Journal of Biomedical Science and Engineering
Vol.5 No.5(2012), Article ID:19409,8 pages DOI:10.4236/jbise.2012.55032
Cellular viability and nitric oxide (NO) production by J774 macrophages in the presence of orthodontic archwires
![]()
1Juiz de Fora Federal University, Juiz de Fora, Brazil
2Institute of Biomedical Sciences, Uberlândia Federal University, Uberlândia, Brazil
3Department of Orthodontics and Pediatrics, Juiz de Fora Federal University, Juiz de Fora, Brazil
4Department of Statistic, Juiz de Fora Federal University, Juiz de Fora, Brazil
5Department of Parasitology, Microbiology and Immunology, Juiz de Fora Federal University, Juiz de Fora, Brazil
Email: *carlostoledo1@hotmail.com
Received 17 January 2012; revised 12 February 2011; accepted 21 March 2012
Keywords: orthodontic wires; cell culture techniques; cell survival; nitric oxid
ABSTRACT
To assess, in vitro, the cellular viability in a murine macrophage cell line J774 with 9 different orthodontic wires and to evaluate the effects of its NO production. To assess cellular viability by MTT: 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide assay in the cell line J774 with 9 different orthodontic wires and quantify NO production by these macrophages. Cell cultures were evaluated at 24, 48 and 72 hours. There was no significant difference of the means of cellular viability between the control and the group of wires in the respective time intervals. In the comparison with the control group, there was significant difference in the NO production in groups 1, 6, and 9 at 24 hours interval. Group 8 showed significant difference in relation to the control group at final time interval. Cellular viability in all groups was higher at the final time interval than at the initial time interval. This increase was significant in the control group. In the material groups, the final mean of cellular viability at 72 hours showed no significant difference when compared with the control group. NO production in all groups was higher at the final time interval than at the initial time interval. This increase was significant in the control group. In the material groups, the final mean of NO production at 72 hours was only significant in group 8 (beta-titanium) when compared with the control group.
1. INTRODUCTION
As in many other fields of biotechnology, dentistry materials face the challenge of the absence of biocompatibility, i.e. the lack of the coexistence of manufactured materials with body tissues and fluids, which should remain in the organism for varied periods of time without harming the soft tissues. Very few dentistry materials, maybe even none, are totally inert from the biological point of view because they have a variety of components with toxic and irritative potentiality [1,2]. Biocompatibility is characterized by the capacity of the materials to not cause toxic, irritative, inflammatory, allergic, mutagenic or carcinogenic effects in tissues [3-6].
The development of biocompatible materials has been one of the greatest challenges in the health field. One of the main parameters for the evaluation of biological response and damage or cell death potential are the cytotoxicity tests, which are the first trial tests used for almost all the materials [7].
The oral environment is favorable for the colonization of a broad spectrum of microorganisms. The placement of orthodontic appliances creates favorable conditions for the accumulation of food residues and microorganisms, which may cause caries and exacerbate any preexisting periodontal disease. Besides the presence of different cytokines, nitric oxide (NO) production has been observed in the microenvironment of the periodontal disease, which acts directly in the maintenance of inflamemation, as well as in the tissue destruction [8-10].
Once the oral environment is particularly ideal for the biodegradation of metals because of its ionic, thermal, microbiologic and enzymatic properties, the patient is likely to be exposed to products of the corrosion process. Previous studies have shown that metallic ions may be released from metallic materials as a result of corrosion [11-15].
Several studies on biocompatibility of the components of orthodontic appliances used different cell lineages: human gingival fibroblasts [16,17], human osteoblasts, fibroblasts and keratinocytes [18], J774 murine macrophages [19,20].
Locci et al. (2000) evaluated, in vitro, the effect of the components of orthodontic appliances exposed and not exposed to the corrosion phenomenon on some cell functions. They concluded that the archwire was the most biocompatible component, and the bracket was the least biocompatible. Sestini et al. (2006) assessed the cytotoxicity of two types of orthodontic wires with different concentrations of nickel and manganese. The results showed that the wires were not toxic to the cells.
Noble et al., in 2008 [21], stated that nickel is a common component in many orthodontic materials and that an allergy to nickel is commonly seen in the population, more frequently in women. Other authors [22-26] reported evidences that the oral exposure to nickel may induce immunological tolerance to it, and thereby reducing the incidence of allergies.
The International Organization for Standardization and the Council on Dental Materials, Instruments and Equipment of the American Dental Association have recommended batteries of in-vitro and in-vivo tests to study the biocompatibility of materials, and, according to the standards, the major tests for the initial evaluation of materials are cellular viability tests. Among these tests, the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma, St. Louis, Mo) assay is an important test to determine the cytotoxicity of materials of different origins on cell cultures [27].
Cytotoxicity might also be related to molecules released by a certain stimulus and cause tissue alteration. Studies have reported a potentially cytotoxic molecule, nitric oxide (NO), in the tissues of the oral cavity and its involvement in inflammatory diseases [28-30]. NO is a regulatory molecule, primarily produced by activated macrophages and is of paramount importance in the processes of immune response, inflammation, osseous metabolism, and apoptosis. This gas appears to be beneficial as well as detrimental. Beneficial effects can include antimicrobial activity and immune modulation. Its detrimental effects are cytotoxic actions toward adjacent host tissues, including alveolar bone [31-33].
The aims of this study were to assess, in vitro, the cellular viability with the MTT assay in a murine macrophage cell line J774 with 9 different orthodontic wires and also their effects in the production of NO by this macrophage line.
2. MATERIAL AND METHODS
The study sample consisted of three 2-mm-long wire sections of 9 different types of orthodontic rectangular (0.017” × 0.025”) archwires (Table 1) for each time interval (24, 48, and 72 hours). They were used as bought commercially and were sterilized with ethylene oxide.
It was used the murine cell line J774 A.1 (ATCC no. TIB-67, American Type Culture Collection, Manassas, Va), which was placed in a plastic bottle with a supplemented culture medium (5% bovine fetal serum, 50 IU per milliliter of penicillin, 1% nonessential amino acid, and 2% L-glutamine (Gibco, Grand Island, NY)), and incubated at 37˚C in 5% carbon dioxide. After transfer to an adequate container, the cells were washed with centrifugation at 1200 rpm, for 10 minutes at 4˚C.
For determination of viability and cell count, Trypan blue stain was used in a 1:1 proportion (stain: culture
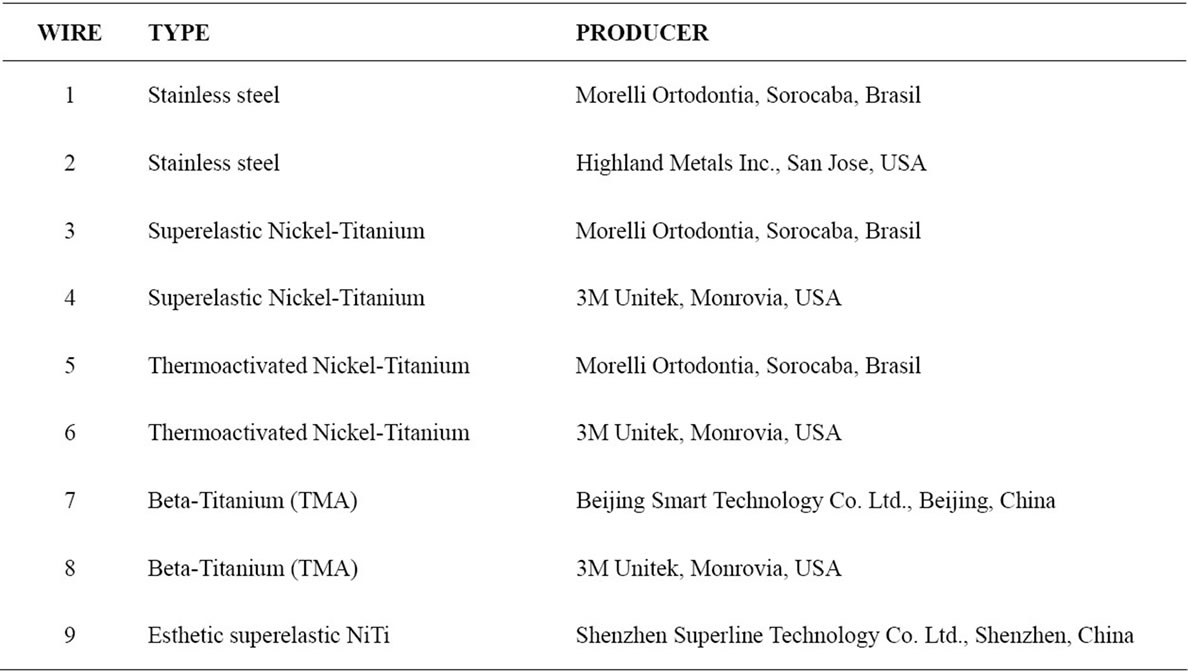
Table 1. Types of orthodontic archwires used in the sample.
medium) in the Newbauer chamber [34].
Culture plates (96 wells) were seeded with 2 × 104 J774 cells per well, in a volume of 100 μL, resuspended in culture medium supplemented with Roswell Park Memorial Institute Supplemented Medium 1640 (RPMI). The wire sections were placed on the cells and kept in the culture for 3 time intervals (24, 48, and 72 hours) in 5% carbon dioxide at 37˚C. After each incubation period, supernatant was collected for posterior NO quantification, and the cells were evaluated for cytotoxicity after wire removal. The control group consisted of J774 murine macrophages, which were seeded in 96 well plates without any materials.
After supernatant removal, to the wires and to the cells that remained in the wells of the plate, 90 μL of RPMIsupplemented medium and 10 μL of MTT solution (50 mg/mL) were added. Cells were incubated for 4 hours in a carbon dioxide oven at 37˚C. After the incubation period, MTT reaction was blocked with 100 μL per well of alcohol-acid solution incubated for 10 minutes at room temperature, and the reading was done at 540 nm with a microplate reader (Spectramax 190-Molecular Device, Sunnyvale, Calif) [34,35]. The analyses of cellular viability were done with the wires in the culture wells, because removing the wires would lead to the loss of cells and lesions of the remaining cells, interfering with the results.
To evaluate NO production, 100 μL of supernatant from each well of the culture plate was transferred to 96 new well plates. The same amount of Griess reagent (1% sulfanilamide, 0.1% naphthylenediamine dihydrochloride, and 2.5% phosphoric acid) was added to the supernatant [36,37]. Nitrite concentrations in the supernatants were obtained by linear regression analysis of the standard curve by using serial double dilutions of sodium nitrite from 200 μmol/L to the eleventh dilution. Absorbance was determined at 540 nm by using the microplate reader.
The statistical analysis was performed with MannWithney test (comparison of the means of each group of wire with the control group in each time interval) e Friedman and Wilcoxon tests (analysis of the variance of means for each material in the time intervals 24 hours, 48 hours and 72 hours).
3. RESULTS
Table 2 gives the means and standard deviations for the analysis of cellular viability for each material at 24, 48 and 72 hours, the P values of the Mann-Whitney test for the comparison of each material with the control group, and the P values of the Friedman and the Wilcoxon tests for the comparison of cellular viability between 24 hours and 48 hours, 24 hours and 72 hours, and 48 hours and 72 hours for each group.
In the analysis of the difference between the time intervals within each group, the control group showed p < 0.05 in the time intervals 24 - 48 hours and 24 - 72 hours. The groups of wires did not show statistically significant differences between the time intervals studied.
There was no statistically significant difference of the means of cellular viability between the control group and the group of wires in the respective time intervals (24 hours, 48 hours and 72 hours).
Table 3 gives the means and standard deviations for the analysis of NO production for each material at 24, 48 and 72 hours, the P values of the Mann-Whitney test for the comparison of each material with the control group, and the P values of the Friedman and the Wilcoxon tests for the comparison of NO production between 24 hours and 48 hours, 24 hours and 72 hours, and 48 hours and 72 hours for each group.
In the analysis of the difference between the time intervals within each group, the control group showed p < 0.05 in the time intervals 24 - 48 hours and 24 - 72 hours. The groups of wires did not show statistically significant differences between the time intervals studied.
In the comparison with the control group, there was statistically significant difference in the NO production in groups 1, 6 and 9 at 24 hours interval. Group 8 showed statistically significant difference in relation to the control group at final time interval (72 hours).
4. DISCUSSION
The first step toward ensuring the biocompatibility of a material to be used in humans is to test its cytotoxicity, which can be evaluated by means of three types of tests: in vitro, using cell cultures, in vivo, using animal experiments, and through clinical tests. A negative result in an in vitro test suggests that the material is free from releasing harmful products or that the amount of such products is insufficient to cause acute effects in isolated cell groups. However, it must be clear that tests of cytotoxicity constitute the first step in the analysis of the material. Conversely, a positive test of cytotoxicity may be indicative that the material contains substances which are released and capable of causing damage or interfere with the cell metabolism [38,39].
In addition to traditional cytotoxicity tests, the study of NO production relative to a specific material is another way to determine the toxicity potential of a material, because cytotoxicity might be related to molecules, which, after being released in response to a certain stimulus, can cause damage to the tissues [30].
In the analysis of cellular viability the means of the control group increased from 24 hours to 48 hours and from 48 hours to 72 hours, being the increase statistically significant in the time intervals 24 - 48 hours and 24 - 72 hours. With the exception of group 7, which presented a
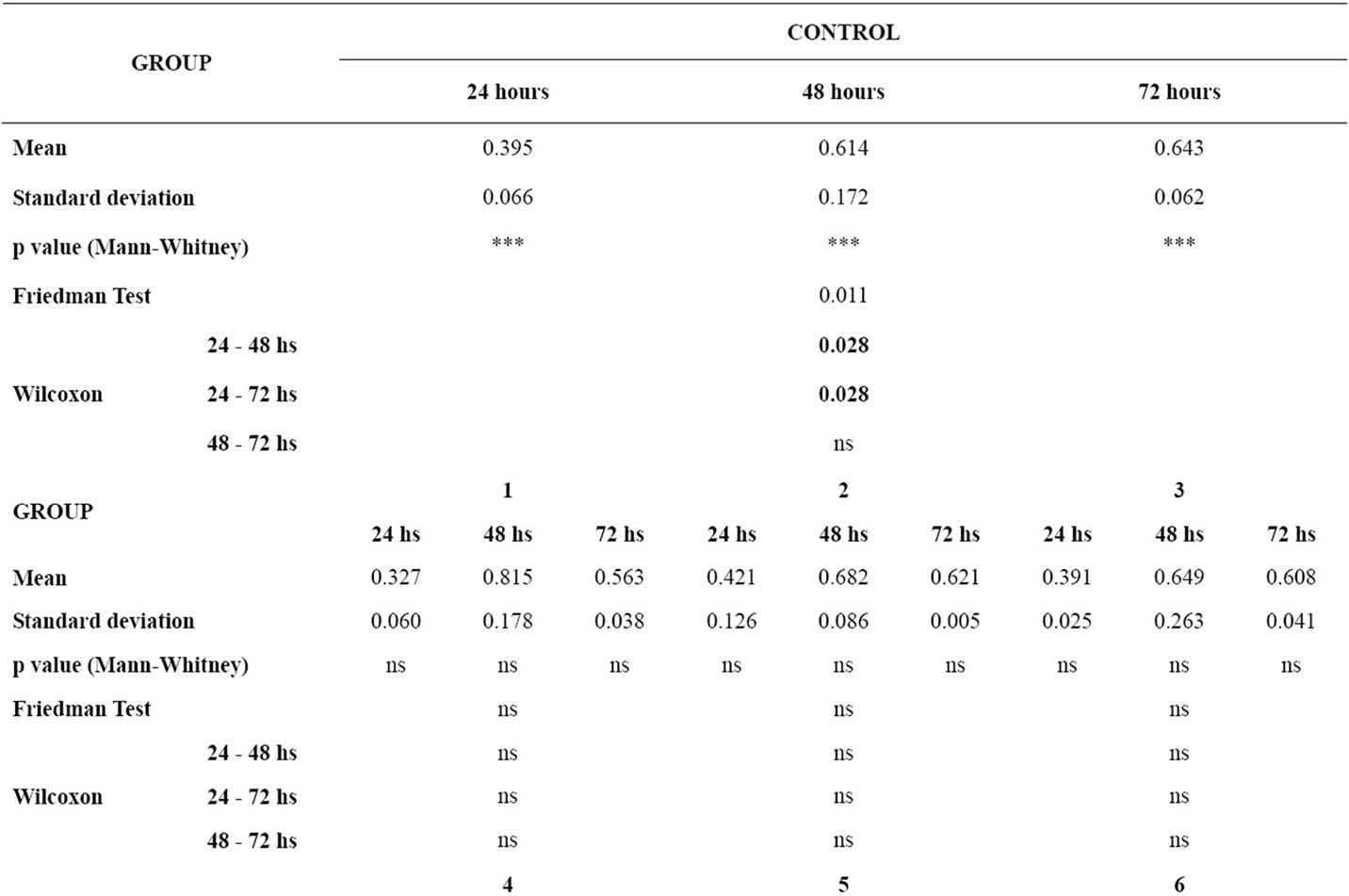
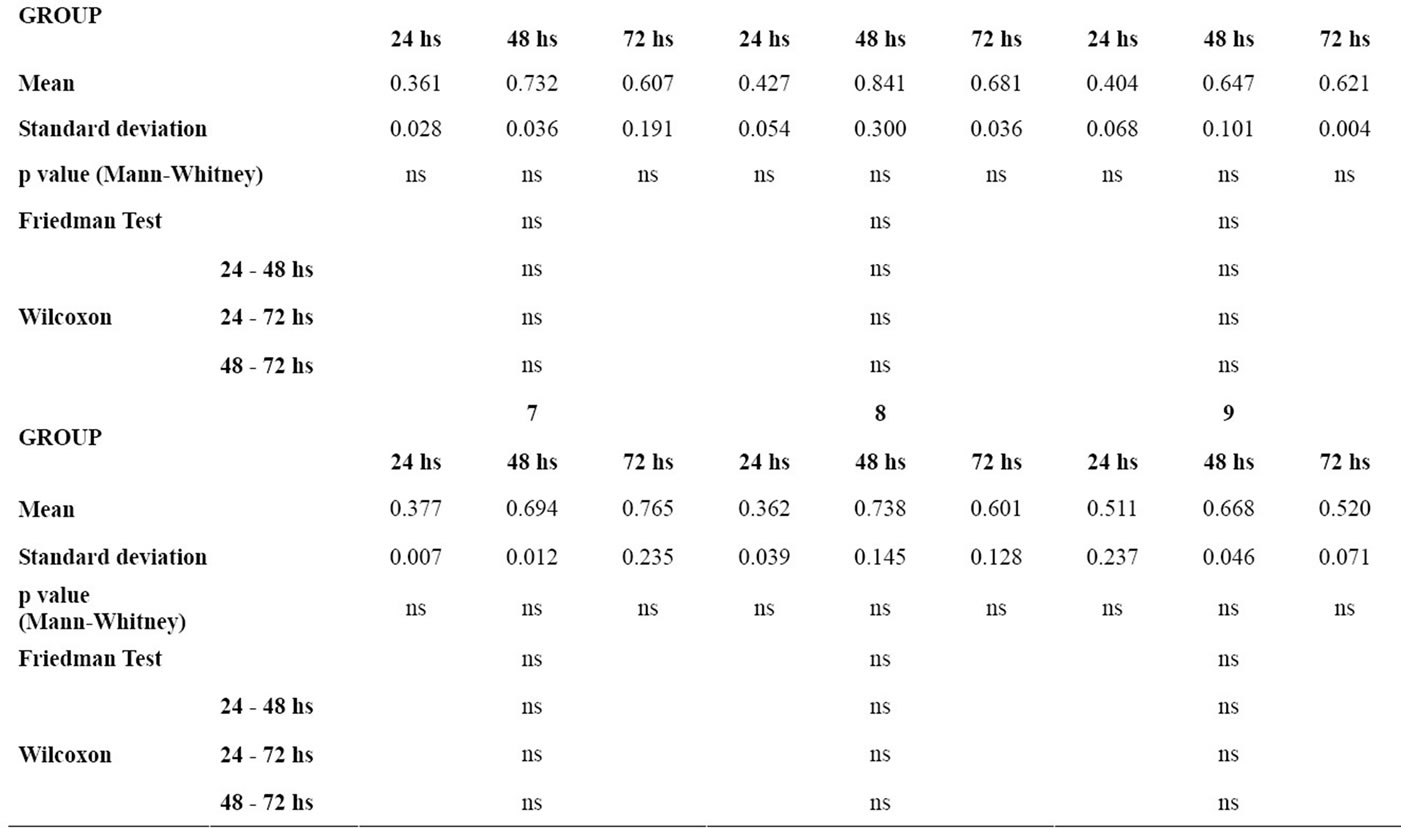
Table 2. p value < 0.05, Mean, standard deviation, p value of the Mann-Whitney test for the comparison of cellular viability of the murine macrophages for each material in relation to the control group in each time interval; p value of the Friedman and the Wilcoxon tests for the comparison of cellular viability of the murine macrophages between the time intervals for each group (ns = non significant).
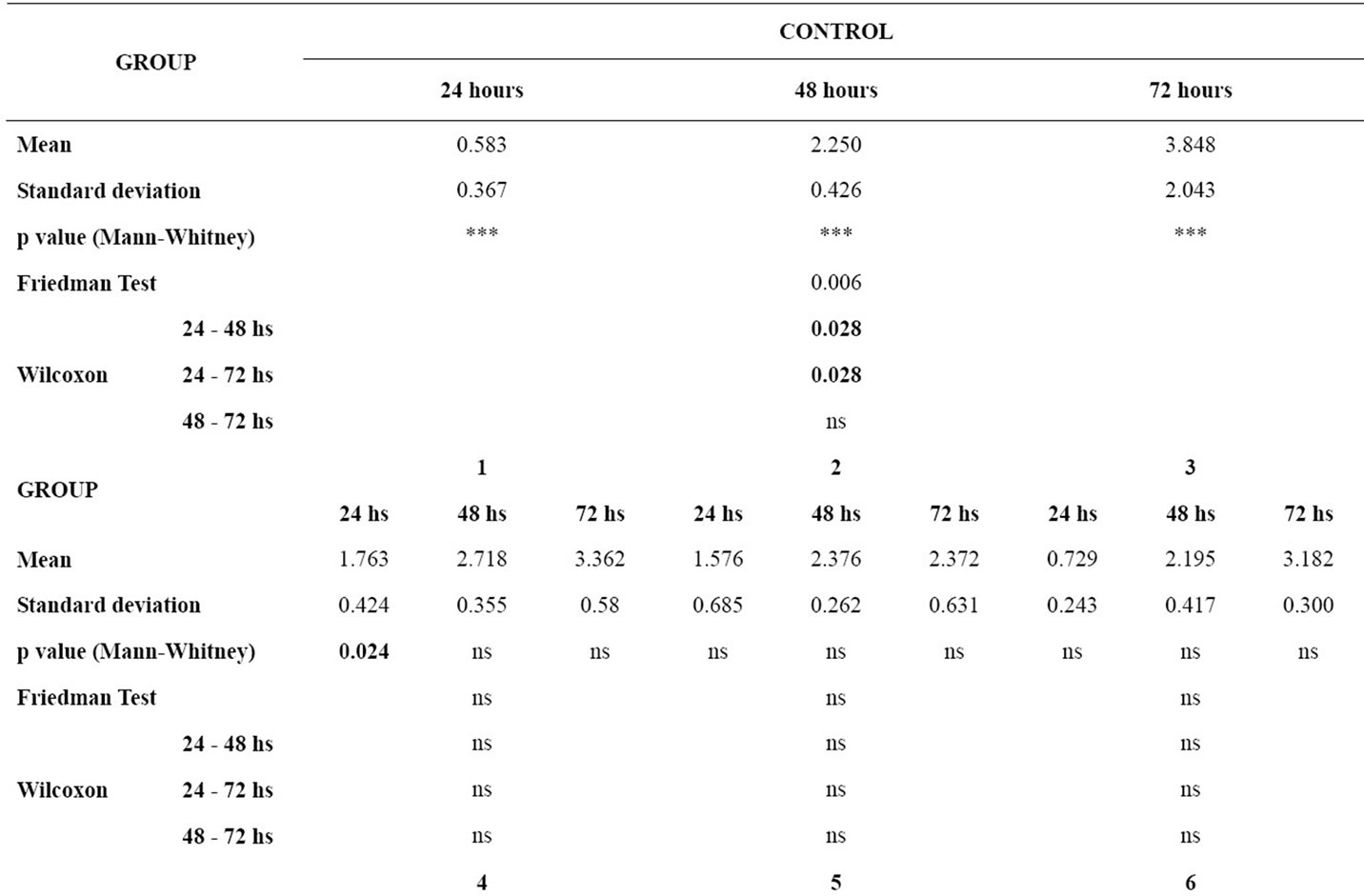
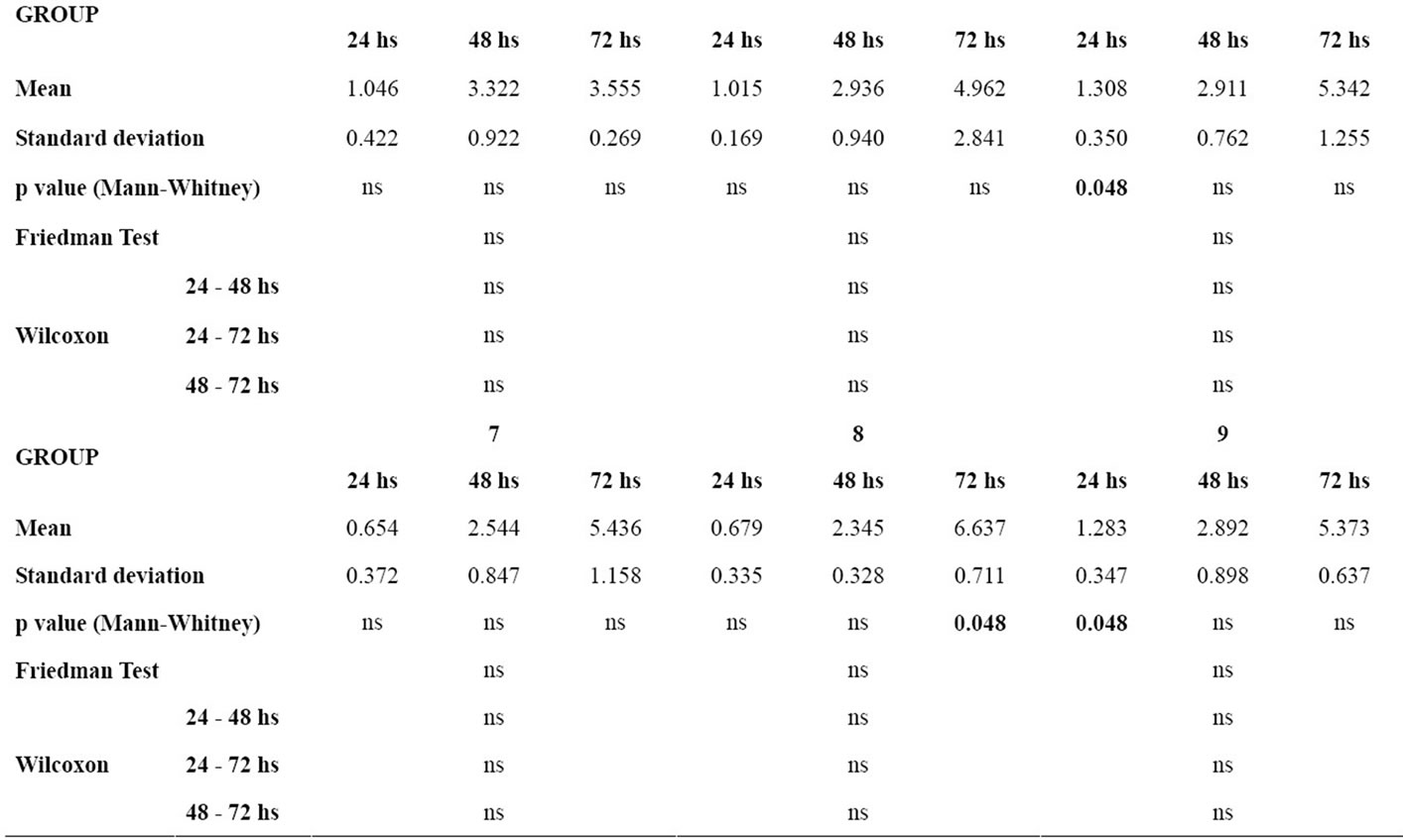
Table 3. p value < 0.05, Mean, standard deviation, p value of the Mann-Whitney test for the comparison of nitric oxide production by the murine macrophages for each material in relation to the control group in each time interval; p value of the Friedman and the Wilcoxon tests for the comparison of nitric oxide production by the murine macrophages between the time intervals for each group (ns = non significant).
similar behavior to that of the control group, the means of cellular viability for the other groups showed an increase from 24 hours to 48 hours and a decrease from 48 hours to 72 hours. However, in none of the groups were these variations statistically significant.
It was also noted that the means of cellular viability of the material groups in each of the time intervals did not show statistically significant differences when compared to the control group.
In the control group only the cells were present in the culture media, allowing cellular proliferation to occur progressively in the time intervals studied, what could be observed through the means shown by the group and the significant increase of such means in the time intervals evaluated. The introduction of the wire sections in the culture media may induce greater cellular proliferation in that the J774 macrophages are cells of immune response. Therefore, it was observed that the means of all groups at the 48 hour interval were greater than that of the control group. However, at 72 hours, there was a decrease in the mean of cellular viability but in none of the groups was this viability, in the final time interval, significantly different from the control group.
Thus, by the results presented, none of the orthodontic archwire groups was capable of affecting significantly cellular proliferation, being hereby considered no cytotoxic in this study.
According to Abbas, Lichtman and Pillai (2007) [40] NO production can be considered the result of normal physiology of cells such as macrophages and could be affected by imune modulatory substances that might increase or decrease the production of these molecules. NO has emerged as a major modulator of cellular function in health and disease and can have both cytotoxic and cytoprotective effects [41].
In the analysis of NO production the means of the control group increased from 24 hours to 48 hours and from 48 hours to 72 hours, being this increase statisticcally significant in the time intervals 24 - 48 hours and 24 - 72 hours. With the exception of group 2, in which there was a decrease in the mean from 48 hours to 72 hours, all the other groups presented similar behavior to that of the control group. However, in none of the groups were these variations statistically significant.
At 24 hours, NO production was higher in all groups of wires when compared with the control group. However, a statistically significant difference was noted only in groups 1, 6 and 9. The contact between the material and the cell might stimulate greater NO production, since the activated macrophages are the primary producers of this molecule of major importance in immune responses and inflammatory processes (33). At 48 hours, the means of NO production did not show statistically significant difference in any of the groups. In the final time interval (72 hours), group 8 (beta-Titanium—Titanium Molybdenum Alloy) showed significant difference in relation to the control group (p = 0.048).
Both in the analysis of the means of cellular viability and in the analysis of NO production it could be observed that, in the final time interval, only the mean of NO production in the beta-titanium group (Titanium Molybdenum Alloy) was statistically significant in relation to the control group. In the other groups differences were not evident in cellular viability, nor were in the NO production in this time interval. Other studies on cytotoxicity of orthodontic materials demonstrated that the archwire was the least cytotoxic component of the orthodontic appliance [17,18].
In the orthodontic literature, it can be found reports of allergic reactions to nickel-titanium archwires owing to the presence of Nickel, Cobalt and Chrome [42]. However, the Niti wires evaluated in this study did not show any significant action on cellular proliferation or NO production at final time of evaluation (72 hours). As for the beta-titanium (Titanium Molybdenum Alloy) archwires, which contain in their composition mainly Titanium and Molybdenum, no cytotoxic reactions have been reported. Titanium is used in dental implants because of its capacity for osseointegration.
The best assessment method for analyzing the problem of corrosion in the oral medium, which releases harmful substances to the tissues [1,12-15], is the in vivo study. Cytotoxicity tests, in which the cells remain in contact with the wire sections for up to 72 hours in the culture medium, do not provide sufficient time for the process of corrosion to occur. The mechanical action of mastication as well as the presence of microorganisms may be factors that, by interacting with the orthodontic wires, lead to reactions in which the final product may be harmful to the surrounding tissues.
5. CONCLUSIONS
1) Cellular viability in all groups was higher at the final time interval than at the initial time interval. This increase was statistically significant in the control group.
2) In the material groups, the final mean of cellular viability at 72 hours showed no statistically significant difference when compared with the control group.
3) NO production in all groups was higher at the final time interval than at the initial time interval. This increase was statistically significant in the control group.
4) In the material groups, the final mean of NO production at 72 hours was only significant in group 8 (betatitanium-Titanium Molybdenum Alloy) when compared with the control group.
![]()
![]()
REFERENCES
- Phillips, R.W. (1993) Materiais dentários. 9th Edition, Editora Guanabara Koogan, Rio de Janeiro.
- Tsuchiya, T., Ikarashi, Y., Arai, T., Ohhashi, J. and Nakamura, A. (1994) Improved sensitivity and decreased sample size in a cytotoxicity test for biomaterials: A modified colony microassay using a microplate and crystal violet staining. Journal of Applied Biomaterials, 5, 361-367. doi:10.1002/jab.770050412
- Arvidson, K., Cottler-Fox, M., Hammarlund, E. and Friberg, U. (1987) Cytotoxic effects of cobalt-chromium alloys on fibroblasts derived from human gingival. Scandinavian Journal of Dental Research, 95, 356-363.
- Jacobsen, N. and Hensten-Pettersen, A. (1989) Occupational health problems and adverse patient reactions in orthodontics. European Journal of Orthodontics, 11, 254-264.
- Vahey, J.W., Simonian, P.T. and Conrad 3rd, E.U. (1995) Carcinogenicity and metallic implants. American Journal of Orthopedics, 24, 319-324.
- Sun, Z.L., Wataha, J.C. and Hanks, C.T. (1997) Effects of metal ions on osteoblast-like cell metabolism and differentiation. Journal of Biomedical Materials Research, 34, 29-37. doi:10.1002/(SICI)1097-4636(199701)34:1<29::AID-JBM5>3.0.CO;2-P
- Anusavice, K.J. (2003) Phillips’ science of dental materials. 11th Edition, WB Saunders, Philadelphia.
- Grimsdottir, M.R. and Hensten-Pettersen, A. (1993) Cytotoxic and antibacterial effects of orthodontic appliances. Scandinavian Journal of Dental Research, 101, 229-231.
- Batista, A.C. (2001) Avaliação da expressão da enzima óxido nítrico-sintase induzível (iNOS) em gengivites associadas à placa bacteriana e periodontites crônicas localizadas. Master’s Thesis, USP, Bauru.
- Anhoury, P., Nathanson, D., Hughes, C.V., Socransky, S., Feres, M. and Chou, L.L. (2002) Microbial profile on metallic and ceramic bracket materials. Angle Orthodontist, 72, 338-343.
- Dobbs, H.S. and Minski, M.J. (1980) Metal ion release after total hip replacement. Biomaterials, 1, 193-198. doi:10.1016/0142-9612(80)90016-2
- Barret, R.D., Bishara, S.E. and Quinn, J.K. (1993) Biodegradation of orthodontic appliances. Part I. Biodegradation of nickel and chromium in vitro. American Journal of Orthodontics & Dentofacial Orthopedics, 103, 8-14. doi:10.1016/0889-5406(93)70098-9
- Grímsdóttir, M.R., Hensten-Pettersen, A. and Kullmann, A. (1994) Proliferation of nickel-sensitive human lymphocytes by corrosion products of orthodontic appliances. Biomaterials, 15, 1157-1160. doi:10.1016/0142-9612(94)90236-4
- Wataha, J.C., Malcolm, C.T. and Hanks, C.T. (1995) Correlation between cytotoxicity and the elements released by dental casting alloys. International Journal of Prosthodontics, 8, 9-14.
- Staffolani, N., Damiani, F., Lilli, C., Guerra, M., Staffolani, N.J., Belcastro, S. and Locci, P. (1999) Ion release from orthodontic appliances. Journal of Dentistry, 27, 449-454. doi:10.1016/S0300-5712(98)00073-6
- Eliades, T., Lekka, M., Eliades, G. and Brantley, W.A. (1994) Surface characterization of ceramic brackets: A multitechnique approach. American Journal of Orthodontics & Dentofacial Orthopedics, 105, 10-18. doi:10.1016/S0889-5406(94)70094-X
- Locci, P., Marinucci, L., Lilli, C., Belcastro, S., Staffolani, N., Bellocchio, S., Damiani, F. and Becchetti, E. C. (2000) Biocompatibility of alloys used in orthodontics evaluated by cell culture tests. Journal of Biomedical Materials Research, 51, 561-568. doi:10.1002/1097-4636(20000915)51:4<561::AID-JBM3>3.0.CO;2-H
- Sestini, S., Notarantonio, L., Cerboni, B., Alessandrini, C., Fimiani, M., Nannelli, P., Pelagalli, A. and Giorgetti, R. (2006) In vitro toxicity evaluation of silver soldering, electrical resistance, and laser welding of orthodontic wires. European Journal of Orthodontics, 28, 567-572. doi:10.1093/ejo/cjl048
- Vitral, J.C., Fraga, M.R., de Souza, M.A., Ferreira, A.P. and Vitral, R.W. (2010) In-vitro study of the cellular viability and nitric oxide production by J774 macrophages with ceramic, polycarbonate, and polyoxymethylene brackets. American Journal of Orthodontics & Dentofacial Orthopedics, 137, 247-253. doi:10.1016/j.ajodo.2008.03.028
- Vitral, J.C., Fraga, M.R., de Souza, M.A., Ferreira, A.P. and Vitral, R.W. (2010) In-vitro study of cellular viability and nitric oxide production by J774 macrophages stimulated by interferon gamma with ceramic, polycarbonate, and polyoxymethylene brackets. American Journal of Orthodontics & Dentofacial Orthopedics, 137, 665-670. doi:10.1016/j.ajodo.2008.07.017
- Noble, J., Ahing, S.I., Karaiskos, N.E. and Wiltshire, W.A. (2008) Nickel allergy and orthodontics, a review and report of two cases. British Dental Journal, 204, 297-300. doi:10.1038/bdj.2008.198
- Vreeburg, K.J., de Groot, K., Von Blomberg, M. and Scheper, R.J. (1984) Induction of immunological tolerance by oral administration of nickel and chromium. Journal of Dental Research, 63, 124-128. doi:10.1177/00220345840630020501
- Van Hoogstraten, I.M., Andersen, K.E., Von Blomberg, B.M., Boden, D., Bruynzeel, D.P., Burrows, D., Camarasa, J.G., Dooms-Goossens, A., Kraal, G., Lahti, A., et al. (1991) Reduced frequency of nickel allergy upon oral nickel contact at an early age. Clinical & Experimental Immunology, 85, 441-445. doi:10.1111/j.1365-2249.1991.tb05746.x
- Van Hoogstraten, I.M., Boos, C., Boden, D., Von Blomberg, M.E., Scheper, R.J. and Kraal, G. (1993) Oral induction of tolerance to nickel sensitization in mice. Journal of Investigative Dermatology, 101, 26-31. doi:10.1111/1523-1747.ep12358502
- Artik, S., Haarhuis, K., Wu, X., Begerow, J. and Gleichmann, E. (2001) Tolerance to nickel: Oral nickel administration induces a high frequency of anergic T cells with persistent suppressor activity. Journal of Immunology, 167, 6794-6803.
- Mortz, C.G., Lauritsen, J.M., Bindslev-Jensen, C. and Andersen, K.E. (2002) Nickel sensitization in adolescents and asociation with ear piercing, use of dental braces and hand eczema. Acta Dermato-Venereologica, 82, 359-364. doi:10.1080/000155502320624096
- Hanks, C.T., Wataha, J.C. and Sun, Z. (1996) In vitro models of biocompatibility: A review. Dental Materials, 12, 186-193. doi:10.1016/S0109-5641(96)80020-0
- Lohinai, Z.M. and Szabo, C. (1998) Role of nitric oxide in physiology and patophysioloy of periodontal tissues. Medical Science Monitor, 4, 1089-1095.
- Matejka, M., Partyka, L., Ulm, C., Solar, P. and Sinzinger, H. (1998) Nitric oxide synthesis is increased in periodontal disease. Journal of Periodontal Research, 33, 517-518. doi:10.1111/j.1600-0765.1998.tb02352.x
- Batista, A.C., Silva, T.A., Chun, J.H. and Lara, V.S. (2002) Nitric oxide synthesis and severity of human periodontal disease. Oral Diseases, 8, 254-260. doi:10.1034/j.1601-0825.2002.02852.x
- Moncada, S., Palmer, R. M. and Higgs, E. A. (1991) Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacological Reviews, 43, 109-142.
- Kröncke, K.D., Fehsel, K. and Kolb-Bachofen V., (1997) Nitric oxide: Cytotoxicity versus cytoprotection—How, why, when, and where? Nitric Oxide, 1, 107-120. doi:10.1006/niox.1997.0118
- Kendall, H.K., Marshall, R.I. and Bartold, P.M. (2001) Nitric oxide and tissue destruction. Oral Disease, 7, 2-10. doi:10.1034/j.1601-0825.2001.70102.x
- Wilson, A.P. (2000) Cytotoxicity and viability assays. In: Masters, J.R.W., Ed., Animal Cell Culture, Oxford University Press, New York, 175-219.
- Mosmann, T. (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65, 55-63. doi:10.1016/0022-1759(83)90303-4
- Green, L.C., Wagner, D.A., Glogowski, J., Skipper, P.L., Wishnok, J.S. and Tannenbaum, S.R. (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry, 126, 131-138. doi:10.1016/0003-2697(82)90118-X
- Yao, D., Vlessidis, A.G. and Evmiridis, N.P. (2004) Determination of nitric oxide in biological samples. Microchimca Acta, 147, 1-20. doi:10.1007/s00604-004-0212-8
- Wataha, J.C. (2001) Principles of biocompatibility for dental practitioners. Journal of Prosthetic Dentistry, 86, 203-209. doi:10.1067/mpr.2001.117056
- Schmalz, G. (2002) Materials science: Biological aspects. Journal of Dental Research, 81, 660-663. doi:10.1177/154405910208101001
- Abbas, A.K., Lichtman, A.H. and Pillai, S. (2007) Cellular and Molecular Immunology. 6th Edition, WB Saunders, Philadelphia.
- Wiley, J.W. (2007) The many faces of nitric oxide: Cytotoxic, cytoprotective or both. Neurogastroenterology & Motility, 19, 541-544. doi:10.1111/j.1365-2982.2007.00958.x
- Rose, E.C., Jonas, I.E. and Kappert, H.F. (1998) In vitro investigation into the biological assessment of orthodontic wires. Journal of Orofacial Orthopedics/Fortschritte der Kieferorthopädie, 59, 253-264. doi:10.1007/BF01321792

