Journal of Biomedical Science and Engineering
Vol.6 No.7A1(2013), Article ID:34677,6 pages DOI:10.4236/jbise.2013.67A1003
Bio-efficacy and persistence evaluation of Dimilin®GR-2% and Mosquiron® 10 EC insect growth regulators against Anopheles gambiae s.l (diptera: Culicidae) larvae
![]()
1Directorate of Public Health and Research, National Malaria Control Centre, Ministry of Health, Lusaka, Zambia
2Department of Biological Sciences, School of Natural Sciences, University of Zambia, Lusaka, Zambia
3Department of Community Medicine, School of Medicine, University of Zambia, Lusaka, Zambia
Email: *emmanuel_chanda@yahoo.co.uk
Copyright © 2013 Emmanuel Chanda et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 9 March 2013; revised 10 April 2013; accepted 26 April 2013
Keywords: Evaluation; Insect Growth Regulators; Integrated Vector Management; Malaria Control
ABSTRACT
Expansive chemical-based vector control has resulted in development of vector resistance to different insecticides harnessed for prevention of disease transmission in public health. The environmentally safe insect growth regulators and microbial larvicides provide potential tools for insecticide resistance management. The efficacy and persistence of Dimilin® GR-2% and Mosquiron® 10 EC insect growth regulators were evaluated against Anopheles gambiae s.l larvae under laboratory and simulated field conditions. In laboratory bio-efficacy trials, complete emergence inhibition was achieved at higher concentrations with 96 hours post exposure of mosquito aquatic stages to the two larvicides. In simulation field trials, persistence of both larvicides at higher concentrations increased gradually with complete inhibition attained at 7 days and maintained up to 21 days. In the quest of deploying non insecticide based interventions for a sustainable environment, insect growth regulators can be recommended for operational scale larviciding for mosquito larval control in the context of integrated vector management.
1. INTRODUCTION
Globally malaria remains a major public health problem and this is epitomized by sub-Saharan African countries [1]. The huge burden of the disease is maintained by strong vectorial potential of Anopheles gambiae Giles, An. arabiensis Patton and An. funestus Giles [2,3]. Indoor residual spraying (IRS) and insecticide-treated nets (ITNs) are expansively being deployed as frontline tools to prevent malaria transmission [4,5]. However, chemicalbased vector control tools have resulted in development of vector resistance to different insecticides in use for public health and agriculture in addition to adverse environmental concerns [6].
To optimize the use of limited available resources for vector control, implementation of the World Health Organization (WHO)-led integrated vector management (IVM) strategy is being advocated to rationalize decision making [7]. To this effect, a Global Plan on Insecticide Resistance Management for Malaria Vectors (GPIRM) has been developed to maintain effectiveness of malaria vector control [8]. The WHO also recommends larviciding with Bacillus thuringiensis var. israelensis (Bti) and the insect growth regulators (IGR) for the purposes of malaria vector control, as a supplementary tool, within the context of IVM in sub-Saharan Africa [9]. The IGRs and Bti, unlike the chemical larvicides, are strictly arthropod-specific, environmentally safe and potential tools for insecticide resistance management [10,11].
There are two types of IGRs, juvenile hormone (JH) analogues (Juvenoids) and the chitin synthesis inhibitors. JH analogues interfere with the transformation of late instar larvae to pupae and then to adult, whereas chitin synthesis inhibitors inhibit cuticle formation and affect all instars and immature stages of the mosquito [12]. Dimilin® GR-2% (Diflubenzuron) and Mosquiron® 10 EC (Novaluron) are benzoyl urea based chitin biosynthesis inhibiting stomach poison compounds that have successfully passed the WHO’s pesticide evaluation scheme (WHOPES’s) and recommended for mosquito larvae control [13]. Many laboratories and simulated field trials have been conducted with chemical larvicides and Bti to evaluate their efficacy and persistence [14,15].
The present study assessed and compared the efficacy of Dimilin® GR-2% and Mosquiron® 10 EC against An. gambiae s.l larvae under laboratory and simulated field conditions. This was an initial step towards programmatic deployment of larviciding as a supplementary vector control intervention in Zambia.
2. MATERIALS AND METHODS
2.1. Experimental Site
Zambia is a landlocked country in southern Africa with an estimated population of 13 million people, 45% are children below 15 years of age [16]. The study was conducted at National Malaria Control Centre (NMCC) using field collected An. gambiae s.l larvae between September and October, 2007. During the study period, annual ambient temperatures varied between an average minimum of 26˚C and maximum of 28˚C and Relative humidity ranged from 47 to 54 (Meteorological Stations).
2.2. Mosquito Collections and Laboratory Processing
Anopheles mosquito larvae were collected from breeding sites using WHO standard 250 mL dippers [17], transported to the NMCC in Lusaka for the evaluation assays. Some immature stages were reared to F0 adults under standard laboratory conditions with a temperature range of 25˚C ± 2˚C and 70% - 80% relative humidity. Mosquitoes were identified morphologically using standard keys for anophelines of southern Africa [18,19].
2.3. Evaluated Products
Dimilin® GR-2% is a granular larvicide, 100 grams of this slow acting product is composed of 2 grams Diflubenzuron and 98 grams Coformulants. The product is manufactured by Rea Industrie Chimiche SRL, SS 87 Km 20.700, 81025 Marcianise (CE), Italy and the Registration Holder is Crompton (Uniroyal Chemical) Registrations Ltd., Ankerweg 18, 1041 Amsterdam, The Netherlands. Dimilin® GR-2% inhibits the synthesis of chitin and, hence, interferes with molting; to a lesser extent, a contact poison and acts by disturbing the molting process of all stages of larvae instars of mosquitoes [20]. Application rates for Anopheles larvae control in clear water is 1.25 - 2.50 kg/Ha.
Mosquiron® 10 EC is an emulsifiable concentrate. 100 grams of this product is composed of Novaluron. The Registration Holder is Makhteshim-Agan SA (Pty) Ltd. Registration number 1992/001741/07 Posbus 498 Brackenfell 7561 Israel. Mosquiron® 10 EC acts by inhibiting chitin biosynthesis resulting in interference with the formation of the cuticle, thereby halting the normal growth and development of larvae. Application rates for Anopheles larvae control in clear water is 10 l water/200 m2 up to 50 cm deep (0.1 ml - 0.5 ml/m3).
The supplies of Dimilin® GR-2% and Mosquiron® 10 EC used in this trial were manufactured in October 2007 with a two-year lifespan.
2.4. Entomological Evaluation
The evaluation was done in accordance with the WHO standard protocol [21]. Biological activity was determined using stock solution (1%) prepared by dissolving 200 mg in 20 ml. Test concentrations were diluted with water and serial dilutions (0.1%, 0.01%, 0.001%, 0.0001%, 0.00001%, 1.0% and 10%) were made in the same manner. The assays were in two different stages; the Laboratory testing phase to determine the inherent bio-potency of the technical materials and the small scale field trials to establish the efficacy levels of the products against target mosquito larvae, under simulated field conditions. Mortality was assessed and recorded. Larvicide formulations were tested at different concentrations and phase one studies guided the dosages chosen for use in phase two trials [21].
2.5. Laboratory Trials
During the laboratory trials, bioassays were conducted for Anopheles pre-imagos. Batches of 25 larvae were introduced in test vessels (0.1%, 0.01%, 0.001%, 0.0001%, 0.00001%, 1.0% and 10%) and fed on a mixture of finely ground yeast extract (one part) and dog biscuit (three parts) at a concentration of 10 mg/l at twoday intervals until mortality counts were made. The food powder was suspended in water and two drops added per test cup including the control. A mixture of secondand third-stage larvae were selected for the experiment as they were large enough and can easily be counted and had three to two stages before becoming adults and therefore, giving investigators enough time to make follow-ups of their development. All the test and control cups were covered with netting to prevent successfully emerged adults from escaping into the environment [21].
The test containers were held at 25˚C - 28˚C for a photoperiod of 12L: 12D. Mortality or survival was counted every other day until the complete emergence of adults. At the end of the observation period, the impact was expressed as IE% based on the number of larvae that do not develop successfully into viable adults. In recording IE% for each concentration, moribund and dead larvae and pupae, as well as adult mosquitoes not completely separated from the pupal case, were considered as “affected”. The experiment stopped when all the larvae or pupae in the controls had died or emerged as adults. The testing room was maintained at 25˚C ± 2˚C and 75% - 80% relative humidity [21].
2.6. Simulated Field Trials
In simulated field trials, multiple plastic tubs of water were placed under imitation field conditions. Plastic tubs with diameter of 60 cm and depth of 15 cm were used with 10 litres of water from the breeding sites where the larvae were collected. The water-filled containers were given at least 24 hrs for conditioning or ageing. A batch of 30 third instar An. gambiae s.l larvae was released into each replicate and fed on alternate days. After 2 - 3 hrs of larval acclimation, separate containers were treated with Dimilin® GR-2% and Mosquiron® 10 EC dosages using pipettes. The containers were covered with netting material to prevent other mosquitoes or other insects from laying eggs and to protect the water from falling debris. The water level in the containers was sustained and a minimum of four replicates of each dosage and four controls were utilized.
To test residual activity, Larval survival was assessed every third day post addition until all larvae had pupated and emerged and pupal skins were counted seven days or more after addition to assess emergence. This process continued until no mortality was noted. Adults not freed from pupal skins were considered dead. The observations were performed at 1, 3, 7, 14 and 21 days post-treatment, by which time all larvae would have pupated and emerged as adults. The test was terminated when there was no statistically significant residual activity in terms of larval mortality or inhibition of adult emergence when comparing the treated (at the highest dosage tested) batches and the untreated controls. The weekly settings of larvae continued until no difference in mortality was recorded between untreated controls and treated batches. Percentage mortality or emergency inhibition (IE %) was calculated [21].
2.7. Data Management and Statistical Analysis
Data were collected from both laboratory and simulation field experiments for Dimilin® GR-2% and Mosquiron® 10 EC, entered in Excel spread sheets (Microsoft Corporation) and statistically analyzed by employing Epi-Info version 3.2.2. The data on the number of live and dead larvae and pupae from all replicates of each dosage on one day was combined and percentage mortality or IE% calculated. The significance of the difference in the mosquito mortalities was analyzed by Chi-square (Χ2) test using a computer software [22] and Odds ratios (OR) and 95% confidence intervals (CI) to assess the efficacy of the given concentration of each larvicide.
3. RESULTS
The effect of Dimilin® GR-2% and Mosquiron® 10 EC at various concentrations within their activity range, on field-collected 2nd and 3rd instar An. gambiae s.l larvae was expressed in terms of the percentage of larvae that do not develop into successfully emerging adults or adult emergence inhibition (IE %).
3.1. Laboratory Trials of Dimilin® GR-2% and Mosquiron® 10 EC
While the 0.01, 0.001, 0.0001 and 0.00001 concentrations (v/v) had exhibited appreciable emergence inhibittion effect, a marked percentage reduction effect was observed at 1.0% and complete killing effect was observed at 10.0% concentration (v/v) for both larvicides. At 1.0% and 10.0% conc. (v/v), 72 hrs and 96 hrs post-application, the inhibition rate was 90% and100% for Dimilin® GR-2% (Figure 1(a)) and 93% and100% for Mosquiron® 10 EC (Figure 1(b)).
3.2. Simulated Field Trials of Dimilin® GR-2% and Mosquiron® 10 EC
Emergence inhibition (IE %) at 0.1%, 1.0% and 10% conc v/v was effective in three weeks. For both Dimilin® GR 2% and Mosquiron® 10 EC percentage emergence inhibition increased gradually from <10% (0.1% and 1.0% conc. v/v) and from <40% (10% conc. v/v) to 90% (0.1% conc. v/v) and 100% (1.0% and 10% conc. v/v) 24 hrs post application (Figures 1(c) and (d)). At 14 and 21 days post-treatment, complete killing effect for 1.0% and 10% conc. v/v was maintained for both larvicides. The 0.1% concentration remained at 98% at 14 days and only killed the larvae fully at 21 days after application (Figures 1(c) and (d)).
3.3. Comparison Efficacy of Dimillin® GR-2% and Mosquiron® 10 EC
According to the simulation field results, there was marked effect of Dimillin® GR-2% and Mosquiron® 10 EC at both 10 and 1.0% from the first day of application to the last day of the study as exemplified by the computed odds ratios and Chi-square P-values of the 0.1% concn. v/v (Table 1) and as determined by the student t-test (t = −0.768, df = 4, P = 0.485).
4. DISCUSSION
Expansive utilization of chemical insecticides in mosquito vector control has not only resulted in the development of vector resistance to different insecticides but also have adverse environmental and ecological effects [11]. In view of this, there have been aggressive efforts
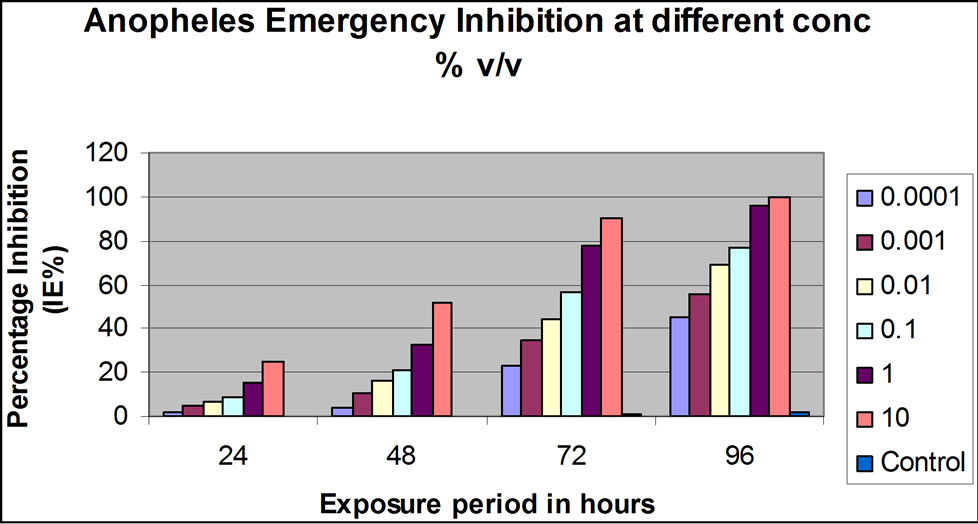 (a)
(a)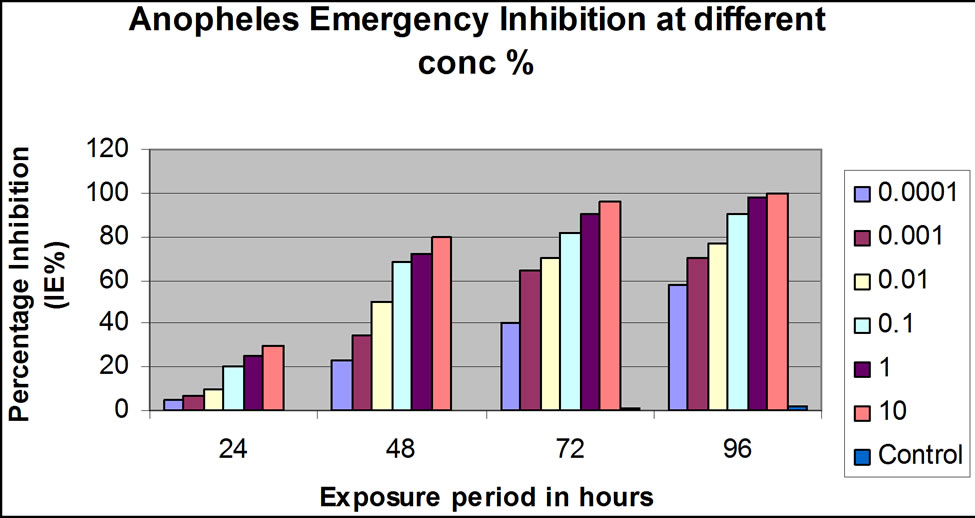 (b)
(b)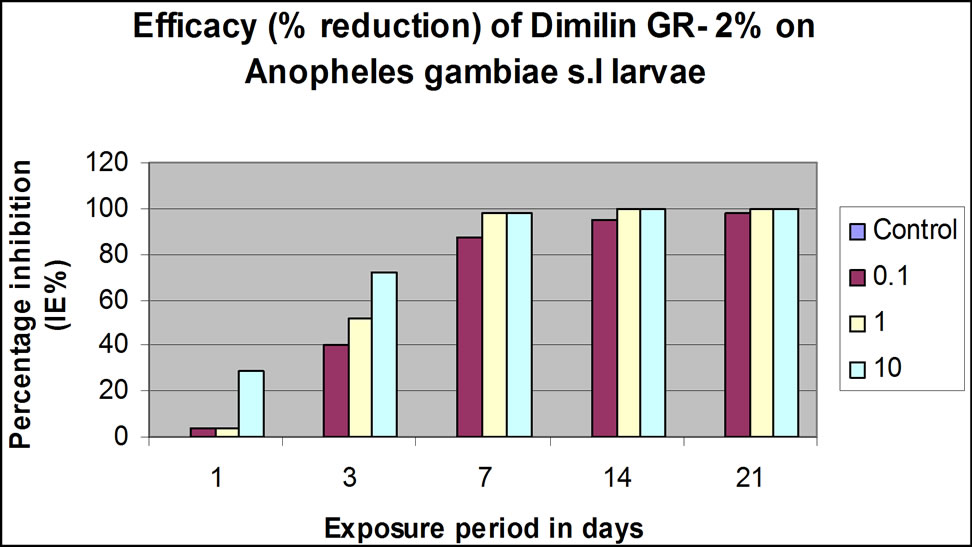 (c)
(c)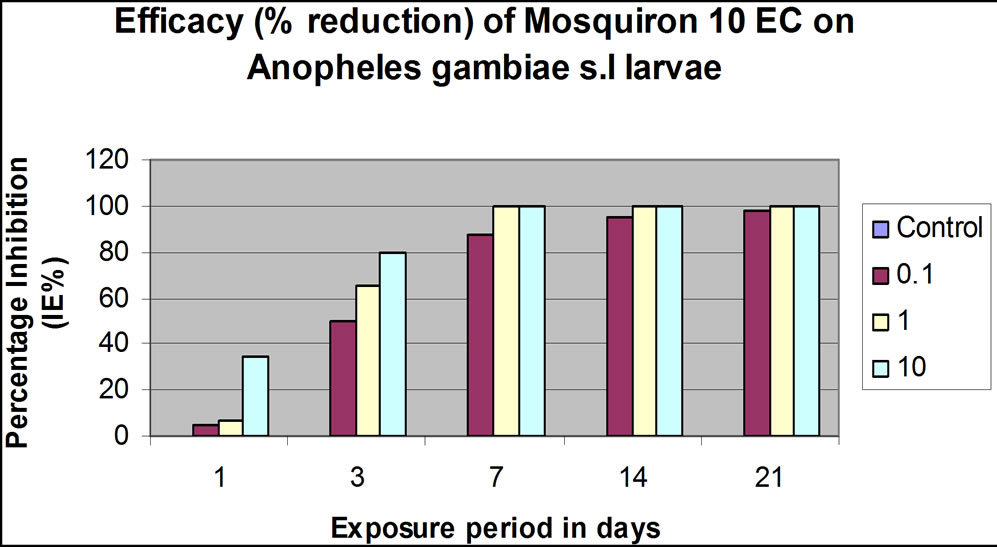 (d)
(d)
Figure 1. Laboratory and simulation field efficacy percentage (%) inhibition results for Dimilin GR-2% and Mosquiron® 10 EC. (a) Dimilin GR-2% laboratory efficacy % inhibition 24, 48, 72 and 96 hrs post-application at different concentrations (v/v); (b) Mosquiron® 10 EC laboratory efficacy % inhibition 24, 48, 72 and 96 hrs post-application at different concentrations (v/v); (c) Results of the simulation field efficacy trial indicating the % inhibition at 0.1%, 1.0% and 10% in three weeks; (d) Results of the simulation field efficacy trial indicating the % inhibition at 0.1%, 1.0% and 10% in three weeks.
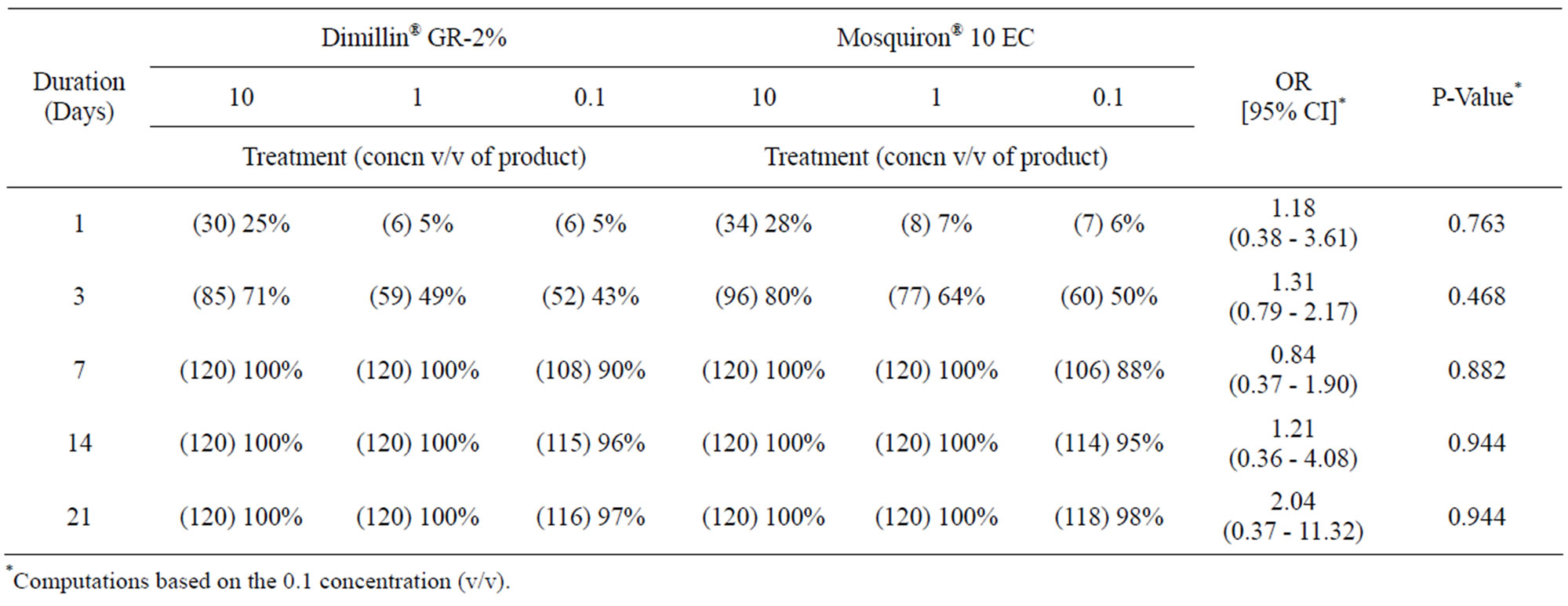
Table 1. Residual activity of Dimillin® GR-2% and Mosquiron® 10 EC and percentage (%) reduction of emergency of An. gambiae s.l lavae.
in research towards alternative interventions, particularly the use of biological larvicides such as B. thuringiensis var. israelensis and B. sphaericus including Insect Growth Regulators [14,15]. Although only a few operational field studies have been conducted, various laboratory and simulated field studies have been conducted with Afro-tropical malaria vector species [11]. With increased funding for malaria vector control, re-evaluating the potential of antilarval measures tailored to local conditions in sub-Saharan Africa is critical [22-24] and has been supported by the increasing interest in integrated vector management [7,25,26].
Examination of data from laboratory trials demonstrated effective bio-potency of Dimilin® GR-2% and Mosquiron® 10 EC for the control of An. gambiae s.l at dosages of 10% and 1% concentration (v/v) achieving very high emergence inhibition rates. The mean larval mortality rates reached 95% and 100% at 24 hrs and 48hrs post-treatment respectively. Although high mortality of larvae and pupae was observed at 24 h after treatment in laboratory trials, daily results indicated that significant mortality of larval An. gambiae s.l was delayed up to 96 and 72 h and after treatment. Delayed effectiveness against larvae of Anopheles species has also been reported [27].
In simulated field trials, both products were found to be effective against An. gambiae s.l at dosages of 0.1% and 1.0% concentration (v/v), with percent emergence inhibition persisting up to 3 wks (336 hrs) post-treatment. The delayed action of IGRs on treated larvae means that mortality is assessed every other day or every three days until the completion of adult emergence. Delayed mortality of larvae appears to be due to the molting of larvae to higher instars and to pupae at various intervals, which may have obscured the mortality associated with individual instars similar to the effect produced by certain IGRs [28].
Although field trials with larvicides in natural breeding places are important than experiments under laboratory conditions due to the difficulty in simulating all the factors that influence their action in the laboratory [29], trials are an inevitable pre-requisite. This efficacy study demonstrates that Dimilin GR-2% and Mosquiron® 10 EC are effective and persistent in simulation field trials and persisted longer at higher doses. For the products to provide effective control their application should be repeated every 3 - 4 weeks. Earlier studies have demonstrated prolonged efficacy of IGRs on malaria vectors [28]. The WHO interim statement on Larviciding in sub Saharan Africa clearly stipulates that this tool should be deployed as a supplementary intervention and confined to areas where breeding sites are few, fixed and findable [9].
In response to the international calls to protect both human health and the environment from DDT through the United Nation Environment Programme (UNEP), Global Environment Facility (GEF) and WHO, Zambia has halted the use of DDT for IRS [30]. In the quest of deploying non insecticide based interventions for a sustainable environment, Larval source management has been integrated in the national Malaria Control Programme [31,32]. Evaluations for larviciding with Bacillus thuringensis var. israelensis (Bti) and Bacillus sphaericus (Bs) have been conducted both at large scale under a collaborative project between the Ministry of Health and a Cuban company Labiofarm [33], and on a small scale under the auspices of Biovision [34]. Operational deployment of Dimilin GR-2% and Mosquiron® 10 EC as supplementary vector control tools in the context of IVM could be useful in Zambia, particularly in urbanized areas.
5. CONCLUSION
The IGRs, Dimilin GR-2% and Mosquiron® 10 EC demonstrated effective and persistent post-treatment activity both in the laboratory and simulation field assays. They are recommended as ideal larvicides for operational malaria control in the context of the WHO-recommended IVM approach.
6. AUTHORS’ CONTRIBUTION
E. Chanda designed and coordinated the study, participated in data collection, analyzed the data, and drafted the paper. A. Kandyata and J. Chanda assisted with data collection, analysis and interpretation. K.S. Baboo critically reviewed the paper.
7. CONFLICT OF INTEREST
The author of the paper does not have a direct financial relation with the commercial identities mentioned in the paper that might lead to a conflict of interest for any of the authors. The authors declare that they have no competing interests.
8. ACKNOWLEDGEMENTS
The project was financially and materially supported by Amiran (Z) Limited, Plot no 9362, MumbwaRoad, P.O. Box 31744, 10101 Lusaka and Cropserve (Z) Limited, Plot no 5055, Mungwi Road, P/Bag E891, 10101 Lusaka. The authors would also like to thank Dr. Peter Mwaba, the permanent secretary Ministry of Health Zambia, for the support.
REFERENCES
- WHO (2012) World malaria report. Geneva.
- Beier, J.C., Killeen, G.F. and Githure, J.I. (1999) Short report: Entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. American Journal of Tropical Medicine and Hygiene, 61, 109-113.
- Hay, S.I., Rogers, D.J., Toomer, J.F. and Snow, R.W. (2000) Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: Literature survey, Internet access and review. Transactions of the Royal Society of Tropical Medicine and Hygiene, 94, 113-127. doi:10.1016/S0035-9203(00)90246-3
- WHO (2009) World malaria report. Geneva.
- Feachem, R. and Sabot, O. (2008) A new global malaria eradication strategy. Lancet, 371, 1633-1635.
- Hargreaves, K., Koekemoer, L.L., Brooke, B.D., Hunt, R.H., Mthembu, J. and Coetzee, M. (2000) Anopheles funestus resistant to pyrethroid insecticides in South Africa. Medical and Veterinary Entomology, 14, 181-189. doi:10.1046/j.1365-2915.2000.00234.x
- WHO (2004) Global strategic framework for integrated vector management. Geneva.
- WHO (2012) Global plan for insecticide resistance management in malaria vectors (GPIRM). Geneva. http://www.who.int/malaria/vector_control/ivm/gpirm/en/index.html.
- WHO (2012) Interim Position Statement. The role of larviciding for malaria control in sub-Saharan Africa. Geneva.
- Jambulingam, P., Sadanandane, C., Boopathi Doss, P.S., Subramanian, S. and Zaim, M. (2008) Field evaluation of an insect growth regulator, pyriproxyfen 0.5% GR against Culex quinquefasciatus, the vector of Bancroftian filariasis in Pondicherry, India. Acta Tropica, 107, 20-24.
- Fillinger, U. and Lindsay, S.W. (2006) Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya. Tropical Medicine and International Health, 11, 1629-1642. doi:10.1111/j.1365-3156.2006.01733.x
- Siddall, J.B. (1976) Insect growth regulators and insect control: A critical appraisal. Environmental Health Perspectives, 14, 119-126. doi:10.1289/ehp.7614119
- Najera, J.A. and Zaim, M. (2002) Malaria vector control. Decision making criteria and procedures for judicious use of insecticide.
- Singh, H. and Tripathi, V.N. (2003) Field Trial of Relative Efficacy of Abate and Bacillus thuringiensis Against Simulium himalayense Larvae (Diptera simulidae). Medical Journal Armed Forces India, 59, 111-113.
- Thiéry, I., Fouque, F., Gaven, B. and Lagneau, C. (1999) Residual efficacy of B. thuringiensis sub sp. medellin and jegathesan on Culex pipiens and Aedes aegypti larvae. Journal of the American Mosquito Control Association, 15, 371-379.
- Central Statistics Office (2010) National census report. Lusaka.
- WHO (1975) Manual on practical entomology in malaria. Part II: Methods and techniques. Geneva.
- Gillies, M.T. and DeMeillon, B. (1968) The Anophelinae of Africa South of the Sahara.
- Gillies, M.T. and Coetzee, M. (1987) A supplement to: The Anophelinae of Africa South of the Sahara.
- Thavara, U., Tawatsin, A., Chansang, C., Asavadachanukorn, P., Zaim, M. and Mulla, M.S. (2007) Simulated field evaluation of the efficacy of two formulations of diflubenzuron, a chitin synthesis inhibitor against larvae of Aedes aegypti (L.) (diptera: Culicidae) in water-storage containers. Southeast Asian Journal of Tropical Medicine and Public Health, 38, 269-275.
- WHO. Guidelines for laboratory and field testing of mosquito larvicides. Geneva.
- Preacher, K.J. (2001) Calculation for the chi-square test: An interactive calculation tool for chi-square tests of goodness of fit and independence. http://quantpsy.org
- Kitron, U. and Spielman, A. (1989) Suppression of transmission of malaria through source reduction: Anti-anopheline measures applied in Israel, the United States, and Italy. Reviews of Infectious Disease, 11, 391-406. doi:10.1093/clinids/11.3.391
- Sachs, J.D. (2002) A new global effort to control malaria. Science, 298, 122-124. doi:10.1126/science.1077900
- WHO (2006) Malaria control: The power of integrated action. Geneva.
- Townson, H., Nathan, M.B., Zaim, M., et al. (2005) Exploiting the potential of vector control for disease prevention. Bulletin of the World Health Organization, 83, 942- 947.
- Msangi, S., Lyatuu, E. and Kweka, J.E. (2011) Field and laboratory evaluation of bioefficacy of an insect growth regulator (dimilin) as a larvicide against mosquito and housefly larvae. Journal of Tropical Medicine, Article ID: 394541, pp. 1-18.
- Batra, C.P., Mittal, P.K., Adak, T. and Subbarao, S.K.. (2006) Efficacy of agnique(r) MMF monomolecular surface film against Anopheles stephensi breeding in urban habitats in India. Journal of the American Mosquito Control Association, 22, 426-432. doi:10.2987/8756-971X(2006)22[426:EOAMMS]2.0.CO;2
- Kroeger, A., Horstick, O., Ried, P.C., Kaiser, A. and Becker, N. (1995)The potential for malaria control with the biological larvicide Bacillus thuringiensis israelensis (Bti) in Peru and Ecuador. Acta Tropica, 60, 47-57. doi:10.1016/0001-706X(95)00101-J
- Chanda, E., Mukonka, V.M., Kamuliwo, M., Macdonald, M. and Haque, U. (2013) Operational scale entomological intervention for malaria control: Strategies, achievements and challenges in Zambia. Malaria Journal, 12, 10. doi:10.1186/1475-2875-12-10
- Lindsay, S.W., Filinger, U., Majambira, S. and Dechant, P. (2008) Feasibility assessment for integrating larval source management in the national malaria control programme in Zambia. Field Report, Ministry of Health, Lusaka.
- Chanda. E. (2012) Integrating larval source management in the national malaria control programme in Zambia.
- Martinez, A.A., Gutierrez, L.T., Hernandez, S.F., Velazquez, F.S., Robiana, R.A., Rogue, R.R., et al. (2011) Implementing the operational use of the biological larvicides Grselesf and Bactivec as a new intervention to reduce the incidence of malaria and other mosquito borne diseases in Zambia. Field Report, Ministry of Health, Lusaka.
- Chanda, E., Zulu, R., Chanda, J., Chabu, I.E., Phiri, T.N. and Kandyata, A. (2011) Monitoring the operational impact of larval source management on malaria in Lusaka and Chongwe districts in Zambia. Field Report, Ministry of Health Report, Lusaka.
NOTES
*Corresponding author.

