Journal of Cancer Therapy
Vol.07 No.07(2016), Article ID:67892,9 pages
10.4236/jct.2016.77049
Outcome of Combination Chemotherapy with Docetaxel, Estramustine Phosphate, and Carboplatin after Docetaxel and Prednisolone Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer
Ryuichi Ito, Shintaro Narita*, Hiroshi Tsuruta, Kazuyuki Numakura, Atsushi Maeno, Mitsuru Saito, Takamitsu Inoue, Norihiko Tsuchiya, Shigeru Satoh, Tomonori Habuchi
Department of Urology, Akita University School of Medicine, Akita, Japan

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 30 May 2016; accepted 27 June 2016; published 30 June 2016
ABSTRACT
We retrospectively reviewed the outcome of docetaxel, EMP, and carboplatin (DEC) as second-line chemotherapy in castration-resistant prostate cancer (CRPC) patients previously treated with docetaxel-prednisolone (DP). Nineteen patients pretreated with DP received a DEC regimen which consisted of a 28-day cycle of docetaxel [60 mg/m2 intravenously (IV) on day 1)], carboplatin (IV to an area under the curve of 5 on day 1), and EMP (560 mg orally daily). The DEC therapy was continued intermittently after two consecutive courses. End points were DEC effect on prostate-specific antigen (PSA), radiographic response, progression-free survival (PFS), and overall survival (OS). All patients received DP before DEC administration with a median of 6 cycles (range, 1 - 12). Mean follow-up duration was 19.0 months after starting DEC therapy; median total number of the therapy cycles was 2 (range, 1 - 11). Thirteen patients (68.4%) showed a PSA decrease; 6 (31.6%) showed a decrease in the PSA level of ≥50%, including 4 with no PSA response to DP. Grade 3/4 neutropenia and febrile neutropenia were observed in 13 (68.4%) and 2 (10.5%) patients, respectively. The median PFS following DEC was 3.7 months. The median OS was 18.0 months. In univariate analyses, patients with ≤12 months from CRPC to DEC had shorter PFS and OS, whereas PSA response to DP was not associated with PFS or OS in CRPC patients treated with DEC after DP. In conclusion, DEC retains some clinical benefits for CRPC patients pretreated with DP, even in patients without any response to DP. Therefore, they may be an effective and feasible treatment option for CRPC patients after first-line docetaxel therapy, particularly for those deemed unfit for novel endocrine and chemotherapeutic drugs.
Keywords:
Chemotherapy, Carboplatin, Docetaxel, Estramustine Phosphate, Prostate Cancer

1. Introduction
Prostate cancer is the second leading cause of cancer mortality among men worldwide [1] . While it initially responds to androgen deprivation therapy, it eventually becomes castration-resistant in most patients. Docetaxel has been a standard first-line treatment in patients with castration-resistant prostate cancer (CRPC) for almost a decade; however, novel drugs have now become available, including enzalutamide, abiraterone acetate, and cabazitaxel, which can be used in pre- and/or post-docetaxel settings [2] . Several studies have indicated that the clinical cross-resistances between docetaxel and novel androgen receptor (AR) signaling-targeted drugs existed [3] [4] . Furthermore, a novel chemotherapy agent cabazitaxel, which has been suggested to have a little cross resistance to new AR signaling-targeted agents, is reported to be accompanied by severe toxicities in a substantial number of patients, particularly in Asian patients [5] [6] .
Platinum compounds, which have historically been believed to have only modest activity for prostate cancer in monotherapy [7] , have also been shown to be effective when combined with taxanes and estramustine phosphate (EMP) in phase I - II studies [8] - [11] . In its pooled analysis, the response of prostate-specific antigen (PSA) levels and 12-month survival estimate were reported to be 69% and 79%, respectively [12] . We previously demonstrated that intermittent chemotherapy with docetaxel, EMP, and carboplatin (DEC) was a feasible option for CRPC based on the outcome with a decrease in the PSA level by ≥30% with 65.7% of patients and the median overall survival (OS) of 17.8 months [13] . These studies only assessed the outcomes for taxanes, EMP, and carboplatin (TEC) in first-line settings; therefore, the efficacy and feasibility of TEC as second-line chemotherapy in patients with CRPC who previously received docetaxel remain unknown.
In this study, we retrospectively assessed the outcome of combination therapy with DEC as second-line chemotherapy in patients with CRPC who were previously treated with docetaxel-prednisolone (DP) therapy.
2. Patients and Methods
2.1. Patients
We included all patients with confirmed CRPC who were previously treated with DP therapy. The definition of CRPC was histologically confirmed adenocarcinoma of the prostate with PSA or radiographic progression despite surgical or medical castration, with a serum testosterone level of 50 ng/dL or less. All patients were pretreated with a 28-day cycle of DP therapy, which consisted of docetaxel (70 mg/m2 on day 1) and oral prednisolone (10 mg/day) in more than 1 cycle. The treatment of DP therapy was applied as an intermittent protocol using our original regimen. Briefly, three consecutive administrations of docetaxel in a 28-day cycle were defined as one course of the DP therapy. If the patient achieved PSA or clinical response during a course of therapy, a treatment holiday was taken until the patient’s PSA level returned to the baseline. The DP therapy was intermittently continued until treatment failure (as defined above), intolerable drug toxicities occurred, or the patient refused further treatment.
2.2. Procedure and Treatment
This study and the DEC protocol were approved by the institutional review board of Akita University School of Medicine. The patients were treated with a DEC regimen that consisted of a 28-day cycle of docetaxel [60 mg/m2 intravenously (IV) on day 1)], carboplatin (IV to an area under the curve of 5 on day 1), and EMP (560 mg orally daily). Pre-medication was with dexamethasone (8 mg IV), which was 30 min before each docetaxel infusion. The detailed regimen has been previously described [13] . Briefly, two consecutive DEC therapies were performed, followed by the assessment of efficacy and toxicity. Before further therapy with the DEC therapy, a chemotherapy holiday was taken until the PSA levels increased to above baseline. Dose-reduction was allowed for elderly patients, those with a low performance status, and those with a history of severe adverse events. A luteinizing hormone-releasing hormone agonist was continued throughout the study. Treatment was stopped for any of the following reasons: progression of disease, severe adverse events, patient’s refusal of further treatment, or at the physician’s discretion. The administration of zoledronic acid or denosumab was allowed for patients with bone metastasis.
2.3. Outcome and Statistical Analysis
The study end points were the PSA response rate, radiographic response, progression free survival (PFS), and OS. PSA and radiographic progression were defined according to the criteria of the Prostate Cancer Clinical Trials Working Group 2 [14] . We measured PSA levels at least every 3 months after starting chemotherapy. A post-therapy PSA response was calculated using the maximum degree of change from baseline within 3 months. PSA response and PFS was defined as a decrease in the PSA level of ≥50% and the period from the initiation of DEC therapy to PSA progression and/or radiographic progression, respectively. Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria version 4.0 (NCI-CTC v4.0).
Regarding statistical analyses, continuous variables are expressed as median values. OS and PFS were estimated using the Kaplan?Meier method. To identify risk factors for PFS and OS, univariate analysis was conducted using the following variables: patient age, Eastern Cooperative Oncology Group performance status, baseline PSA, baseline alkaline phosphatase, baseline lactate dehydrogenase, baseline hemoglobin, PSA response to the previous DP therapy, total cycles of previous DP therapy, duration from CRPC to the DEC therapy, visceral metastasis presence, and Gleason score at biopsy. All reported p values were two-sided, with statistical significance considered at p < 0.05.
3. Results
3.1. Patient Characteristics
In total, 19 patients received DEC therapy after first-line chemotherapy with DP from May 2010 to March 2014. Table 1 summarizes the baseline patient characteristics before DEC therapy. The median age was 65 years (range, 51 - 79 years), and most patients (89.5%) had a performance status of 0 or 1. In addition, 73.7% of the patients had a Gleason score of ≥8, and the median baseline PSA level was 67.0 ng/mL (range, 9.1 - 1102 ng/mL). A total of 18 (94.7%) patients had bone metastasis, whereas visceral metastasis was observed in 5 (26.3%) patients. The median time from diagnosis to docetaxel administration was 27.0 months (range, 3.0 - 1313 months), whereas the median time from docetaxel administration to DEC therapy was 13 months (range, 4.0 - 29 months). The median time from CRPC to DEC was 17 months (range, 5.0 - 57 months). All patients received a median of 6 cycles (range, 1 - 12) of DP therapy before receiving DEC therapy. In addition, 12 (63.2%), 3 (15.8%), and 16 (84.2%) patients had previously received flutamide, chlormadinone acetate, and EMP, respectively.
3.2. Treatment Outcome
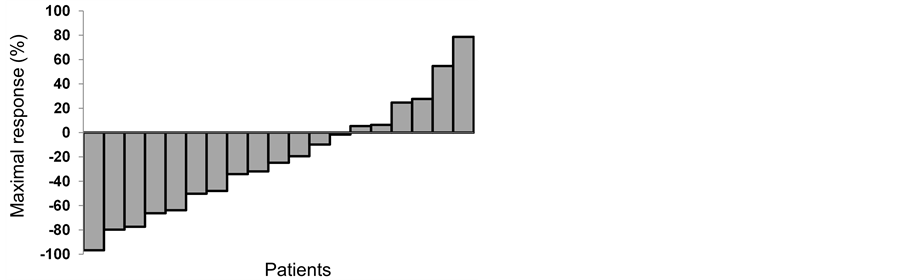
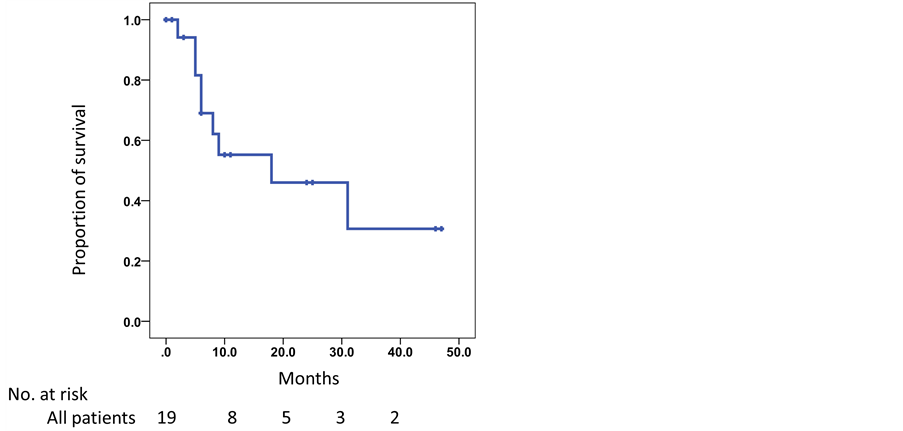
The mean follow-up period was 19.0 months, and patients received a median of 2 (range, 1 - 11) cycles of DEC therapy. The maximum response to DEC therapy is shown in Figure 1 and Table 2. Of the 19 patients, 13 (68.4%) had a PSA decrease, with 6 (31.6%) having a decrease of ≥50%. All patients were withdrawn from DEC therapy during the study period because of disease progression, except for one patient who experienced grade 3 general fatigue as a side effect. The median PFS was 3.7 months (range, 0.5 - 20.4 months). In 10 patients (52.6%) with evaluable extraosseous metastases, 1 patient (10%) achieved partial response and 6 patients (60%) achieved stable disease according to the Response Evaluation Criteria in Solid Tumors. Ten patients (52.6%) survived to the end of the study period, and the median OS was 18.0 months (Figure 2). The 1- and 2-year survival rates were 55.2% and 46%, respectively.
Table 1. Patient characteristics.
ECOG = Eastern Cooperative Oncology Group, DEC = docetazel, estramustine phosphate, and carboplatin, DP = docetaxel and prednisolone, PSA = prostate specific antigen, EMP = estramustine phosphate.
Table 2. Clinical outcomes.
PSA = prostate specific antigen, PR = partial response, SD = stable disease, pts = patients.
Figure 1. Waterfall graph of maximal changes in prostate-specific antigen levels after the administration of docetaxel, estramustine phosphate, and carboplatin in castration-resistant prostate cancer after first-line docetaxel and prednisolone therapy.
Figure 2. Kaplan-Meier curve for overall survival in patients with castration-resistant prostate cancer who were treated with docetaxel, estramustine phosphate, and carboplatin after docetaxel and prednisolone therapy.
3.3. Toxicity
A detailed toxicity profile during the study period is shown in Table 3. The most frequent grade 3 or 4 adverse events were neutropenia (68.4%), anemia (21.1%), thrombocytopenia (10.5%), and febrile neutropenia (10.5%). Non-hematologic adverse event exceeding grade 3 was observed in 2 patients (10.5%). There were no deaths due to treatment-related adverse event.
3.4. Pretreatment Variables for Clinical Outcome
We assessed the relationship between PSA response to DP therapy and treatment outcome from DEC therapy. Among the 13 patients without a PSA response to DP therapy, 4 (30.8%) achieved a PSA response to DEC therapy. In addition, among the 6 patients who had a PSA response to DEC therapy, 4 (66.7%) had no prior PSA response to DP therapy. On univariate analyses (Table 4), a duration from CRPC to DEC of ≤12 months was a significant risk factor of both PFS [hazard ratio (HR) = 3.055, *p = 0.047] and OS (HR = 5.790, *p = 0.033). Furthermore, the presence of visceral metastasis was significantly associated with a poor OS (HR = 6.139, *p = 0.027). The PSA response to DP therapy had no statistically significant impact on PFS or OS in patients with CRPC receiving DEC therapy after DP therapy.
Table 3. Overall toxicities according to grade (CTCAE v4.0).
Table 4. Univariate analysis for progression free survival and overall survival in patients with castration-resistant prostate cancer treated with docetaxel, estramustine phosphate and carboplatine therapy after docetaxel and prednisolone therapy.
4. Discussions
Recently the outcomes have been reported for several combination chemotherapies and novel drugs as second- line treatment after docetaxel in CRPC [6] [15] - [20] (Table 5). Based on the large randomized trials, two novel drugs targeting AR signaling and chemotherapy with cabazitaxel achieved a PSA decrease of ≥50% in 29.4% - 54% of patients and a median OS of 15.1 - 18.4 months. Several combination chemotherapeutic regimens have also been reported to achieve a PSA decrease of ≥50% in 18% - 60% of patients and a median OS of 12.4 - 19
Table 5. Summary of the outcome of second-line therapy in patients with castration-resistant prostate cancer after docetaxel therapy.
EMP: estramustine phosphate, PFS: progression free survival, OS: overall survival, N/A: not assessed.
months [6] [15] - [20] . It is not valid to directly compare the outcomes between the previous reports and the current study because of the substantial differences in patient backgrounds and treatment regimens; however, we showed that DEC therapy is equivalent to other second-line treatments administered to patients after first-line docetaxel-based chemotherapy. Although the survival rate in some groups of the pateints in the current study seems to be worse than that in the previous study, most of the patients in the current study were previously heavily treated with second or thrid-line hormonal therapy (flutamide, 63.2%; chlormadinone acetate, 15.8%) and EMP (84.2%) in addition to docetaxel administration. Therefore, the outcomes in patients treated with our DEC therapy with a PSA decrease of ≥50% in 31.6% of patients and a median OS of 18.0 months could be feasible enough in this setting.
In a study evaluating the efficacy of second-line docetaxel-based chemotherapy, Nakabayashi et al. showed that docetaxel plus carboplatin had a modest effect, with PSA levels decreasing up to ≥50% in 20% of patients and a median OS of 14.9 months [21] . In another study of combination chemotherapy with TEC as second-line therapy, Sella et al. demonstrated the efficacy of combination paclitaxel, carboplatin, and EMP in 15 patients previously treated with docetaxel [18] . In the study, the PSA level decreased to ≥50% in 60% of patients, and a partial response was observed in 40%. Based on the results of the second-line taxane-based combination chemotherapies, including our result, the studies that included EMP and carboplatin in combination with a taxane appeared to have relatively better outcomes, potentially exploiting specific advantages of each drug for CRPC [18] . In vitro study has also shown the synergistic effect among these three drugs [22] . However, in the study using paclitaxel, one patient died due to brain hemorrhage following prolonged thrombocytopenia and four patients withdrawn from therapy due to toxicity [18] . Further study is warranted to determine which taxane is the best candidate partner for EMP and carboplatin to maximize the therapeutic effect and avoid severe adverse events.
Cabazitaxel is a promising second-line chemotherapeutic agent for patients with CRPC who fail to respond to first-line docetaxel chemotherapy. However, the problem with cabazitaxel treatment is the high incidence of severe side effects such as neutropenia and febrile neutropenia [5] [6] . Recent studies have shown that the rate of febrile neutropenia was extremely high in Asian patients (31% - 54.5%) [5] [6] . In our study, the incidence of febrile neutropenia was 10.5%, equivalent to the incidence in an international landmark trial of cabazitaxel (8%) [15] but lower than the rate in the phase I trial of cabazitaxel in Japanese patients (54.5%) [6] . Nevertheless, DEC therapy achieved a decrease in the PSA level of ≥50% in 31.6% of patients, which was not inferior to the outcomes of that phase I study (29.3%) [6] . These results indicate that patients who are expected to be unfit for cabazitaxel, particularly Asian patients, will be good candidates for the DEC therapy.
Another important issue in the treatment of patients with CRPC is to identify the best candidate for TEC therapy. Regan et al. conducted a meta-analysis that included seven trials using TEC and revealed that the absence of extra-skeletal metastases, a higher hemoglobin, and lower performance status, lactate dehydrogenase, alkaline phosphatase, and PSA at enrollment were associated with longer survival [12] . Our previous study also showed that serum lactate dehydrogenase was an independent prognostic factor for OS in patients with CRPC who received DEC therapy [13] . As these trials examined the prognostic factors for TEC therapies in the first- line setting, these needed to be carefully applied to second-line settings, which we did in the current study. In second-line therapy, we showed that short duration from CRPC to DEC and the presence of visceral metastasis were significant risk factors of OS. Because these results imply that patients with these risk factors cannot achieve good response to current therapeutic options [23] [24] , novel therapeutic approaches need to be developed for CRPC with these aggressive phenotypes.
We also focused on the impact of the PSA response to previous DP therapy on the PSA response to current DEC therapy. Our study revealed that the PSA response to previous DP therapy had no impact on any clinical outcomes, including the PSA response, PFS, and OS, in patients treated with the second-line DEC therapy. Therefore, it is possible that DEC therapy could be a feasible second-line chemotherapy option, even in patients with no PSA response to first-line docetaxel-based chemotherapy.
This study has some limitations, including a retrospective design, short follow-up duration, and small sample size. A Randomized trial should be needed to compare our results with those in the patients treated by classical methods. In addition, DP and DEC therapies were administered intermittently, according to our original protocol [13] . Therefore, the effect of continuous administration of DEC therapy, which is a more common protocol of taxane administration, remains unclear. Furthermore, none of the patients had received therapy from the new classes of drugs such as abiraterone acetate, enzalutamide, and cabazitaxel. The advantage of implementing DEC therapy before or after these novel drugs therefore also remains unclear and should be resolved in a future study.
5. Conclusion
We show that DEC therapy is feasible and beneficial in patients with CRPC after DP therapy. It produced relatively modest side effect and comparable response rates compared with previous studies evaluating the effects of second-line chemotherapy options. Thus, DEC may be an effective and feasible treatment option for patients with CRPC after the failure of first-line DP, particularly those deemed unfit for novel endocrine and chemotherapeutic drugs.
Acknowledgements
We thank Yoko Mitobe, Yuka Izumida, Yukiko Sugiyama, and Saeko Nakamura for technical assistance.
Cite this paper
Ryuichi Ito,Shintaro Narita,Hiroshi Tsuruta,Kazuyuki Numakura,Atsushi Maeno,Mitsuru Saito,Takamitsu Inoue,Norihiko Tsuchiya,Shigeru Satoh,Tomonori Habuchi, (2016) Outcome of Combination Chemotherapy with Docetaxel, Estramustine Phosphate, and Carboplatin after Docetaxel and Prednisolone Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer. Journal of Cancer Therapy,07,471-479. doi: 10.4236/jct.2016.77049
References
- 1. Siegel, R., Ma, J., Zou, Z. and Jemal, A. (2014) Cancer Statistics, 2014. CA: A Cancer Journal for Clinicians, 64, 9-29.
- 2. Lorente, D., Mateo, J., Perez-Lopez, R., de Bono, J.S. and Attard, G. (2015) Sequencing of Agents in Castration-Resistant Prostate Cancer. The Lancet Oncology, 16, e279-e292.
- 3. Mezynski, J., Pezaro, C., Bianchini, D., et al. (2012) Antitumour Activity of Docetaxel Following Treatment with the CYP17A1 Inhibitor Abiraterone: Clinical Evidence for Cross-Resistance? Annals of Oncology, 23, 2943-2947.
- 4. van Soest, R.J., van Royen, M.E., de Morree, E.S., et al. (2013) Cross-Resistance between Taxanes and New Hormonal Agents Abiraterone and Enzalutamide May Affect Drug Sequence Choices in Metastatic Castration-Resistant Prostate Cancer. European Journal of Cancer, 49, 3821-3830.
- 5. Lee, J.L., Park, S.H., Koh, S.J., et al. (2014) Effectiveness and Safety of Cabazitaxel plus Prednisolone Chemotherapy for Metastatic Castration-Resistant Prostatic Carcinoma: Data on Korean Patients Obtained by the Cabazitaxel Compassionate-Use Program. Cancer Chemotherapy and Pharmacology, 74, 1005-1013.
- 6. Nozawa, M., Mukai, H., Takahashi, S., et al. (2015) Japanese Phase I Study of Cabazitaxel in Metastatic Castration-Resistant Prostate Cancer. International Journal of Clinical Oncology, 20, 1026-1034.
- 7. Yagoda, A., Watson, R.C., Natale, R.B., et al. (1979) A Critical Analysis of Response Criteria in Patients with Prostatic Cancer Treated with Cis-Diamminedichloride Platinum II. Cancer, 44, 1553-1562.
- 8. Berry, W., Friedland, D., Fleagle, J., et al. (2006) A Phase II Study of Weekly Paclitaxel/Estramustine/Carboplatin in Hormone-Refractory Prostate Cancer. Clinical Genitourinary Cancer, 5, 131-137.
http://dx.doi.org/10.3816/CGC.2006.n.029 - 9. Kikuno, N., Urakami, S., Nakamura, S., et al. (2007) Phase-II Study of Docetaxel, Estramustine Phosphate, and Carboplatin in Patients with Hormone-Refractory Prostate Cancer. European Urology, 51, 1252-1258.
http://dx.doi.org/10.1016/j.eururo.2006.12.030 - 10. Oh, W.K., Hagmann, E., Manola, J., et al. (2005) A Phase I Study of Estramustine, Weekly Docetaxel, and Carboplatin Chemotherapy in Patients with Hormone-Refractory Prostate Cancer. Clinical Cancer Research, 11, 284-289.
- 11. Kelly, W.K., Curley, T., Slovin, S., et al. (2001) Paclitaxel, Estramustine Phosphate, and Carboplatin in Patients with Advanced Prostate Cancer. Journal of Clinical Oncology, 19, 44-53.
- 12. Regan, M.M., O’Donnell, E.K., Kelly, W.K., et al. (2009) Efficacy of Carboplatin-Taxane Combinations in the Management of Castration-Resistant Prostate Cancer: A Pooled Analysis of Seven Prospective Clinical Trials. Annals of Oncology, 21, 312-318.
- 13. Narita, S., Tsuchiya, N., Yuasa, T., et al. (2012) Outcome, Clinical Prognostic Factors and Genetic Predictors of Adverse Reactions of Intermittent Combination Chemotherapy with Docetaxel, Estramustine Phosphate and Carboplatin for Castration-Resistant Prostate Cancer. International Journal of Clinical Oncology, 17, 204-211.
- 14. Scher, H.I., Halabi, S., Tannock, I., et al. (2008) Design and End Points of Clinical Trials for Patients with Progressive Prostate Cancer and Castrate Levels of Testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. Journal of Clinical Oncology, 26, 1148-1159.
http://dx.doi.org/10.1200/JCO.2007.12.4487 - 15. de Bono, J.S., Oudard, S., Ozguroglu, M., et al. (2010) Prednisone plus Cabazitaxel or Mitoxantrone for Metastatic Castration-Resistant Prostate Cancer Progressing after Docetaxel Treatment: A Randomised Open-Label Trial. The Lancet, 376, 1147-1154.
- 16. Fizazi, K., Scher, H.I., Molina, A., et al. (2012) Abiraterone Acetate for Treatment of Metastatic Castration-Resistant Prostate Cancer: Final Overall Survival Analysis of the COU-AA-301 Randomised, Double-Blind, Placebo-Controlled Phase 3 Study. The Lancet Oncology, 13, 983-992.
- 17. Scher, H.I., Fizazi, K., Saad, F., et al. (2012) Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. The New England Journal of Medicine, 367, 1187-1197.
- 18. Sella, A., Yarom, N., Zisman, A. and Kove,l S. (2009) Paclitaxel, Estramustine and Carboplatin Combination Chemotherapy after Initial Docetaxel-Based Chemotherapy in Castration-Resistant Prostate Cancer. Oncology, 76, 442-446.
- 19. Loriot, Y., Massard, C., Gross-Goupil, M., et al. (2009) Combining Carboplatin and Etoposide in Docetaxel-Pretreated Patients with Castration-Resistant Prostate Cancer: A Prospective Study Evaluating also Neuroendocrine Features. Annals of Oncology, 20, 703-708.
- 20. Ross, R.W., Beer, T.M., Jacobus, S., et al. (2008) A Phase 2 Study of Carboplatin plus Docetaxel in Men with Metastatic Hormone-Refractory Prostate Cancer Who Are Refractory to Docetaxel. Cancer, 112, 521-526.
- 21. Nakabayashi, M., Sartor, O., Jacobus, S., et al. (2008) Response to Docetaxel/Carboplatin-Based Chemotherapy as First- and Second-Line Therapy in Patients with Metastatic Hormone-Refractory Prostate Cancer. BJU International, 101, 308-312.
- 22. Engblom, P., Rantanen, V., Kulmala, J. and Grenman, S. (1999) Carboplatin-Paclitaxel- and Carboplatin-Docetaxel-Induced Cytotoxic Effect in Epithelial Ovarian Carcinoma in Vitro. Cancer, 86, 2066-2073.
- 23. Antoun, S., Bayar, A., Ileana, E., et al. (2015) High Subcutaneous Adipose Tissue Predicts the Prognosis in Metastatic Castration-Resistant Prostate Cancer Patients in Post Chemotherapy Setting. European Journal of Cancer, 51, 2570-2577.
- 24. Shiota, M., Yokomizo, A., Adachi, T., et al. (2014) The Oncological Outcomes and Risk Stratification in Docetaxel Chemotherapy for Castration-Resistant Prostate Cancer. Japanese Journal of Clinical Oncology, 44, 860-867.
NOTES
*Corresponding author.