Journal of Biomedical Science and Engineering
Vol.7 No.1(2014), Article ID:42405,9 pages DOI:10.4236/jbise.2014.71003
Effects of laminin on hard tissue formation by bone marrow cells in vivo and in vitro
![]()
Department of Endodontics, Osaka Dental University, Osaka, Japan
Email: yosikawa@cc.osaka-dent.ac.jp, kakigi@cc.osaka-dent.ac.jp, yabuuchi8@yoshie-gdc.com, hayashi@cc.osaka-dent.ac.jp
Copyright © 2014 Masataka Yoshikawa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Masataka Yoshikawa et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received 5 December 2013; revised 1 January 2014; accepted 13 January 2014
ABSTRACT
The effect of laminin on hard tissue formation using rat bone marrow cells was assessed. Rat bone marrow cells were obtained from femora of 6-week-old male Fischer 344 rats. In this in vivo examination, porous cylindrical hydroxyapatite scaffolds with a hollow center were immersed in 100 (g/ml laminin solution and air-dried. Rat bone marrow cells in 200 (l culture medium at 1 × 106 cells/ml were seeded in the scaffolds. The scaffolds were implanted into the dorsal subcutis of 7-week-old male Fischer 344 rats for 6 weeks. The scaffolds were then removed and examined histologically. For in vitro examinations, 1 × 105 rat bone marrow cells in 2 ml culture medium were then cultured with the addition of dexamethasone and laminin. Rat bone marrow cells were also cultured in laminin-coated culture plates. In vitro examinations showed the effectiveness of laminin for hard tissue formation from the results of biochemical and immunochemical analysis. From the in vivo examination, laminin coating of the scaffolds induced hard tissue in the pores with the cells. It is concluded that laminin is useful for bone formation, as in an in vitro culture study using bone marrow cells, in hydroxyapatite scaffolds in vivo.
KEYWORDS
Laminin; Bone Marrow Cells; Scaffold; Hydroxyapatite; Osteogenesis
1. INTRODUCTION
It has recently been stated that undifferentiated mesenchymal stem cells in bone marrow and periosteum can subsequently exhibit the characteristics of vascular endothelial cells, nerve cells, smooth muscle cells and cardiac muscle cells [1]. In addition, stem cells could differentiate into chondrocytes, fat cells or osteoblasts [2]. Among these cells, multi-potent progenitor cells derived from mesenchymal bone marrow stem cells (BMCs) have been applied for bone regeneration [3-5]. Previously, porous HA scaffolds with laminin coating were prepared and used in an in vivo examination [6]. To culture rat BMCs (rBMCs), laminin solution was added to the culture medium as a supplement. In this study, confirmation of the effect of laminin on the possibility of osteogenesis by BMCs in vivo and in vitro was attempted. Culture plates with laminin-coated wells were used for rBMC culture. Effects of laminin on rBMC differentiation and hard tissue formation in vivo and in vitro were assessed.
For tooth regeneration or restoration in dentistry, stem cells could be used to create a dentin-pulp complex by using a porous scaffold. It is desirable for the regeneration of this complex that the stem cells originate from a tooth. However, it may be difficult to achieve this. It is known that dentition occurs twice in the human life span. The numbers of human deciduous teeth and permanent teeth are 20 and 28 - 32, respectively. The volume of dental pulp cavity in teeth is small. The number of cells in dental pulp may thus also be small. In addition, it may be impossible to obtain dental pulp from sound teeth. For these reasons, it would be difficult to obtain stem cells derived from autologous dental pulp for use in the regeneration of dentin-pulp complex. It is also considered that the effects on the body of the extraction of stem cells from bone marrow for tooth regeneration may be too severe. Therefore, more studies and active discussion to establish the optimal technique are necessary. The major problems for tooth regeneration might be establishment of means to achieve effective proliferation of the cells in order to obtain them in high numbers and the definition of sources of mesenchymal stem cells that can be used without surgical stress on the body.
It was considered that the use of porous hydroxyapatite with hard tissue regenerated by autologous stem cells in the pores might be useful as a practical method for the restoration or regeneration of a tooth root or tooth crown instead of dentin [6]. For in vivo osteogenesis in porous HA scaffold without subculture, bone marrow cell suspension should generally be prepared at 1 × 107 cells/ml or more [7]. In dentistry, it is considered that the source of stem cells should be tooth pulp or oral mucosa [8]. However, the number of stem cells obtained from these tissues may be too small to be seeded in the scaffold for osteogenesis or odontogenesis. To increase the number of stem cells obtained from oral tissues, promotion of the proliferation of the cells is important for odontogenesis or osteogenesis. Moreover, bone formation must also be attempted using a smaller number of BMCs. To promote hard tissue formation using a small number of stem cells, various supplements [9,10] might be useful. It is considered that laminin may be suitable for hard tissue formation. In this study, the potential effect of laminin on the formation of hard tissue was assessed using a small number of rBMCs in vivo and in vitro.
As undifferentiated mesenchymal stem cells and odontoblasts in dental pulp actually induce secondary dentin [11], the cells from dental pulp may be appropriate for the regeneration of dentin or dentin-pulp complex by seeding in porous HA scaffolds. However, dental pulp has important functions to provide tooth nutrition via blood vessels [12] and to form reparative dentin [13]. Thus, extirpation of the pulp from a normal tooth must be avoided to maintain the soundness of the tooth. Moreover, the total number of pulp cells obtained from one permanent tooth would be insufficient for tooth generation. A long period is needed for pulp cell proliferation and differentiation to osteoblasts and/or odontoblasts [14]. Under the present conditions, stem cells of dental origin were not available in this study. Therefore, instead of tooth pulp cells, bone marrow cells of rats (rBMCs) were used in these in vivo and in vitro studies. It was considered that laminin might be a cofactor to promote cell differentiation and bone formation in pores of the scaffold. The potential of laminin to form hard tissue was examined using rBMCs in the pores of the HA scaffold. As there is a hollow with dental pulp surrounded by hard tissue in a tooth, it was considered that an HA scaffold with a hollow center would be suitable for tooth regeneration.
2. MATERIALS AND METHODS
2.1. Isolation of rBMCs and Preparation of the Cell Suspension
The Animal Welfare Committee of Osaka Dental University approved the experimental procedures on the use and care of animals. This study was performed in the Laboratory Animal Facilities in the Institute of Dental Research under the Guidelines for Animal Experimentation of Osaka Dental University.
rBMC plugs were obtained from the bone shaft of femurs of 6-week-old male Fischer 344/N Slc rats (Japan SLC Inc., Shizuoka, Japan) after euthanasia by halothane suffocation. Primary culture of rBMCs was performed as described previously [6]. Briefly, primary culture of rBMCs was performed in a cell culture flask (T75; BD Biosciences, MA, USA) containing 15 ml minimum essential medium (MEM; Nacalai Tesque, Inc., Kyoto, Japan) supplemented with fetal bovine serum (FBS; SAFC Biosciences, KS, USA) and antibiotics (100 U/ml penicillin, 100 mg/ml streptomycin and 0.25 mg/ml amphotericin B; Sigma-Aldrich Co., MO, USA) in an incubator at 5% CO2 and 95% relative humidity at 37˚C for 7 days. The prepared culture medium was changed to remove non-adherent cells and subsequently renewed 2 times during the period. After the primary culture, confluent rBMCs were released from their culture substratum with trypsin-EDTA solution (0.5 mg/ml trypsin, 0.53 M/ml EDTA; Nacalai Tesque, Inc.). The cells were centrifuged at 900 rpm for 5 minutes at room temperature and re-suspended at 1 ´ 106 cells/ml in the culture medium for in vivo and 1 ´ 105 cells/ml in the medium for in vitro examination.
2.2. Preparation of Porous HA Scaffolds for Use in Vivo Examination
Porous columnar HA scaffolds with a hollow center had been designed for our studies (Figure 1(a)). The macro and scanning electron microscopic images of the scaffold were identical to those in our previous study [6]. The scaffolds were manufactured by HOYA CORPORATION (Tokyo, Japan). Total porosity was 55% and the scaffolds were 8 mm diameter and 10 mm height with a hollow center of 2 mm diameter. The surface and inner micro-structure of the HA scaffolds were observed under a scanning electron microscope (SEM; S-4000; Hitachi, Ltd., Tokyo, Japan). The diameter of apertures on the surface of the HA scaffold (Figure 1(b)) was approximately 350 mm. In the internal structure of the scaffold (Figure 1(c)), the interconnected pores were 200 - 250 mm in diameter. All pores connected with neighbouring pores. The scaffolds were divided into four groups with 6 scaffolds each. The HA scaffolds were sterilized with ethylene oxide gas.
Immersion in 100 mg/ml laminin (from mouse sarcoma; Merck-Millipore Corp., ChemiconÒ, MA, USA) solution of 6 HA scaffolds was performed to coat the surface of pores, which were then air-dried. Laminin-
 (a)
(a) (b)
(b) (c)
(c)
Figure 1. (a) Macro photographic image of porous hydroxyapatite (HA) scaffold with a hollow center used in this study. Apertures of pores are seen on the surface of the scaffold. The scaffolds were 8 mm diameter and 10 mm high with 55% total porosity. Diameter of the hollow center was 2 mm. (b) SEM image of surface of porous HA scaffold. Apertures of pores are shown. They are connected with other pores. (c) SEM image of internal structure on split surface of the porous HA scaffold. Each pore is mutually connected with its neighbors through apertures. Interconnected pores of 70 to 310 mm diameter are shown.
treated scaffolds had been immersed in 2 ml suspension with rBMCs of 1 ´ 106 cells/ml in our previous study [6]. However, in this study, 0.3 ml rBMC suspension with 1 ´ 106 cells/ml was poured to each scaffold to seed rBMCs in accurate quantity (Group 1). As a control, the other 6 scaffolds were immersed in the culture medium and then received 0.3 ml suspension (Group 2), the same as those of Group 1. The other two groups of 6 scaffolds each were soaked in laminin solution (Group 3) or the medium (Group 4) and did not receive rBMCs seeding. All scaffolds were kept in 5% CO2 at 99% relative humidity for 3 hours at 37˚C before experimental use. The concentration of laminin solution used in this study was 1/5-fold that in our previous study [6].
2.3. In Vivo Examination of Bone Formation in Subcutaneously Implanted Laminin-Coated Porous HA Scaffolds by rBMCs
This in vivo study was performed as mentioned in our previous study using 7-week-old male Fischer 344 rats [6]. All procedures were performed under general anaesthesia by intra-peritoneal injection of sodium pentobarbital (DS Pharma Biomedical Co., Ltd, Osaka, Japan) at a rate of 4 mg per 100 g body weight. The backs of the rats were shaved and disinfected with povidone iodide solution (ISODINE solution 10%; Meiji Seika Pharma Co., Ltd., Tokyo, Japan). On the back, subfascial pockets were made alongside the vertebrae. All rats received 2 scaffolds. A scaffold with laminin coating and rBMC seeding was inserted into the left subcutaneous pocket and a scaffold without laminin coating but with rBMCs was inserted into the right pocket. A scaffold with or without laminin coating and with no rBMCs was also inserted into each side of the subcutaneous pocket, respectively, of the other rats. The incised wound was sutured, and then adhesive (Aron alpha; Toagosei Co., Ltd., Tokyo, Japan) was applied over the wound.
The scaffolds were removed from the backs of animals 6 weeks postoperatively after euthanasia by intra-peritoneal injection of an excess dose of sodium pentobarbital. Three of 6 specimens in each group were fixed in 10% buffered formalin solution and decalcified in 10% formic acid solution. After dehydration by ethanol, they were permeated with xylene for embedding in paraffin. The paraffin-embedded specimens were serially cut into 8- mm-thick sections. The sections were stained with hematoxylin-eosin for histological examination under an optical microscope.
2.4. Calculation of the Ratio of the Pores with Bone to the Total Number of Pores in a Section of Porous HA Scaffolds
To show the effect of laminin on bone formation objectively, the ratio of pores including bone to the total pores was calculated to compare the level of bone formation in the scaffolds with or without immersion in laminin solution. Ten sections per scaffold were randomly selected from Groups 1 and 2. The number of total pores and that of those containing bone were counted in the sections under an optical microscope. Data are presented as the mean ± standard deviation. Statistical comparisons between the mean values for implanted scaffolds in each group were performed using two-way unrepeated ANOVA followed by post hoc analysis using the TukeyKramer test. Differences of p < 0.01 were considered significant.
2.5. Quantitative Examination of ALP Level and Osteocalcin in Subcutaneously Implanted Porous HA Scaffolds
The quantity of ALP in the porous HA scaffolds was measured biochemically in this study. The supernatant was reacted with p-nitro-phenylphosphate (Zymed Laboratories Inc., CA, USA) as a substrate at 37˚C for 30 minutes. Absorbance of the reactant was measured at 405 nm. The value of ALP activity is presented per scaffold as mM of p-nitrophenol released after 30 minutes of incubation at 37˚C.
Three scaffolds in each group with or without laminin coating and with or without rBMC seeding were used for quantitative analysis of osteocalcin immunochemically. After removal of the scaffolds, they were immediately frozen in liquid nitrogen. Then, they were crushed using a Mixer Mill (MM 301; Retsch Co., Ltd., Tokyo, Japan) and homogenized in 1 ml of a 10-fold concentration of TNE solution (pH 7.4; 10 mM Tris-HCl, 1 mM EDTA, 100 mM NaCl) using a Mixer Mill (MM 301; Retsch Co., Ltd., Tokyo, Japan) and sonicated using BIORUPTOR (UCW-201; Tosho Denki Co., Ltd., Yokohama, Japan). The homogenized samples were sonicated for 30 seconds at 3˚C. The emulsified sample was passed through a column (PD-10 desalting column; GE Healthcare UK Ltd., Buckinghamshire, UK) to collect the osteocalcin adhered to HA. Then, each emulsion was centrifuged for 1 minute at 16,000 ´ g. The supernatants were used for quantitative analysis of osteocalcin using Rat Osteocalcin ELISA kit DS (DS Pharma Biomedical Co., Ltd., Osaka, Japan).
Data are presented as the mean ± standard deviation. Statistical comparisons between the mean values in implanted scaffolds in each group were performed using two-way unrepeated ANOVA followed by post hoc analysis using the Tukey-Kramer test. Differences of p < 0.01 were considered significant.
2.6. In Vitro Assessment of Effects on Laminin for Calcified Nodule Formation by rBMCs
2.6.1. rBMCs Culture in Culture Plate with Laminin-Coated Bottom
For laminin coating of wells in culture plates (6-well culture plate: Bio coat; BD Biosciences, MA, USA), 2 ml laminin solution at 100 mg/ml concentration was poured to each well and left for 1 hour at room temperature. The wells were air dried after suctioning out of the solution. In each well of both laminin-coated 6-well culture plates and uncoated 6-well culture plates as a control, rBMC suspension in 2 ml MEM at 1 × 105 cells/ml was seeded and cultured for 2 weeks in MEM supplemented with FBS and ABs in an incubator at 5% CO2 and 95% relative humidity at 37˚C. To assess rBMC differentiation and calcified nodule formation in the well with and without laminin coating on the bottom of the culture plate, 10 nM dexamethasone (Dex; Sigma-Aldrich Co.), 1 mM b-glycerophosphate (b-GP; EMD Biosciences, Inc., CA, USA) and 82 mg/ml ascorbic acid (Vc; SigmaAldrich Co.) were also added to the culture medium. Only b-GP was added to the medium for rBMC culture as a control. The medium was replaced 3 times each week.
2.6.2. rBMCs Culture in the Medium with Laminin Supplementation
The effect of laminin addition to the culture medium on calcified nodule formation by rBMCs was assessed. rBMCs in 2 ml culture medium at 5 × 104 cells/ml were seeded in the wells (Multi-well culture plate, 6-well; BD Biosciences,) and cultured for 2 weeks as secondary culture in the culture medium. The medium was replaced 3 times each week. Each time, 20 ml laminin solution at 500 mg/ml was also added to each well. As a positive control, Dex, b-GP and Vc without laminin solution were added to the medium for rBMC culture. As a negative control, rBMCs were cultured with b-GP and Vc in the medium.
2.7. Assessment of Nodule Formation by rBMCs in Vitro
2.7.1. Phase Contrast Inverted Microscopic Examination and Von Kossa’s Staining
Calcified nodule formation was examined under a phase contrast inverted microscope after subculture for 2 weeks. To confirm the presence of calcified product on the well bottom, von Kossa’s staining was performed. After fixation using paraformaldehyde solution, the cells were immersed in acetone-ethanol equivalent compound liquid for 1 minute. The cells were then reacted with 5% nitric acid silver solution for 60 minutes, followed by rapid exposure to 5% sodium thiosulfate solution. Then, the stained calcium deposition was observed macroscopically.
2.7.2. Immunochemical and Histochemical Examination of Calcified Deposition by rBMCs in Culture Plates
The supernatant in each well of all culture plates was collected and the quantity of osteocalcin was measured immunochemically using Rat Osteocalcin ELISA kit DS (DS Pharma Biomedical Co., Ltd.). Each 15 ml supernatant from the culture media was poured into wells of a 96-well micro-plate, the bottom of which was coated with anti-rat osteocalcin antibody. Peroxidase-conjugated anti-rat osteocalcin polyclonal antibody was added to each well. An equivalent mixture of peroxidase substrate and hydrogen peroxide water was then added, followed by incubation in the dark. Absorbance at 450 nm was measured using a spectrophotometer (SPECTRA max PLUS; Nihon Molecular Devices Corporation, Tokyo, Japan).
Then, the layer was scraped off in 1 ml of 0.05 M sodium phosphate buffer including 2 mM EDTA and 2 M NaCl and washed three times with phosphate-buffered saline solution without Ca2+ and Mg2+ (PBS(-); Nacalai Tesque, Inc.) in centrifuge tubes. The cells in 1.0 ml of PBS(-) were sonicated and 0.1 ml of supernatant was used for DNA measurement. Salmon sperm DNA (Life Technologies Corp., Invitrogen, CA, USA) was used as the standard. The quantity of DNA was measured by fluorescence emission at 460 nm in the presence of 0.5 mg/ml Hoechst 33258 (Wako Pure Chemical Industries Ltd.). The other sonicated cell suspension was centrifuged at 16,000 ´ g to measure ALP activity. To 0.1 ml of the supernatant, p-nitrophenylphosphate was added as a substrate. ALP activity, as the ALP/DNA ratio, is presented as mM of p-nitrophenol released after 30 minutes of incubation at 37˚C. The results are expressed as the mean ± standard deviation. Statistical comparisons between the mean values in geometrically different implanted scaffolds were performed using two-way unrepeated ANOVA followed by post hoc analysis using the Tukey-Kramer test. Differences of p < 0.01 were considered significant.
3. RESULTS
3.1. Hard Tissue Formation in Porous HA Scaffolds in Vivo
In the porous scaffolds immersed in laminin that received rBMC seeding before implantation (Group 1), bone-like tissue was identified in many pores 6 weeks postoperatively (Figure 2). In the scaffold without immersion in laminin but that received rBMC seeding before implantation (Group 2), hard tissue was found in a smaller number of pores than in Group 1. Without bone marrow cell seeding, no hard tissue was found in the pores of scaffolds that had been immersed in laminin solution (Group 3) or in MEM (Group 4). Infiltration of viable connective tissue into the pores was observed in both Group 3 and Group 4.
3.2. Calculation of the Ratio of Pores with Hard Tissue to the Total Number of Pores in a Section of Porous HA Scaffolds
Using serial paraffin sections of implanted HA scaffold, the average ratio of pores with hard tissue to the total number of pores in a serial section was calculated. The total number of pores observed on a section was approximately 250 on average in each group. There were no statistically significant differences in the number of pores in each section between the scaffolds in the groups. The ratio of pores with hard tissue to the total number of pores in a section is shown in Figure 3. In Group 1, the ratio of pores with hard tissue to total pores was 26.7%. This showed a statistically significant difference from the ratio calculated in Group 2 (p < 0.01). In Groups 3 and 4, the ratio of pores with hard tissue to total pores could not be shown because of the absence of hard tissue in the pores.
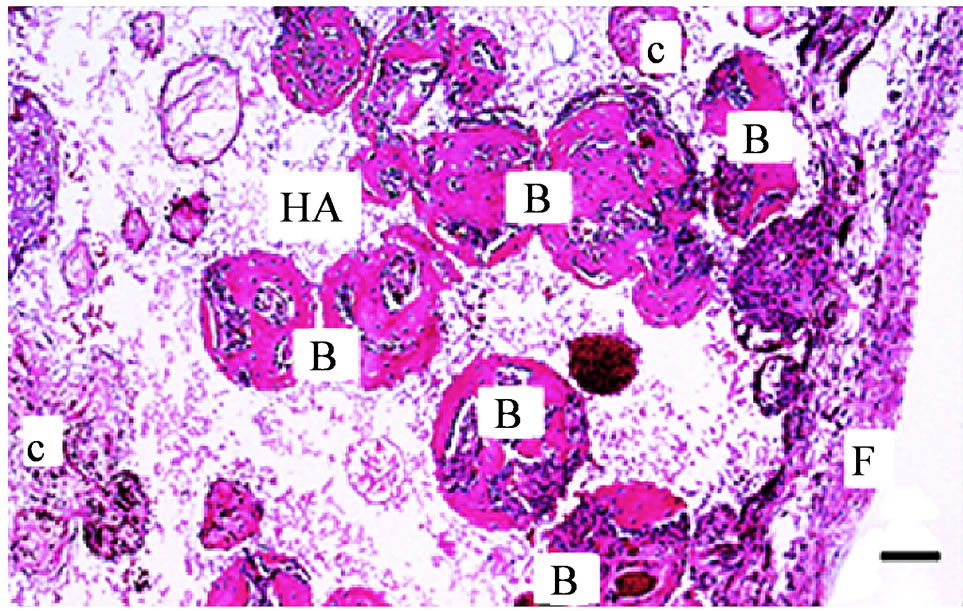
Figure 2. Optical microscopic image of a histological section of a porous hydroxyapatite (HA) scaffold implanted in the dorsal subcutis of rat 6 weeks postoperatively. The HA scaffold was immersed in laminin solution followed by seeding with rat bone marrow cells. Many pores are filled with newly formed bone-like hard tissue (B). In some pores, infiltration of connective tissue (c) is observed. F: Fibrous connective tissue around the HA scaffold. (Bar: 200 μm)
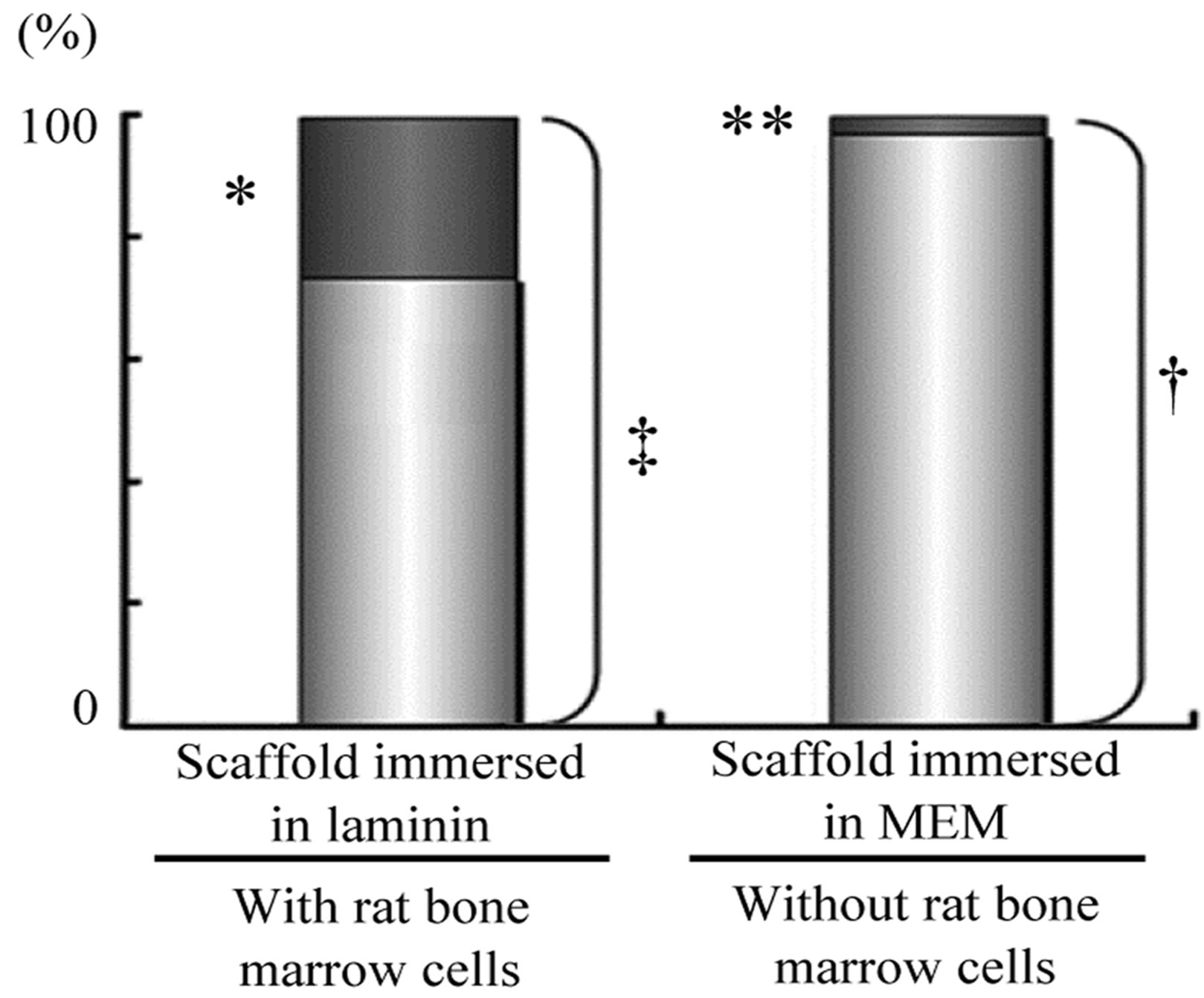
Figure 3. Effect of laminin on bone formation in porous HA scaffolds with rat bone marrow cells (rBMCs) in vivo. Ratio of pores with new bone-like hard tissue to total pores in the HA scaffold implanted in the dorsal subcutis of rats is shown. There is no significant difference in the average total number of pores in each section (n = 10, ‡ vs. †). Many pores with bone-like tissue was observed in sections of porous HA scaffold with immersion in laminin solution. The number of pores with bonelike tissue is statistically different in the sections of the scaffold without immersion in laminin solution (n = 10, *p < 0.01 vs. **).
3.3. ALP Level and Quantity of Osteocalcin in the Scaffolds Implanted in Subcutis of Rats
The level of ALP activity in subcutaneously implanted HA scaffolds in each group due to hard tissue formation in the pores was analyzed biochemically. The results are shown in Figure 4. With rBMC seeding, the level of ALP activity in subcutaneously implanted HA scaffolds immersed in laminin solution was 149 ± 28 mM/scaffold (n = 3). It was significantly higher than the activity of subcutaneously implanted scaffolds immersed in MEM (n = 3, p < 0.01). The level of ALP activity in the scaffolds without rBMCs was significantly lower as shown in Figure 4.
In subcutaneously implanted HA scaffolds in each group, the quantity of osteocalcin in the pores was measured immunochemically. The laminin-treated porous scaffolds with rBMC seeding showed a significant difference in the quantity of osteocalcin in the scaffolds compared with the other scaffolds in vivo (n = 3, p < 0.01). The results are shown in Figure 5. The quantity of osteocalcin produced by rBMCs in HA scaffolds with immersion in laminin solution (Group 1) was 0.32 ± 0.01 ng/scaffold. The data showed a significant difference in comparison with the quantity in HA scaffolds in other groups (p < 0.01). The scaffolds with rBMCs but not laminin immersion (Group 2) showed a statistically lower level than those in Group 1 (p < 0.01). In HA scaffolds implanted in subcutis without receiving rBMCs, osteocalcin was scarcely produced (Groups 3 and 4).
3.4. Effect of Laminin on Calcified Nodule Formation by rBMCs in Vitro
Two weeks later, many mineralized areas in the cultured
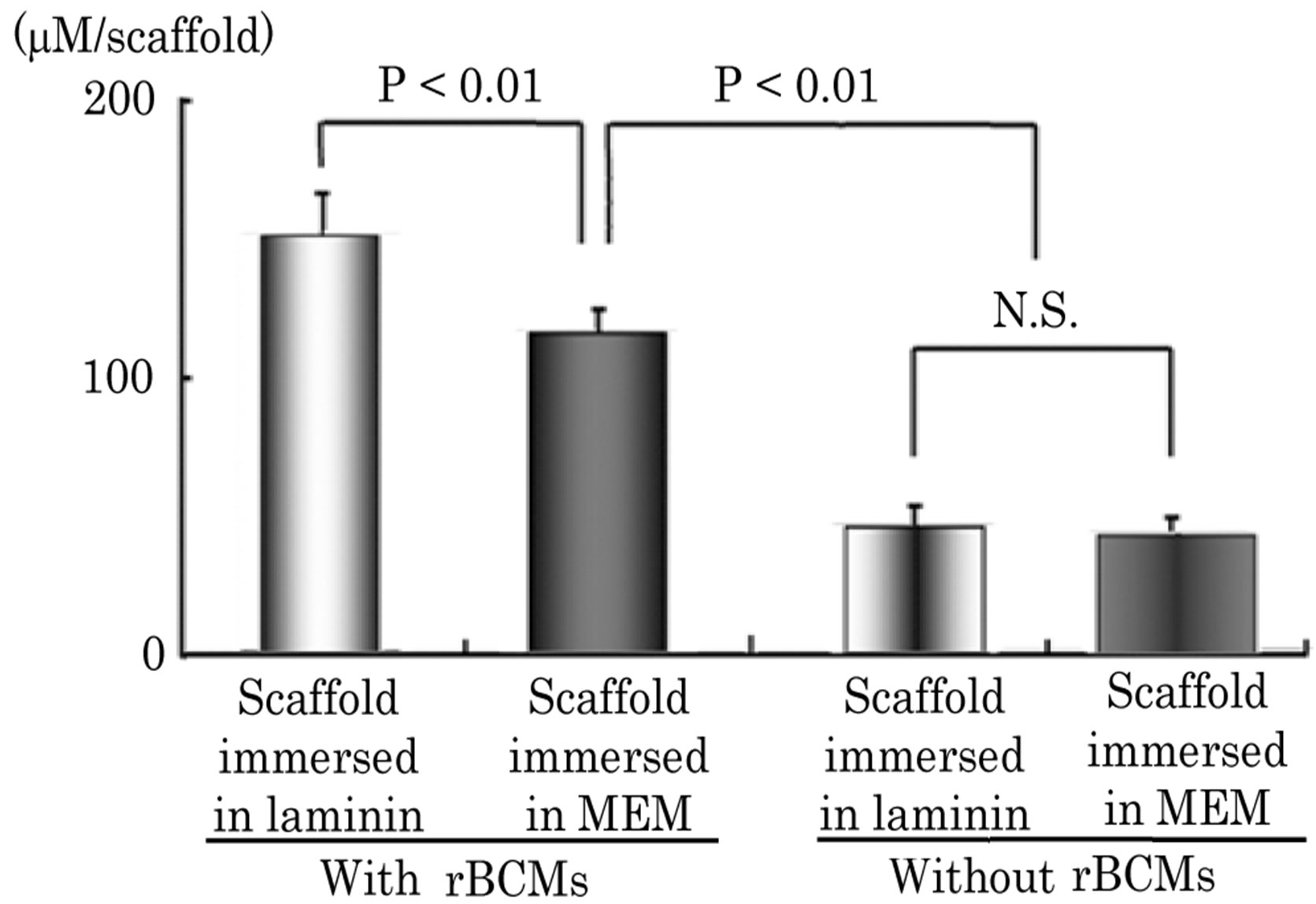
Figure 4. ALP activity in scaffold implanted subcutaneously Immersion in laminin solution of porous HA scaffolds with rBMCs shows a significant difference in the level of ALP activity of the scaffolds immersed in MEM. The activity of cells in the connective tissue shows no significant difference. Immersion in laminin solution of HA scaffolds without rBMCs shows no significant difference in the level of ALP activity from the scaffolds immersed in MEM (n = 10).

Figure 5. Quantity of osteocalcin in scaffold implanted subcutaneously. The quantity of osteocalcin in the scaffolds immersed in laminin followed by rBMC seeding showed a significant difference from that of scaffolds without immersion in laminin followed by cell seeding (n = 3, p < 0.01). There is a significant difference in the quantity of osteocalcin in the scaffolds with rBMCs compared with those without cell seeding (n = 6, p < 0.01).
rBMCs were observed on laminin-coated and uncoated plates with Vc, b-GP and Dex (Figure 6).
The results of biochemical analysis for the level of ALP activity, as the ALP/DNA ratio after 2 weeks of rBMC culture in wells with or without laminin coating, are shown in Figure 7. In the wells with laminin coating, rBMCs cultured in the medium with Vc, b-GP and Dex showed a significantly high level of ALP activity. rBMCs cultured in laminin-coated well without Vc and Dex showed no significant difference in the level of ALP activity compared with that of such cells cultured in laminin-added medium with no Vc and Dex or that of cells cultured in the medium with addition of Vc, b-GP and Dex but not laminin. The level of ALP activity of rBMCs cultured in the medium with addition of laminin, Vc, b-GP and Dex was lower than that of those cultured in the medium without addition of Vc and Dex.
4. DISCUSSION
The effect of laminin coating on HA scaffolds for osteogenesis in the pores was described in our previous report from optical microscopic findings [6]. This study was performed to show the potential of laminin objectively on bone formation in HA scaffolds in vivo. The number of pores with bone was counted to culture the ratio of the pores containing bone in the section in this study. An osteogenic effect on the scaffold by laminin was also examined by the quantitative analysis of osteocalcin. The quantity of osteocalcin in the scaffolds measured in this study was the same as the results obtained in the previous study [6]. However, in this study, the emulsified samples of the HA apatites were passed through a column to collect the osteocalcin adhered to HA, so the amount of osteocalcin was measured accurately in this

Figure 6. Microscopic image of nodule in culture plate. In the laminin-coated culture plate, 2 weeks of subculture of rBMCs with dexamethasone in culture medium was performed. Nodules of calcium deposition are shown (×40).
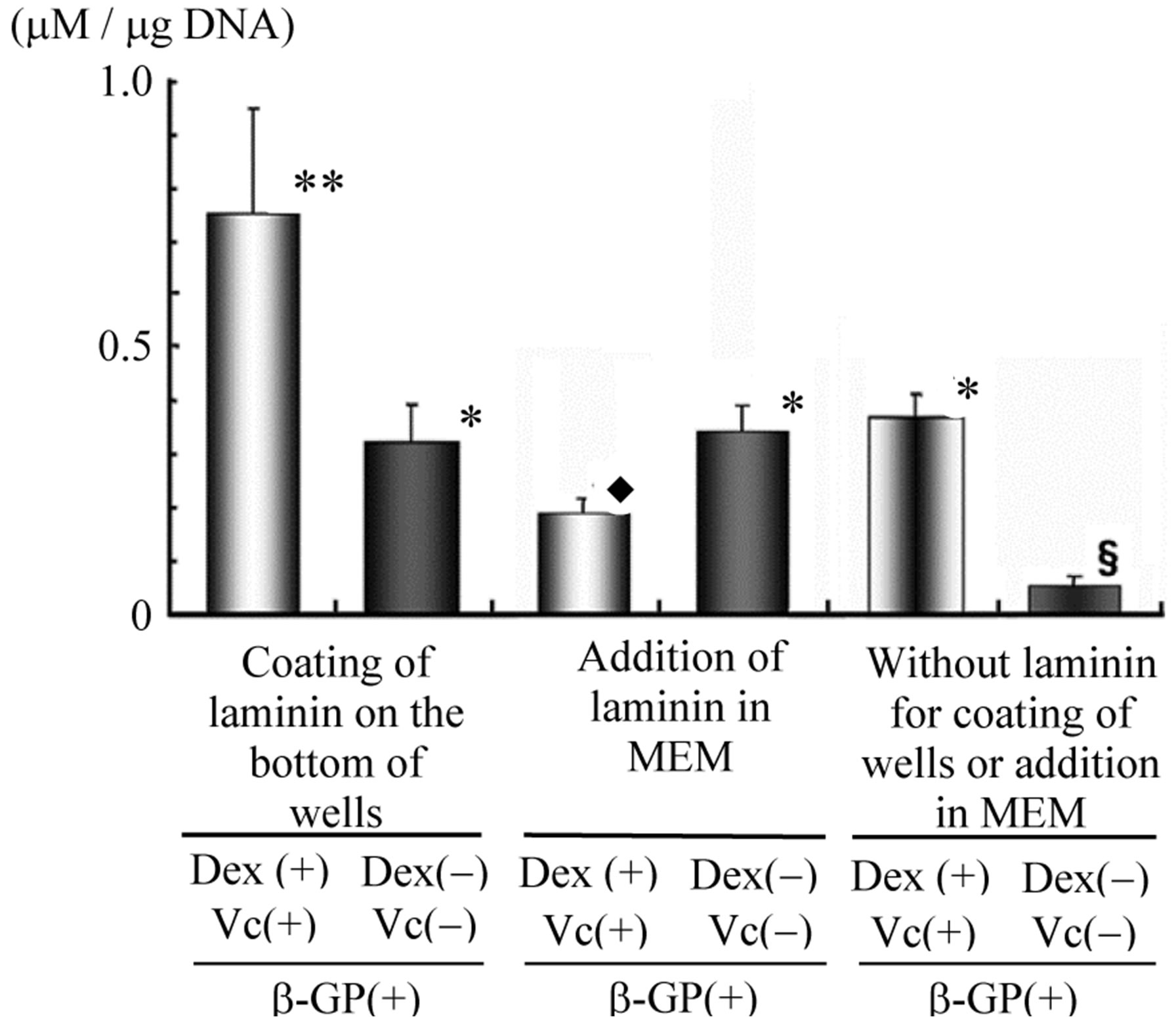
Figure 7. ALP activities in vitro as ALP (mM)/DNA (mg) ratio of rBMCs. After 2 weeks of rBMC culture, laminin coating of plate in wells showed significantly high level of ALP with addition of Dex and Vc. Addition of laminin showed no significant difference to the level of ALP activity (**p < 0. 01 vs. *, *p < 0.01 vs.¨, ¨p < 0.01 vs. §).
study.
ALP activity was not measured in our previous study. The value in the scaffolds in this study revealed clearly that laminin promoted osteogenesis in the pores of the scaffolds. rBMCs was cultured in a culture plate with the laminin coat by the bottom and produced calcium deposition. In this study, the effect of laminin was confirmed from the result of measured ALP activity. However, the addition of laminin solution as a culture supplement by adding to the culture medium induced little deposition of calcium by rBMCs. The effect of laminin was to adhere the stem cells to the bottom of the culture plate. It is therefore clear that laminin contributes to the proliferation and differentiation of stem cells. The formation of calcium deposition might be promoted by the effect of laminin.
It is well known that laminin is a glycoprotein that contributes to the proliferation of osteoblast-like cells [15]. It was reported that, in a cell suspension, 1 × 107 cells/ml or more is needed for in vivo examination in a porous scaffold for hard tissue formation by bone marrow cell seeding [7]. However, hard tissue formation was found in the pores of HA scaffolds with a smaller number of rBMCs in this in vivo examination. It was also suggested that laminin promotes osteogenesis by bone marrow cells in the HA scaffold. Adhesion of the cells to a substrate might be essential for the growth and differentiation of the cells [16,17]. Tissue fluid infiltrates the pores in the scaffold implanted in subcutaneous tissue. rBMCs seeded in the pores of the HA scaffold may be released from the pores by the flow of tissue fluid before adherence of the cells to the pore wall. Seeded rBMCs attached to the pore wall of the scaffold by the effects of laminin in this study. Many cells could therefore remain in the pores and could differentiate to osteoblastic progenitor cells or osteoblasts in the pores. As it is said that mesenchymal stem cells in the bone marrow and periosteum are multipotent cells [2], they might differentiate into chondrogenic, adipogenic or osteogenic lineages [1]. Bone marrow mesenchymal stem cells might be used for osseous regeneration [3-5]. Specifically, the cells could be used for bone repair [18]. The effect of laminin was examined in this study as a factor that may promote the differentiation of stem cells in rBMCs and hard tissue formation in the pores of a scaffold in vivo. In addition, the potential of laminin for hard tissue formation was investigated using a small number of rBMCs in the pores of HA scaffolds pre-treated by immersion in laminin solution.
Porous HA structures with a hollow center have been designed and manufactured as scaffolds for the regeneration of dentin-pulp complex [6]. In vivo examination of osteogenesis showed that an appropriate number of bone marrow cells in suspension might be 1 ´ 107 cells/ml or more [7,19]. However, in this study, rBMCs in the suspension at 1 ´ 106 cells/ml were seeded into a porous HA scaffold with a hollow center after immersion in laminin or MEM. The results from this in vivo study indicated that the effect of laminin might induce hard tissue formation in the pores of the scaffold with a smaller number of rBMCs. Adherence to the substrate and development of focal adhesions are essential for cellular growth and differentiation [16]. Active differentiation of rBMCs might form hard tissue in the pores of scaffolds. A previous study showed that laminin isoforms in bone marrow are adhesive substrates to bone marrow stem and progenitor cells and influence progenitor cell migration in vitro [17]. The effects of laminin on calcified nodule formation by bone marrow cells were also confirmed in an in vitro examination by conspicuous calcified nodule deposition in a laminin-coated plate [6]. However, the results were only macroscopic observation of deposited calcified nodules. In this in vitro study, osteocalcin and ALP activity were measured quantitatively and the results were assessed objectively.
Laminin should well support osteogenic differentiation of bone marrow cells in vitro. It was reported that contact with laminin-5 is sufficient to stimulate osteogenic differentiation in human mesenchymal stem cells [20]. This previous report supports the results obtained in this study. The results in this study showed high ALP activity of rBMCs in the laminin-coated culture plates. It is clearly suggested that rBMCs cultured in laminin-coated plates were stimulated and differentiated into cells acting as osteoblast-like cells. Laminin may promote cellular attachment for differentiation and bone regeneration of bone marrow stem cells. It is considered that laminin must directly stimulate the osteogenic differentiation of mesenchymal stem cells.
Laminin might be an adhesive substrate for bone marrow stem cells and progenitor cells, according to previous research [17]. rBMCs cultured in this in vitro study with or without Dex showed significantly higher ALP activity in a laminin-coated plate than in an uncoated plate. It was reported that human mesenchymal stem cells were stimulated to differentiate into osteogenic cells by contact with laminin [20]. Therefore, the results in this study suggest the possibility that ALP activity of rBMCs increased due to laminin. High ALP activity of the rBMCs suggested that differentiation to osteogenic cells from bone marrow was promoted by laminin on the culture plate. Laminin added to MEM would cover the surface of rBMCs and contribute to attachment of the cells on the bottom of wells and might act as a substrate for the growth and differentiation of cells. Moreover, the results of this study indicated that the interaction of laminin and Dex improves hard tissue formation of rBMCs.
5. CONCLUSION
As a supplement for rBMC differentiation and hard tissue formation by the cells, it was confirmed that laminin might be effective for hard tissue formation in the porous scaffold by seeding with mesenchymal stem cells. In subcutaneous implantation of a porous HA scaffold after immersion in laminin solution and seeding of bone marrow cells at 1 × 106 cells/ml, new hard tissue was formed in many pores of the scaffold. It was shown by the result of monolayer culture of rBMCs in vitro that rBMC differentiation and hard tissue formation would be promoted by the effects of laminin. In addition, laminin is useful as a co-factor of Dex and β-GP to promote cell differentiation and bone formation in the pores of a scaffold.
ACKNOWLEDGEMENTS
This study was performed in the Morphological Research Facilities, Biomaterials Research Facilities, Low-Temperature Facilities, Tissue Culture Facilities, Laboratory Animal Facilities and Photograph-Processing Facilities, Institute of Dental Research, Osaka Dental University.
This study was supported in part by 2008-2010 (C: 20592246) and 2011-2013 (C: 23592820) Grants-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science.
REFERENCES
- Pittenger, M.F. and Martin, B.J. (2004) Mesenchymal stem cells and their potential as cardiac therapeutics. Circulation Research, 95, 9-20. http://dx.doi.org/10.1161/01.RES.0000135902.99383.6f
- Barry, F.P. and Murphy, J.M. (2004) Mesenchymal stem cells: Clinical applications and biological characterization. The International Journal of Biochemistry & Cell Biology, 36, 568-584. http://dx.doi.org/10.1016/j.biocel.2003.11.001
- Petite, H., Viateau, V., Bensaid, W., Meunier, A., De Pollak, C., Bourguignon, M., Oudina, K., Sedel, L. and Guillemin, G. (2000) Tissue-engineered bone regeneration. Nature Biotechnology, 18, 959-963. http://dx.doi.org/10.1038/79449
- Caplan, A.I. and Bruder, S.P. (2001) Mesenchymal stem cells: Building blocks for molecular medicine in the 21st century. Trends in Molecular Medicine, 7, 259-264. http://dx.doi.org/10.1016/S1471-4914(01)02016-0
- Derubeis, A.R. and Cancedda, R. (2004) Bone marrow stromal cells (BMSCs) in bone engineering: Limitations and recent advances. Annals of Biomedical Engineering, 32, 160-165. http://dx.doi.org/10.1023/B:ABME.0000007800.89194.95
- Yoshikawa, M., Tsuji, N., Shimomura, Y., Hayashi, H. and Ohgushi, H. (2008) Osteogenesis depending on geometry of porous hydroxyapatite scaffolds. Calcified Tissue International, 83, 139-145. http://dx.doi.org/10.1007/s00223-008-9157-y
- Akahane, M., Ohgushi, H., Yoshikawa, T., Sempuku, T., Tamai, S., Tabata, S. and Dohi, Y. (1999) Osteogenic phenotype expression of allogeneic rat marrow cells in porous hydroxyapatite ceramics. Journal of Bone and Mineral Research, 14, 561-568. http://dx.doi.org/10.1359/jbmr.1999.14.4.561
- Huang, G.T., Gronthos, S. and Shi, S. (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. Journal of Dental Research, 88, 792-806. http://dx.doi.org/10.1177/0022034509340867
- Sengupta, S., Park, S.H., Patel, A., Carn, J., Lee, K. and Kaplan, D.L. (2010) Hypoxia and amino acid supplementation synergistically promote the osteogenesis of human mesenchymal stem cells on silk protein scaffolds. Tissue Engineering Part A, 16, 3623-3634. http://dx.doi.org/10.1089/ten.tea.2010.0302
- Huang, C.H., Tseng, W.Y., Yao, C.C., Jeng, J.H., Young, T.H. and Chem, Y.J. (2010) Glucosamine promotes osteogenic differentiation of dental pulp stem cells through modulating the level of the transforming growth factor-b type I receptor. Journal of Cellular Physiology, 225, 140- 151. http://dx.doi.org/10.1002/jcp.22206
- Bohl, K.S., Shon, J., Rutherford, B. and Mooney, D.J. (1998) Role of synthetic extracellular matrix in development of engineered dental pulp. Journal of Biomaterials Science, Polymer Edition, 9, 749-764. http://dx.doi.org/10.1163/156856298X00127
- Srisuwan, T., Tilkorn, D.J., Al-Benna, S., Vashi, A., Penington, A., Messer, H.H., Abberton, K.M. and Thompson, E.W. (2012) Survival of rat functional dental pulp cells in vascularized tissue engineering chambers. Tissue and Cell, 44, 111-121. http://dx.doi.org/10.1016/j.tice.2011.12.003
- Leye Benoist, F., Gaye Ndiaye, F., Kane, A.W., Benoist, H.M. and Farge, P. (2012) Evaluation of mineral trioxide aggregate (MTA) versus calcium hydroxide cement (Dycal®) in the formation of a dentine bridge: A randomised controlled trial. International Dental Journal, 62, 33-39. http://dx.doi.org/10.1111/j.1875-595X.2011.00084.x
- Yoshikawa, M., Tsuji, N. and Toda, T. (2004) Hard tissue formation by cultured dental pulp cells and bone marrow cells. Journal of Osaka Dental University, 38, 119-125.
- Santiago, J.A., Pogemiller, R. and Ogle, B.M. (2009) Heterogeneous differentiation of human mesenchymal stem cells in response to extended culture in extracellular matrices. Tissue Engineering Part A, 15, 3911-3922. http://dx.doi.org/10.1089/ten.tea.2008.0603
- Burridge, K., Fath, K., Kelly, T., Nuckolls, G. and Turner, C. (1988) Focal adhesions: Transmembrane junctions between the extracellular matrix and the cytoskeleton. Annual Review of Cell Biology, 4, 487-525. http://dx.doi.org/10.1146/annurev.cb.04.110188.002415
- Gu, Y.C., Kortesmaa, J., Tryggvason, K., Persson, J., Ekblom, P., Jacobsen, S.E. and Ekblom, M. (2003) Laminin isoform-specific promotion of adhesion and migration of human bone marrow progenitor cells. Blood, 101, 877-885. http://dx.doi.org/10.1182/blood-2002-03-0796
- D’Ippolito, G., Schiller, P.C., Ricordi, C., Roos, B.A. and Howard, G.A. (1999) Age-related osteogenic potential of mesenchymal stromal cells from human vertebral bone marrow. Journal of Bone and Mineral Research, 14, 1115-1122. http://dx.doi.org/10.1359/jbmr.1999.14.7.1115
- Ohgushi, H., Dohi, Y., Yoshikawa, T., Tamai, S., Tabata, S., Okunaga, K. and Shibuya, T. (1996) Osteogenic differentiation of cultured marrow stromal stem cells on the surface of bioactive glass ceramics. Journal of Biomedical Materials Research, 32, 341-348. http://dx.doi.org/10.1002/(SICI)1097-4636(199611)32:3<341::AID-JBM6>3.0.CO;2-S
- Klees, R.F., Salasznyk, R.M., Kingsley, K., Williams, W.A., Boskey, A. and Plopper, G.E. (2005) Laminin-5 induces osteogenic gene expression in human mesenchymal stem cells through an ERK-dependent pathway. Molecular Biology of the Cell, 16, 881-890. http://dx.doi.org/10.1091/mbc.E04-08-0695

