Journal of Biomedical Science and Engineering
Vol.09 No.03(2016), Article ID:64972,10 pages
10.4236/jbise.2016.93012
13C Protein Oxidation in Breath: Is It Relevant for the Whole Body Protein Status?
Gerlof A. R. Reckman1*, Martijn Koehorst1, Marion Priebe1, Henk Schierbeek2, Roel J. Vonk1
1University Medical Center Groningen, Groningen, The Netherlands
2Academic Medical Center, Amsterdam, The Netherlands

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 10 February 2016; accepted 21 March 2016; published 24 March 2016
ABSTRACT
Introduction: The kinetics of protein oxidation, monitored in breath, and its contribution to the whole body protein status is not well established. Objectives: To analyze protein oxidation in various metabolic conditions we developed/validated a 13C-protein oxidation breath test using low enriched milk proteins. Method/Design: 30 g of naturally labeled 13C-milk proteins were consumed by young healthy volunteers. Breath samples were taken every 10 min and 13CO2 was measured by Isotope Ratio Mass Spectrometry. To calculate the amount of oxidized substrate we used: substrate dose, molecular weight and 13C enrichment of the substrate, number of carbon atoms in a substrate molecule, and estimated CO2-production of the subject based on body surface area. Results: We demonstrated that in 255 min 20% ± 3% (mean ± SD) of the milk protein was oxidized compared to 18% ± 1% of 30 g glucose. Postprandial kinetics of oxidation of whey (rapidly digestible protein) and casein (slowly digestible protein) derived from our breath test were comparable to literature data regarding the kinetics of appearance of amino acids in blood. Oxidation of milk proteins was faster than that of milk lipids (peak oxidation 120 and 290 minutes, respectively). After a 3-day protein restricted diet (~10 g of protein/day) a decrease of 31% ± 18% in milk protein oxidation was observed compared to a normal diet. Conclusions: Protein oxidation, which can be easily monitored in breath, is a significant factor in protein metabolism. With our technique we are able to characterize changes in overall protein oxidation under various meta- bolic conditions such as a protein restricted diet, which could be relevant for defining optimal protein intake under various conditions. Measuring protein oxidation in new-born might be rele- vant to establish its contribution to the protein status and its age-dependent development.
Keywords:
Protein Oxidation, Protein Status, Milk Proteins, Stable Isotopes, Breath Test

1. Introduction
The effects of low and high protein diets are extensively discussed [1] - [4] . The metabolic advantages of high protein diets are limited. Recommendations about the upper limit of protein intake are lacking [5] - [7] . Recent reports described negative metabolic effects of high protein intake [8] - [11] . It is therefore relevant to extend our basic knowledge about protein/amino acid metabolism. The contribution of protein oxidation to the whole-body protein status is not well defined. Moreover, the rate limiting step of amino acid oxidation, its effecting factors and its interrelation with the whole-body nitrogen metabolism is not clear. To monitor amino acid oxidation we developed an easy to apply protein oxidation breath test and validated it. Our analysis indicated that the oxidation rate of amino acids is a relevant factor in protein metabolism.
2. Materials and Methods
2.1. Participants
Data on technical variation were obtained with 1 healthy male, age 27, height 1.81 meters, weight 70 kilogram. Data on variation between individuals were obtained from 8 young healthy students: 3 male, 5 female. Average age 22, average height 1.81 meters ± 0.12, average weight 72 ± 12. The subjects recruited were biomedical science students. Breath test experiments were approved by the local Ethics Committee.
2.2. Acquisition of 13C Substrate
By restricting the diet of cows to naturally enriched maize [12] exclusively, 13C enriched milk was obtained with a delta value of −14 compared to ~−21/22 of a baseline grass based diet [13] . Five different food-grade fractions from this milk were separated: milk protein, whey, casein, lactose and milkfat. Milkfat was collected as a separate soluble fraction, the other fractions were all stored as a powder. Before consumption of any of the powdered substrates they were dissolved in 500 ml of water.
2.3. Equipment, Isotope Ratio Mass Spectrometers (IRMS) and Gas Chromatograph (GC)
Breath samples were introduced in splitless mode into the GC injector and a Porabond Q capillary column (length 25 m, ID 0.32 mm, Df 8 mm, Agilent, Amsterdam, The Netherlands) was used for the chromatographic separation. The GC used was an Agilent 6890 series from Agilent Technologies (Amsterdam, The Netherlands). The GC oven was kept isothermal at 80˚C. The injection temperature was set at 110˚C and the injection volume was 20 µl. Measurement of CO2 was performed on a Delta XL (Thermo Fisher, Bremen, Germany). The IRMS was operated at an accelerating voltage of 5 kV. The ion source was held at a pressure of 3.0 × 10−6 Torr, and ions generated by electron impact at 70 eV. Three faraday cup detectors monitored simultaneously and continuously the 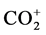 signals for the three major ions at m/z 44 (12CO2), m/z 45 (13CO2 and 12C17O16O) and m/z 46 (12C18O16O). The dynamic range of the instrument is between 0.2 and 50 V. The CO2 working reference gas quality 5.3 (Linde, Schiedam, Netherlands) was calibrated with known reference gases (Messer Griesheim, Krefeld, Germany) against δ 13CVPDB (Vienna Pee Dee Belemnite).
signals for the three major ions at m/z 44 (12CO2), m/z 45 (13CO2 and 12C17O16O) and m/z 46 (12C18O16O). The dynamic range of the instrument is between 0.2 and 50 V. The CO2 working reference gas quality 5.3 (Linde, Schiedam, Netherlands) was calibrated with known reference gases (Messer Griesheim, Krefeld, Germany) against δ 13CVPDB (Vienna Pee Dee Belemnite).
2.4. Calculations
2.4.1. Body Surface Area (BSA) and Substrate Properties
The acquired delta values of all the breath samples were used to calculate the amount oxidized taking into account the subjects BSA calculated using the formula, Equation (1), from Haycock [14] , enrichment of substrate, amount of substrate, molecular weight of substrate (g/mol) and amount of carbon atoms in a single molecule of substrate. We assumed CO2 production to be 300 mmol/h.
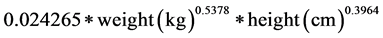 (1)
(1)
2.4.2. 13C Breath Calculations from the IRMS Results
Calculations were performed as described in Lefebvre [15] , Evenepoel [16] and Chleboun [17] . Because our protein is overall naturally labeled with 13C, we could not use the weight and numbers of C atoms of leucine as our substrate for the calculations. The next paragraph will explain how substrate weight and number of C atoms present in our substrate were calculated.
2.4.3. Calculations of Milk Substrates
Normally, in isotope studies one well defined labeled substrate is used. For our calculations we also need one value for weight of e.g. casein and one number for the amount of carbon atoms for casein. However, casein is a collection of proteins and each of these proteins has a different amino acid composition and therefore a different mass and a different number of C atoms. By taking into account the seven most abundant casein proteins we accounted for over 90% of all casein proteins. Of these seven proteins the amino acid content was searched for in an online database at www.ncbi.nlm.gov/protein. For all seven proteins a weighted average amino acid and weighted average number of C atoms was calculated. Then all seven proteins were calculated into 1 weighted average amino acid weight and number of C atoms. In the case of casein we ended up with non-integer numbers 131.37 g/mol and 5.13 C atoms per molecule of casein. We used the same procedure for the constituents of whey, complete milk protein (whey plus casein) and milkfat.
2.5. Human Studies
2.5.1. Day(s) before Breath Test
In order to keep the 13C level in each test subject as low as possible before the test, consumption of naturally enriched 13C products should be eliminated at least one day before the test. In contrast to subjects from Western European countries subjects from North America need to refrain from 13C products for a longer period of time [18] . Exercise can influence the fate of the test substrate. Therefore, exercise should be standardized at least one day before the test and during the test day. To measure only the oxidation of the test substrate, subjects should arrive in a fasting state at the day of testing. Therefore, consumption of food and drinks is not allowed from 22:00 the day before the test. Drinking water or coffee without sugar and/or milk were allowed. Instead of the skipped breakfast the subjects consume the test drink.
2.5.2. Day of Breath Test
Basal breath samples were taken a few minutes before the consumption of the test drink. The test drink is consumed at 09:15 within 5 minutes and breath samples are taken from 09:25 every 10 minutes until 14:45 (Figure 1).
2.5.3. Sampling
Breath samples were collected as described in Dubuc [19] and Tanis [20] except our exetainer® tubes (Labco Limited) are 12 ml instead of 20 ml. The breath samples are ideally measured as soon as possible; however the samples have a shelf life of at least 4 weeks [21] - [23] .
2.6. Statistics
All values are shown as mean ± standard deviation (SD). Two-tailed student’s t-tests (significance level set at 0.05) have been applied.
2.7. Technical Limitations
The largest technical uncertainty in the breath test is the assumption that a subject under resting conditions (sitting in a chair) produces 300 mmol/hour of CO2 the body surface area [17] .
Uncertainty in the 13C baseline and the inaccuracy of the isotope ratio mass spectrometer are deemed to be much smaller than that in CO2 production. We collect the baseline sample three times and average the results. The isotope ratio mass spectrometer is calibrated twice before each run and twice after every tenth sample.
3. Results
3.1. Oxidation of Protein (Whey) versus Carbohydrate (Glucose)
Glucose is considered as one of the fastest substrates in terms of digestion and uptake. When comparing 30 g of glucose with 30 g of whey there is a trend towards higher peak oxidation for whey compared to glucose (Figure 1 and Table 1). Both substrates are oxidized equally fast during the first hour.
3.2. Oxidation Profiles of Whey and Casein (Breath Data versus Blood Data)
Milk protein is comprised of whey and casein. Boirie [24] measured the appearance of 13C-whey and 13C-casein in blood via labeled 13C-leucine (Figure 2(a)). Their results served as our template for what to expect when measuring the appearance of 13C in 13CO2, derived from overall labeled amino acids, in exhaled breath. Boirie [24] found that whey appears quickly compared to casein and has a clear peak in appearance rate. Casein however forms a plateau after less than 1 hour and after 5 hours starts to diminish more slowly compared to the rate of decrease seen in whey.
The oxidation kinetics measured in exhaled breath of whey and casein are displayed in Figure 2(b). Each substrate was given as a 30 g dose diluted in 500 ml of bottled water. Based on literature Figure 2(a), whey was expected to yield a curve with a fast increase in appearance rate ending in a peak after which the appearance rate should diminish sharply. In comparison, casein was expected to yield a curve with slow increase in appearance rate followed by a plateau which slowly decreases. These expected characteristics are seen in the breath oxidation results for both substrates respectively.
3.3. Different Doses of Milk Proteins
The effect of different dosages of milk protein in shown in Table 2. A very low dose of milk protein (10 g) results in a large standard deviation. On the other end a large dose of milk protein (70 g) also reveals an increased standard deviation compared to the 30 and 50 g dose. For 50 g the standard deviation is only 0.49%. The time point of maximal oxidation rate seems to be related by the dose of milk protein; a low dose reaches maximal oxidation rate faster than a higher dose. Interestingly, more g of milk protein are oxidized when given 50 g of milk protein compared to 70 g of milk protein.
3.4. Oxidation of Different Substrates (Milk Protein, Whey, Casein, Milkfat and Lactose)
The following graph (Figure 3) shows the oxidation kinetics of different substrates: whey, casein, milk protein, milkfat and lactose. Strikingly, whey is oxidized faster than lactose. A clear difference in kinetics is observed between whey and casein; whey peaks fast and then decreases to baseline. Casein ascends slowly, forms a plateau and slowly decreases towards baseline. Milk protein which consists of 80% casein and 20% whey behaves more similar as casein, although milk protein has a slightly higher maximum than casein. Milkfat is most slowly oxidized, a flattened maximum is reached between 4 and 5 h.
Figure 1. Oxidation rate of 30 g whey (blue; n = 4) and 30 g glucose (orange; n = 8). X-axis shows time in minutes, y-axis shows oxidation speed in %/hour.


Figure 2. (a) Slow and fast dietary proteins differently modulate postprandial protein accretion Boirie et al. [24] ; (b) 13CO2 oxidation kinetics of 2 substrates: whey (red; n = 4) and casein (green; n = 4). X-axis shows time in minutes, y-axis shows oxidation speed in %/hour. Two-tailed student’s t-test (significance level set at 0.05)
Table 1. Oxidation characteristics of 30 g of glucose (n = 8) and 30 g of whey (n = 4).
3.5. Cumulative Oxidation Characteristics of Different Milk Substrates
The cumulative oxidation values of different substrates varies between 22.40 for milkfat and 32.74 for lactose (Table 3). Although lactose is the only substrate given at a higher dose (50 g) compared to all other substrates (30 g), lactose is still the fastest substrate regarding reaching maximum oxidation. Surprisingly, milk protein which consists of 80% casein and 20% whey reaches peak oxidation just as fast as whey protein. Comparing maximum oxidation levels of milk protein versus casein is difficult, because casein reaches a plateau instead of a clearly defined peak. Maximum oxidation of casein happens to occur at 190 min, but all values from time
Figure 3. Different substrates result in different oxidation kinetics. Milk protein (30 g; n = 6), whey (30 g; n = 4), casein (30 g; n = 4), milkfat (30 g; n = 4) and lactose (50 g; n = 4). X-axis shows time in minutes, y-axis shows oxidation rate in %/hour.
Table 2. Oxidation characteristics of whey protein given at different dosages. 10, 50 and 70 g (n = 4), 30 g was (n = 6). Note the less than 1% deviation at dose 50 g. Cumulative oxidation at endpoint is significantly different between 30 g versus 70 g and 50 g versus 70 g. Two-tailed student’s t-test (significance level set at 0.05).
Table 3. Oxidation characteristics of different milk substrates.
point 130 min until 240 min are close to the value at time point 190 minutes. The largest deviation at cumulative endpoint oxidation is found with “fast” whey protein.
3.6. Influence of Protein Restriction
A 3-day low protein diet (~10 g of protein/day) compared to control (>100 g of protein per day) leads to a reduction in oxidation of the 30 g milk protein (Table 4).
4. Discussion
Establishing optimal protein intake and the associated protein status is highly relevant in various metabolic situations like clinical nutrition, ageing, sport performance and baby food composition. Defining optimal intake of
Table 4. Habitual diet versus protein restricted diet. Two-tailed student’s t-test found no difference (significance level set at 0.05).
proteins in each situation is difficult to perform. The various methods to estimate the nitrogen balance have specific shortcomings. Fecal loss of nitrogen has been debated recently [8] [25] . Moreover, often the molecular targets to evaluate the adverse effects are not clear. Therefore, upper limits of dietary protein intake are not clearly defined in the various metabolic situations.
Recently, more attention has been paid to the adverse effects of an excess intake of protein: evidence has been described about the hyperactivation of mTORC1 system associated with increased risk associated for age-related disorders such as type 2 diabetes mellitus and cancer [11] . This might also be relevant in the new-born: too high protein intake might lead to increased risk for obesity in later life [8] - [10] and a possible relation to development of colon cancer [26] . These observations lead to the re-evaluation of pathways involved in protein (amino acids) turnover. Amino acid (re)-incorporation into whole body proteins, renal protein excretion and urea metabolism are well characterized. Less attention has been paid to the contribution of the amino acid oxidation to the whole- body protein status. Therefore, we developed a non-invasive and easy to apply milk protein breath test.
Applying this test in healthy young volunteers we observed that the amount of milk protein oxidized within 330 min is considerable: 24.2% of the dose of 30 g is oxidized. This indicates that protein oxidation is a relevant metabolic step for the amino acid metabolism. Therefore, monitoring protein oxidation and analyzing the factors influencing this in varying metabolic conditions might be relevant.
In this paper we describe how we used a naturally low overall enriched 13C-protein breath test, which implies that all amino acids are labeled with 13C. With this protein breath test we assess directly the amount of exogenous protein derived amino acids which are oxidized. This protein breath test is easy to apply and economically affordable due to the relative low price of the substrate.
As can be concluded from Figure 1, the technical variation in the outcome of the analyses is low and reproducibility in a single person is high, which indicates that the test is reliable and the protein oxidation rate measured with this breath test is a biological entity which can be practically determined.
We observed (Table 2) that the application of different doses of proteins lead to different cumulative recoveries in breath over 330 minutes. The expectation was higher relative oxidation at higher doses of protein. Applying 10 g of milk protein yields a high standard deviation compared to higher doses, which is understandable when assuming that absolute biological variation is a similar factor in all doses. The dose of 30 to 50 g of protein lead to a higher relative oxidation. From 50 to 70 g of protein there is no additional relative oxidation. The reason could be limited capacity of oxidation or limitation in downstream processes.
The optimal dose to use in a breath test seems to be in the range of 30 to 50 g of protein. 30 g comes most close to a physiological dose of protein, it has an acceptable standard deviation of around 1% over 330 minutes and is easy to dissolve in 500 ml of water. We concluded that this was the most suitable dose in the test.
What could be the effect of the 11 h fast before the breath test? A rough estimation of protein loss can be based upon the nitrogen data from Raguso [27] . They state that the obligatory nitrogen loss is 54 mg N/kg/day for an adult male. Applying the Jones’ Factor of 6.25 on the 54 mg N/kg/day for a 70 kg adult male results in 23.6 g of protein loss per day. An 11 h fast would then result in 10.8 g of protein loss. In theory, giving a dose of 10 g of protein during the breath test would lead to 0% oxidation. This does not take into account small losses in digestion and uptake, nor does it take into account that a night fasting also lowers energy stores. Lower glycogen stores can shift amino acid incorporation towards oxidation to fulfill energy needs.
The application of a 13C-protein derived breath test has been described before [1] [17] [28] - [30] . In all these studies the 13C-label used was only present as 13C-leucine. This implies that measuring “protein oxidation” means measuring the oxidation of 13C-leucine. It is very likely that the oxidation of this essential amino acid with specific metabolic functions does not represent the oxidation characteristics of all (non-essential) amino acids. In some of these studies 13C-leucine was used as part of a diet for hens to obtain 13C-leucine eggs [17] [28] [31] [32] . In our case we used naturally enriched 13C-maize. Maize is a cheap and a widespread crop, which we utilized to feed cows whereby the cow’s milk becomes naturally low and overall 13C-enriched. This procedure makes the substrate and, by consequence, the test economically affordable.
Measuring protein oxidation reflects the ultimate metabolic fate of amino acids. Naturally enriched 13C-milk proteins and the 13C-whey and 13C-casein fractions were used in our experiments. Applying the test with the13C-whey and 13C-casein fractions we observed that the difference between fast protein (whey proteins) and slow proteins (casein), as described by Boirie [24] also can be observed when analyzing the kinetics of oxidation of both proteins. We consider this as a relevant validation of our breath test. Moreover, this suggests that under conditions of normal physiology and administration of 30 g of substrate, the rate limiting step for overall oxidation of milk proteins is the small intestinal absorption. The fast oxidation of amino acids from whey proteins might not be surprising as it is known that for amino acids no specific storage capacity in the body is available, only in the form of body proteins. During the postprandial phase when exogenous amino acids enter the cellular free amino acid pool in relative high amounts, the size of the amino acid pool remains relatively constant. When the amino acid influx exceeds the protein synthesis capacity, the surplus in amino acids will be removed by oxidation [32] . However, our experiments with varying dose suggest a limitation in the oxidative capacity.
Whey proteins turn out to be a very effective substrate in energy metabolism: the oxidation kinetics of whey and glucose are quite comparable. This is an interesting observation in the light of the use of whey proteins as a nutritional supplement to support muscle function. Compared to other milk components whey proteins are also much faster oxidized than milk sugar (lactose) and milkfat. Difference in kinetics between these substrates are most probably due to stomach emptying and subsequent digestion, based on our comparison between whey and casein oxidation measured in breath compared to appearance of whey and casein derived 13C-leucine in blood [24] which suggest that the small intestinal fate (gastric emptying, hydrolysis and absorption reflects the overall-rate of oxidation under certain conditions).
To analyze if the protein oxidation rate is relevant for the whole body protein metabolism, we applied the condition of protein restricted food intake. We applied an extreme intervention of restricted protein intake to 10 g protein/kg bodyweight/day for 3 days and simultaneously we kept up the energy intake at normal values. The expectation was that protein restriction would lead to a shortage of cellular amino acids which then would be reflected by reduced oxidation. This could be monitored in our breath test. In this condition a reduction of protein oxidation was indeed observed; compared to a normal diet, protein restriction caused a ~20% reduction in oxidation. This observation points into the direction that the oxidation rate is related to the whole body protein status.
In general, it is important to determine what the oxidation outcome in terms of oxidation rate and cumulative oxidation actually represents. Measuring oxidation via breath reflects the outcome of several subsequent metabolic steps such as gastric emptying, small intestinal digestion and uptake, transport in the bloodstream and in addition cellular uptake of amino acids and cellular oxidation, which can be considered as an intrinsic limitation of the technique. However, by changing only one variable in the system, we can estimate the effect of that variable on the final outcome (oxidation). The comparison of fast (whey) proteins and slow (casein) proteins in blood and breath indicates that the rate-limiting step under the condition of a dose of 30 g of proteins and normal physiology is the small intestinal absorption. The effects of restriction of dietary protein intake on oxidation of amino acids suggests that under conditions of restricted intake the rate-limiting step shifts to another level. Also the non-linear behavior in oxidation kinetics under a varying dosage regime suggests that the rate-limiting step shifts under this condition.
It is necessary to analyze this in more detail. It is important to establish if the oxidation step of amino acids is a significant missing link in the understanding of sarcopenia and the adverse effects of high protein intake in relation with cancer and development of obesity-susceptibility in early life. Especially concerning this last aspect the age-dependent development of the oxidation capacity might be highly interesting to reveal.
5. Conclusion
In conclusion: amino acid oxidation and subsequent excretion of CO2 in breath are relevant pathways in the catabolism of proteins. The described protein breath test using low-enriched 13C milk proteins is a suitable tool to monitor protein oxidation. It is easily applicable in clinical and epidemiological studies. It is yet not clear what the biological relevance is of variations in protein oxidation capacity and its age-dependent (early life and during ageing) development.
Conflicts of Interest
Roel J. Vonk is CEO of HanzeNutrition BV, the company which donated the naturally enriched substrates.
Cite this paper
Gerlof A. R.Reckman,MartijnKoehorst,MarionPriebe,HenkSchierbeek,Roel J.Vonk, (2016) 13C Protein Oxidation in Breath: Is It Relevant for the Whole Body Protein Status?. Journal of Biomedical Science and Engineering,09,160-169. doi: 10.4236/jbise.2016.93012
References
- 1. Jackson, A.A. (1999) Limits of Adaptation to High Dietary Protein Intakes. European Journal of Clinical Nutrition, 53, S44-S52.
http://dx.doi.org/10.1038/sj.ejcn.1600743 - 2. Santesso, N., Akl, E.A., Bianchi, M., Mente, A., Mustafa, R., Heels-Ansdell, D. and Schünemann, H.J. (2012) Effects of Higher-versus Lower-Protein Diets on Health Outcomes: A Systematic Review and Meta-Analysis. European Journal of Clinical Nutrition, 66, 780-788.
http://dx.doi.org/10.1038/ejcn.2012.37 - 3. Pedersen, A.N., Kondrup, J. and Borsheim, E. (2013) Health Effects of Protein Intake in Healthy Adults: A Systematic Literature Review. Food & Nutrition Research, 57, 21245.
http://dx.doi.org/10.3402/fnr.v57i0.21245 - 4. Cuenca-Sánchez, M., Navas-Carrillo, D. and Orenes-Pinero, E. (2015) Controversies Surrounding High-Protein Intake: Satiating Effect and Kidney and Bone Health. Advances in Nutrition, 6, 260-266.
http://dx.doi.org/10.3945/an.114.007716 - 5. Durnin, J.V.G.A., Garlick, P., Jackson, A.A., Schürch, B., Shetty, P.S. and Waterlow, J.C. (1999) Report of the IDECG Working Group on Lower Limites of Energy and Protein and Upper Limits of Protein Intakes. European Journal of Clinical Nutrition, 53, S174-S176.
http://dx.doi.org/10.1038/sj.ejcn.1600758 - 6. Metges, C.C. and Barth, C.A. (2000) Metabolic Consequences of a High Dietary-Protein Intake in Adulthood: Assessment of the Available Evidence. Journal of Nutrition, 130, 886-889.
- 7. FAO (2013) Dietary Protein Quality Evaluation in Human Nutrition. FAO Food and Nutrition Paper 92.
- 8. Brands, B., Demmelmair, H. and Koletzko, B. (2014) How Growth Due to Infant Nutrition Influences Obesity and Later Disease Risk. Acta Pediatrica, 103, 578-585.
http://dx.doi.org/10.1111/apa.12593 - 9. Weber, M., Grote, V., Closa-Monsaterolo, R., Escribano, J., Langhendries, J., Dain, E., Giovannini, M., Verduci, E., Gruszfeld, D., Socha, P. and Koletzko, B. (2014) Lower Protein Content in Infant Formula Reduces BMI and Obesity Risk at School Age: Follow-Up of a Randomized Trial. American Journal of Clinical Nutrition, 99, 1041-1051.
http://dx.doi.org/10.3945/ajcn.113.064071 - 10. Grunewald, M., Hellmuth, C., Demmelmair, H. and Koletzko, B. (2015) Excessive Weight Gain during Full Breast- Feeding. Annals of Nutrition and Metabolism, 64, 271-275.
http://dx.doi.org/10.1159/000365033 - 11. Melnik, B.C. (2015) Milk—A Nutrient System of Mammalian Evolution Promoting mTORC1-Dependent Translation. International Journal of Molecular Sciences, 16, 17048-17087.
http://dx.doi.org/10.3390/ijms160817048 - 12. Smith, B.N. and Epstein, S. (1971) Two Categories of 13C/12C Ratios for Higher Plants. Plant Physiology, 47, 380-384.
http://dx.doi.org/10.1104/pp.47.3.380 - 13. Camin, F., Perini, M., Colombarie, G., Bontempo, L. and Versini, G. (2008) Influence of Dietary Composition on the Carbon, Nitrogen, Oxygen and Hydrogen Stable Isotope Ratios of Milk. Rapid Communications in Mass Spectrometry, 22, 1690-1696.
http://dx.doi.org/10.1002/rcm.3506 - 14. Haycock, G.B., Schwartz, G.J. and Wisotsky, D.H. (1978) Geometric Method for Measuring Body Surface Area: A Height-Weight Formula Validated in Infants, Children, and Adults. Journal of Pediatrics, 93, 62-66.
http://dx.doi.org/10.1016/S0022-3476(78)80601-5 - 15. Lefebvre, P., Mosora, F., Lacroix, M., Luyckx, A., Lopez-Habib, G. and Duchesne, J. (1975) Naturally Labeled 13C- Glucose Metabolic Studies in Human Diabetes and Obesity. Diabetes, 24, 185-189.
http://dx.doi.org/10.2337/diab.24.2.185 - 16. Evenepoel, P., Geypens, B., Luypaerts, A., Hiele, M., Ghoos, Y. and Rutgeerts, P. (1998) Digestibility of Cooked and Raw Egg Protein in Humans as Assessed by Stable Isotope Techniques. Journal of Nutrition, 128, 1716-1722.
- 17. Chleboun, J. and Kocna, P. (2005) Isotope Selective Nondispersive Infrared Spectrometry Can Compete with Isotope Ratio Mass Spectrometry in Cumulative 13CO2 Breath Tests: Assessment of Accuracy. Klinická Biochemie a Metabolismus, 13, 92-97.
- 18. Wagenmakers, A.J., Rehrer, N.J., Brouns, F., Saris, W.H. and Halliday, D. (1993) Breath 13CO2 Background Enrichment during Exercise: Diet-Related Differences between Europe and America. Journal of Applied Physiology, 74, 2353-2357.
- 19. Dubuc, M.C., Sébastien, H. and Brazier, J.L. (2000) 13C Basal Abundance of Expired CO2-Definition of Pre-Requisites for Kinetic Breath Tests. Isotopes in Environmental and Health Studies, 36, 177-188.
http://dx.doi.org/10.1080/10256010008032941 - 20. Tanis, A.A., van den Berg, J.W., Kroneman, R., Wattimena, J.L., Rietveld, T., Nieland, B.H. and Swart, G.R. (1998) Human Liver Glycogen Metabolism Assessed with a 13C-Enriched Diet and a 13CO2 Breath Test. European Journal of Clinical Investigation, 28, 466-474.
http://dx.doi.org/10.1046/j.1365-2362.1998.00316.x - 21. Hirschl, A.M. and Makristathis, A. (2007) Methods to Detect Helicobacter pylori: From Culture to Molecular Biology. Helicobacter, 12, 6-11.
http://dx.doi.org/10.1111/j.1523-5378.2007.00560.x - 22. McCue, M.D., Sivan, O., McWilliams, S.R. and Pinshow, B. (2009) Tracking the Oxidative Kinetics of Carbohydrates, Amino Acids and Fatty Acids in the House Sparrow Using Exhaled 13CO2. Journal of Experimental Biology, 213, 782- 789.
http://dx.doi.org/10.1242/jeb.039842 - 23. Perets, T.T., Shporn, E., Boltin, D., Dickman, R. and Niv, Y. (2015) Stability of 13C-Urea Breath Test Samples over Time in the Diagnosis of Helicobacter pylori. Journal of Clinical Laboratory Analysis.
- 24. Boirie, Y., Dangin, M., Gachon, P., Vasson, M., Maubois, J. and Beaufrère, B. (1997) Slow and Fast Dietary Proteins Differently Modulate Postprandial Protein Accretion. Physiology, 94, 14930-14935.
http://dx.doi.org/10.1073/pnas.94.26.14930 - 25. Wierdsma, N.J., Peters, J.H.C., van Bokhorst-de van der Schueren, M.A.E., Mulder, C.J.J., Metgod, I. and van Bodegraven, A.A. (2014) Bomb Calorimetry, the Gold Standard for Assessment of Intestinal Absorption Capacity: Normative Values in Healthy Ambulant Adults. Journal of Human Nutrition and Dietetics, 27, 57-64.
http://dx.doi.org/10.1111/jhn.12113 - 26. Kim, E., Coelho, D. and Blachier, F. (2013) Review of the Association between Meat Consumption and Risk of Colorectal Cancer. Nutrition Research, 33, 983-994.
http://dx.doi.org/10.1016/j.nutres.2013.07.018 - 27. Raguso, C.A., Pereira, P. and Young, V.R. (1999) A Tracer Investigation of Obligatory Oxidative Amino Acid Losses in Healthy, Young Adults. American Journal of Clinical Nutrition, 70, 474-483.
- 28. Evenepoel, P., Claus, D., Geypens, B., Maes, M., Hiele, M., Rutgeerts, P. and Ghoos, Y. (1998) Evidence for Impaired Assimilation and Increased Colonic Fermentation of Protein, Related to Gastric Acid Suppression Therapy. Alimentary Pharmacology & Therapeutics, 12, 1011-1019.
http://dx.doi.org/10.1046/j.1365-2036.1998.00377.x - 29. Ghoos, Y. and Beaufrère, B. (1998) 13C Protein Breath Tests. Gut, 43, S23-S24.
http://dx.doi.org/10.1136/gut.43.2008.s23 - 30. Bammens, B., Evenepoel, P., Verbeke, K. and Vanrenterghem, Y. (2004) Impairment of Small Intestinal Protein Assimilation in Patients with End-Stage Renal Disease: Extending the Malnutrition-Inflammation-Atherosclerosis Concept. American Journal of Clinical Nutrition, 80, 1536-1543.
- 31. Bujko, J., Schreurs, V.V., Nolles, J.A., Verreijen, A.M., Koopmanschap, R.E. and Verstegen, M.W. (2007) Application of a [13CO2] Breath Test to Study Short-Term Amino Acid Catabolism during the Postprandial Phase of a Meal. British Journal of Nutrition, 97, 891-897.
http://dx.doi.org/10.1017/S0007114507433049 - 32. Nolles, J.A., Verreijen, A.M., Koopmanschap, R.E., Verstegen, M.W. and Schreurs, V.V. (2008) Postprandial Oxidative Losses of Free and Protein-Bound Amino Acids in the Diet: Interactions and Adaptation. Journal of Animal Physiology and Animal Nutrition, 93, 431-438.
http://dx.doi.org/10.1111/j.1439-0396.2008.00820.x
NOTES
*Corresponding author.





