, R. V. Bellamkonda, T. R. Gengenbachm, D. I. Finkelstein, M. K. Horne and J. S. Forsytie, “Polylysine-Functionalised Thermoresponsive Chitosan Hydrogel for Neural Tissue Engineering,” Bio- materials, Vol. 28, 2007, pp. 441-449. [12] K. Shanmuganathan, J. R. Capadona, S. J. Rowan and Chr. Weder, “Stimuli-Responsive Mechanically Adaptive Polymer Nanocomposites,” Applied Materials & Inter- faces, Vol. 2, No. 1, 2010, pp. 165-174. [13] F. Xia, H. Ge, Y. Hou, T. L. Sun, L. Chen, G. Z. Zhang and L. Jiang, “Multiresponsive Surfaces Change between Superhydrophilicity and Superhydrophobicity,” Advanced Material, Vol.19, 2007, pp. 2520-2524. [14] L. Qiaofang, L. Pengxiao, G. Ying, Zh. Yongjun, “Ther- mally Induced Phase Transition of Glucose-Sensitive Core-Shell Microgels,” Applied Materials & Interfaces, March 2010. Copyright © 2010 SciRes. JBNB  Water—A Key Substance to Comprehension of Stimuli-Responsive Hydrated Reticular Systems29 [15] F. D. Jochum and P. Theato, “Temperature and Light- Responsive Polyacrylamides Prepared by a Double Polymer Analogous Reaction of Activated Ester Poly- mers,” Macromolecules, Vol. 42, 2009, pp. 5941-5945. [16] J. H. Kang, J. H. Moon S. K. Lee, S. G. Park, S. G. Jang, S. Yang and S. M. Yang, “Thermoresponsive Hydrogel Photonic Crystals by Three-Dimensional Holographic Lithography,” Advanced Material, Vol. 20, 2008, pp. 3061-3065. [17] J. F. Mano, “Stimuli-Responsive Polymeric Systems for Biomedical Applications,” Advanced Engineering Mate- rials, Vol. 10, No. 6, 2008, pp. 515-527. [18] M. Yoshida, R. Langer, A. Lendlein and J. Lahan, “From Advanced Biomedical Coatings to Multi-Functionalized Biomaterials,” Journal of Macromolecular Science: Part C: Polymer Reviews, Vol. 46, 2006, pp. 347-3756. [19] G. Santaneel, N. Arup, C. Yang, T. Cai, Somesree GhoshMitra, D. Diercks and H. Zhibing, “Thermorespon- sive Hydrogel Microvalve Based on Magnetic Nano- heaters for Microfluidics,” In: J. Cheng, A. Khadem- hosseini, H.-Q. Mao, M.Stevens and C. Wang, Eds., Re- sponsive Biomaterials for Biomedical Applications, Ma- teries Reseach Society, Vol. 1095E, Warrendale, PA, 2008. [20] D. J. Beebe, J. S. Moore, J. M. Bauer, Q. Yu, R. H. Liu, C. Devadoss and B.-H. Jo, “Functional Hydrogel Structures for Autonomous Flow Control inside Microfluidic Chan- nels,” Nature, Vol. 404, 2000, pp. 588-590. [21] N. Idota, A. Kikuchi, J. Kobayashi, K. Sakai and T. Okano, “Microfluidic Valves Comprising Nanolayered Thermoresponsive Polymer-Grafted Capillaries,” Ad- vanced Material, Vol. 17, 2005, pp. 2723-2727. [22] H. Yang, Y.-H. Han, X.-W. Zhao, K. Nagai and Z.-Z Gu, “Thermal Responsive Microlens Arrays,” Applied Physic Letters, Vol. 89, 2006, pp. 111-121. [23] D. Chandra, J. A. Taylor and S. Yang, “Replica Molding Of High-Aspect-Ratio (Sub) Micron Hydrogel Pillar Ar- rays and Their Stability in Air and Solvents,” Softmatter, Vol. 4, 2008, pp. 979-984. [24] M. E. Harmon, M. Tang and C. W. Frank, “A Microflu- idic Actuator Based on Thermoresponsive Hydrogels,” Polymer, Vol. 44, 2003, pp. 4547-4556. [25] J. Kim, S. Yun and Z. Ounaies, “Discovery of Cellulose as a Smart Material,” Macromolecules, Vol. 39, 2006, pp. 4202-4206. [26] P. M. Mendes, “Stimuli-Responsive Surfaces for Bio-Aplications,” Chemical Society Review, Vol. 37, 2008, pp. 2512-2529. [27] H. Kanazawa, K. Yamamoto, Y. Matsushima, N. Takai, A. Kikuchi and Y. Sakurai, “Temperature-Responsive Chromatography Using Poly (N-Isopropylacrylamide) -Modified Silica,” Analytical Chemistry, Vol. 68, No. 1, 1996, pp. 100-105. [28] H. Kanazawa, Y. Matsushima and T. Okano, “Tempera- ture- Responsive Chromatography,” Advances in Chro- matography, Vol. 41, 2001, pp. 311-336. [29] A. Kikuchi and T. Okano, “Intelligent Thermoresponsive Polymeric Stationary Phases for Aqueous Chromatogra- phy of Biological Compounds,” Progress in Polymer Science, Vol. 27, 2002, pp. 1165-1193. [30] H. Kanazawa, T. Sunamoto, Y. Matsushima, A. Kikuchi and T. Okano, “Temperature-Responsive Chromatographic Separation of Amino Acid Phenylthiohydantions Using Aqueous Media as the Mobile Phase,” Analytical Chemis- try, Vol. 72, 2000, pp. 5961-5966. [31] H. Kanazawa, K. Yamamoto, Y. Y. Kashiwase, Y. Ma- tsushima, N. Takai, A. Kikuchi, Y. Sakurai and T. Okano, “Analysis of Peptides and Proteins by Tempera- ture-Responsive Chromatographic System Using N-Isopropylacrylaide Polymer-Modified Columns,” Journal of Pharmaceutical Biomedical Analysis, Vol. 15, 1997, pp. 1545-1550. [32] M. Gewehr, K. Nakamura, N. Ise and H. Kitano, “Gel permeation Chromatography Using Porous Glass Beads Modified with Temperature-Responsive Polymers,” Makromolekulare Chemie, Vol. 193, 1992, pp. 249-256. [33] K. Hosoya, E. Sawada, K. Kimata, T. Araki, N. Tanaka and J. M. J. Frechet, “In Situ Surface Selective Modifica- tion of Uniform Size Macroporous Polymer Particles with Temperature-Responsive Poly-N-Isopropylacrylamide,” Macromolecules, Vol. 27, 1994, pp. 3973-3976. [34] H. Kanazawa, Y. Kashiwase, K. Yamamoto, Y. Matsu- shima, A. Kikuchi, Y. Sakurai and T. Okano, “Tempera- ture-Responsive Liquid Chromatography. 2. Effects of Hydrophobic Groups in N-Isopropylacrylamide Copoly- mer-Modified Silica,” Analytical Chemistry, Vol. 69, 1997, pp. 823-830. [35] H. Lakhiari, T. Okano, N. Nurdin, C. Luthi, P. Descouts, D. Muller and J. Jozefonvicz, “Temperature-Responsive Size-Exclusion Chromatography Using Poly (N-Isopropy- lacrylamide) Grafted Silica,” Biochimica et Biophysica Acta, Vol. 1379, 1998, pp. 303-313. [36] H. Kanazawa, T. Sunamoto, E. Ayano, Y. Matsushima, A. Kikuchi and T. Okano, “Temperature-Responsive Chro- matography Using Poly (N-Isopropylacrylamide) Hy- drogel- Modified Silica,” Analytical Sciences, Vol. 18, 2002, pp. 45-48. [37] E. Ayano, Y. Okada, C. Sakamoto, H. Kanazawa, T. Okano, M. Ando and T. Nishimura, “Analysis of Herbi- cides in Water Usingtemperature-Responsive Chroma- tography and an Aqueous Mobile Phase,” Journal of Chromatography A, Vol. 1069, 2005, pp. 281-285. [38] M. Lutecki, B. Strachotova, M. Uchman, J. Brus, J. Ples- til, M. Slouf, A. Strachota and L. Matejka, “Thermosensi- tive PNIPA-Based Organic-Inorganic Hydrogels,” Poly- mer Journal, Vol. 38, No. 6, 2006, pp. 527-541. [39] X.-Z. Zhang, F.-J. Wang, C. C. Chu, “Thermoresponsive Hydrogel with Rapid Response Dynamics,” Journal of Materials Sciences, Materials in Medicine, Vol. 14, 2003, pp. 451-455. [40] H. Hou, W. Kim, M. Grunlan and A. Han, “A Thermore- sponsive Hydrogel Poly (N-Isopropylacrylamide) Mi- cropatterning Method Using Microfliudic Techniques,” Copyright © 2010 SciRes. JBNB  Water—A Key Substance to Comprehension of Stimuli-Responsive Hydrated Reticular Systems Copyright © 2010 SciRes. JBNB 30 Journal of Micromechanics Microengineering, Vol. 19, 2009, pp. 1-6. [41] R. M. P. da Silva, J. F. Mano and R. L. Reis, “Smart Thermoresponsive Coatings And Surfaces for Tissue En- gineering: Switching Cell-Material Boundaries,” Trends in Biotechnology, Vol. 25, No. 12, 2006, pp. 577-583. [42] H. Hatakeyma, A. Kichuchi, M. Yamato and T. Okano, “Bio-Functionalized Thermoresponsive Interfaces Facili- tating Cell Adhesion and Proliferation,” Biomaterials, Vol. 27, 2006, pp. 5069-5078. [43] X. Xin-Cai, C. Liang-Yin, C. Sen-Mei and Z. Jia-Hua, “Monodispersed Thermoresponsive Hydrogel Micro- spheres with a Volume Phase Transition Driven by Hy- drogen Bonding,” Polymer, Vol. 46, 2005, pp. 3199- 3209. [44] J. Shi, N. M. Alves and J. F. Mano, “Thermally Respon- sive Biomineralization on Biodegradable Substrates,” Advanced Functional Materials, Vol. 17, 2007, pp. 3312-3318. [45] Z. Ding, R. B. Fong, C. J. Long, P. S. Stayton and A. S. Hoffman, “Size-Dependent Control of the Binding of Biotinylated Proteins to Streptavidin Using a Polymer Shield,” Nature, Vol. 411, 2001, pp. 59-62. [46] S. Ohya, Y. Nakayama and T. Matsuda, “Thermorespon- sive Artificial Extracellular Matrix for Tissue Engineering: Hyaluronic Acid Bioconjugated with Poly- (N-Isopropy- lacrylamide) Grafts,” Biomacromolecules, Vol. 2, 2001, pp. 856-863. [47] S. Ohya and T. Matsuda, “Poly (N-Isopropylacrylamide) (PNIPAM)-Grafted Gelatin as Thermoresponsive Three-Dimensional Artificial Extracellular Matrix: Mo- lecular and Formulation Parameters vs. Cell Proliferation Potential,” In: Polym. Ed., Journal of Biomaterials Sci- ence, Vol. 16, 2005, pp. 809-827. [48] J. A. Jaber and J. B. Schlenoff, “Polyelectrolyte Multi- layers with Reversible Thermal Responsivity,” Macro- molecules, Vol. 38, 2005, pp. 1300-1326. [49] S. A. Sukhishvili, “Responsive Polymer Films and Cap- sules via Layer-by-Layer Assembly,” Current Opinion in Colloid & Interface Science, Vol. 10, 2005, pp. 37-44. [50] K. Edelmann, “Lehrbuch der Kolloidchemie,” Band I. VEB Deutscher Verlag der Wissenschaften, Berlin, 1962, pp. 353-358. [51] M. Milichovský, “Behaviour of Hydrophilic Components in Papermaking Suspension. Part II. Experimental Hy- drated Hydrophilic Modeling System-Its Properties and Behaviour,” Scientific Papers, University of Pardubice, Vol. 56, 1992, pp. 155-182. [52] M. Milichovský, “A New Concept of Chemistry Refining Processes,” TAPPI J., Vol. 73, No. 10, 1990, pp. 221-232. [53] M. Milichovský, “The Role of Hydration in Papermaking Suspension,” Cellulose Chemistry Technology, Vol. 26, No. 5, 1992, pp. 607-618. [54] M. Milichovský, “O Mechanizme Vzaimodejstvij V Bumagoobrazujustschich Gidrofilnych Sistemach,” Chimija Drevesiny, No. 1, 1990, pp. 69-78. [55] M. Milichovský, “Chemische Aspekte der Mahlung von Zellstoff,” Zellstoff und Papier, Vol. 38, No. 1, 1989, pp. 17-23. [56] M. Milichovský, “Nowe Poglady Na Wlasciwosci Papierniczy Zawiesin Wodnych,” Przeglad Papierniczy, Vol. 46, No. 12, 1990, pp. 418-422. [57] M. Milichovský, “Klíčová role vody při výrobě a užití papíru a papírenských výrobků (Water as Key Substance in Production and Utilisation of Paper Products),” Papír a celulóza, Vol. 55, No.11, 2000, pp. 302-308. [58] M. Milichovský, “Voda – klíčový fenomén při výrobě a užití papíru a papírenských výrobků (Water – the Key Phenomenon in Production and Utilisation of Paper Products),” Chemické listy, Vol. 94, No. 9, 2000, pp. 875-878. [59] M. Milichovský, “Způsob dějů a jejich hodnocení probíhajících v papírenských suspenzích (Evaluation of phenomena taking place in paper suspension),” Papír a celulóza, Vol. 33, No. 7-8, 1978, pp.V61-V64. [60] M. Milichovský and Bř. Češek, “Rheosedimentation – Typical and Characteristic Phenomenon of Paper Matter,” Cellulose Chemistry Technology, Vol. 38, No. 5-6, 2004, pp. 385-397. [61] M. Fišerová, J. Gigac and J. Balberčák, “Sedimentation Properties of Hardwood Kraft Pulp Suspensions,” Papír a celulóza, Vol. 64, No. 11-12, 2009, pp. 362-364. [62] M. Milichovský, “Behaviour of Hydrophilic Components in Papermaking Suspension. Part I. Interactions among Hydrated Particles – Theory of Structural Changes in Hydrated Layers,” Scientific Papers, University of Par- dubice, Vol. 56, 1992, pp. 123-154. [63] M. Milichovský, “Teorie chování hydrofilních dis- perzních soustav III (Theory of behaviour of hydrophilich dispersion systems III. Experimental evidence of SCHL theory),” Scientific Papers, University of Pardubice, Vol. 51, 1988, pp. 149-168. [64] B. Menaa, F. Menaa, C. Aiolfi-Guimaraes and O. Sharts, “Silica-Based Nanoporous Sol-Gel Glasses: From Bioenca-Psulation to Protein Folding Studies,” Inter- national Journal of Nanotechnology, Vol. 7, No. 1, 2010, pp. 1-45. |

