Journal of Cancer Therapy
Vol.07 No.04(2016), Article ID:65475,16 pages
10.4236/jct.2016.74027
Epigenetic Tumor Response to Hypoxia: An Epimutation Pattern and a Method of Multi Targeted Epigenetic Therapy (MTET)
M. A. Nezami1, Steven Hager2, Jessica Garner1
1Pacific Medical Center of Hope, Fresno, CA, USA
2cCARE Cancer Treatment Center, Fresno, CA, USA

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 18 February 2016; accepted 10 April 2016; published 13 April 2016
ABSTRACT
In most cases, cancer develops as a result of non-inheritable somatic mutations (epimutations), acquired by the individual adult cell, during the evolution of the cell, and propagated into an expanding clone of progeny of the cells by natural selection [1] . The role of microenvironment in selection for such acquired mutations, or epimutations, is a focus of scientific research in carcinogenesis [2] . Here we describe a defective DNA response to hypoxia due to epigenetic aberrancies, in cancer cellular biology [3] . We also summarize a literature review on hypoxia mediated epigenetic responses, and its role in carcinogenesis and metastasis. Further, we review a novel method of treating hypoxic solid tumors with a combination of epigenetic modifiers with both in vitro and in vivo results in human, translating to an improved prognosis and clinical outcome. We propose that this approach both independently and synergistically (with the current standard of care) can provide an improved outcome.
Keywords:
Epigenetic, Hypoxia, Epimutations, Hypoxia Inducible Factor 1, Circulatory Tumor DNA, Circulatory Tumor Cells

1. Biologic Background
Given its central role in tumor progression and resistance to therapy, tumor hypoxia might well be considered the best validated target that has yet to be exploited in oncology [1] [4] - [20] . Normal tissue oxygen saturation is always above 7 - 9 percent. That said, hypoxic tumors with the greatest tendency to metastasize have an oxygen saturation below 0 - 5 percent, which can activate hypoxia inducible factor 1 (HIF-1) [4] [6] [19] [21] - [27] . Technically, we can call the environment hypoxic any time the tissue oxygen saturation falls to less than two percent [1] [3] . In hypoxic conditions, HIF does not bind to VHL, and therefore accumulates [4] . HIF-1α then binds to hypoxia response elements (HRE) along with co-factors HIF-1β, which activates more than 100 genes involved with metastasis, tumor growth, and metabolic activity [4] [6] [28] . The degree of HIF expression correlates with angiogenesis, resistance to treatment, and overall patient outcome [29] [30] . In early stage lung cancer and pancreatic endocrine cancers, markers of hypoxia have outperformed traditional histopathological staging for predicting prognosis [9] .
There are a few studies which have investigated genome-wide DNA methylation changes that occur in hypoxic environments. Two of these studies showed an increase in global hypomethylation in glioblastoma, sarcoma, colorectal cancer and melanoma cell lines when cultured in hypoxic conditions and compared to normoxic culture [14] [31] [32] . In addition, hypoxia regulated miRNAs are implicated in both renal cell cancer and glioblastoma development [33] [34] . For example, miR-210 is known to be regulated by hypoxia and has found to be a diagnostic marker in pancreatic adenocarcinoma, and a prognostic marker in breast cancer and in late stage non-small cell lung cancer, resulting in caspase 3/7 activity, followed by mitochondrial dysfunction [5] [33] [35] - [37] . Therapeutically, this is important, as the over expression of miRNAs has been implicated in the development of glioblastoma (which is known to have extensive regions of hypoxia and necrosis), and knock- down of this miRNA in glioblastoma cell lines lead to apoptosis, suggesting a role in tumor survival [1] [5] [35] [38] .
There is global increase in DNA methylation in normal cells following a period of hypoxia, however the opposite is found in cancer cells, which show global demethylation following cyclic intratumoral hypoxia [39] . This can be evident in as few as 48 hours [17] . This may be related to broken feedback loops that exist in cancerous cells, as a result of epigenetic dysregulation, where increased DNMT3 as well as MbD1 (methyl-CpG binding domain protein 1) and H3K9 histone acetylation is seen [40] . Histone acetylation is most commonly associated with repression of transcription, whereas histone methylation is associated with both transcriptional activation and repression. Hypoxia is known to induce alterations in chromatin, including global deacetylation, as well as changes in histone methylation and acetylation (through demethylation of lysine residues of histone tails) in the promoter regions of hypoxia related genes (vicious cycle) [18] . Most importantly, hypoxia regulates the activity of histone demethylases. These series of enzymes are also mutated in the event of exposure to carcinogens (such as smoking) and inactivating mutations in such enzymes controlling histone modification have been detected in variety of solid tumors including clear cell renal carcinoma (ccRC), clear cell ovarian cancer, and pancreatic neuroendocrine tumors [41] [42] . Similar epimutations have been suggested in hypoxia associated targets.
One other major effect of hypoxia is the activation of androgen receptor (AR) and regulated AR target genes [1] [3] . Further epigenetic response to hypoxia, as well increases the expression of genes involved in self-re- newal, are targets for the transcription factor Oct-4 in embryonic stem cells. It is very likely that different cell and tissue types would require varying periods of hypoxia before “normal” hypoxic cells become “pre-cancerous” or “cancerous” hypoxic cells.
In gastric cancer, a hypoxia-induced epigenetic silencing of the tumor suppressor RUNX3 through histone H3-lysine 9 dimethylation and decreased H3 acetylation during disease progression has been observed, which is reversible by administration of cytosine-methylation inhibitor 5-aza-2-deoxycytidine and Trichostatin A [43] . This demonstrated that the concept of combination therapy with both HDACI and DNMTI could reverse the histone methyltransferase and HDAC1 up-regulation following hypoxia (increase in the repressive histone mark H3K9me2 and a decrease in the transcriptionally active H3K9 acetylation marks) [40] [44] .
This is a significant finding, as it implies that HDAC I-II inhibitors may have a therapeutic effect in VHL deficient tumors (such a renal cell cancer and pancreatic neuroendocrine tumors) [45] . The class III HDAC inhibitor SirtI has also been found to deacetylate HIF-1α and HIF-2α, thus repressing HIF activity [46] [47] .
2. Therapeutic Background
Intratumoral hypoxia followed by stabilization/activation of hypoxia-inducible factor 1 (HIF-1) and its downstream transcriptional factors is one of the most important mechanisms inducing epithelial-mesenchymal transition (EMT), which has been widely accepted as a crucial step to generate early stage of tumor metastasis [15] [21] [48] .
There have been great efforts directed in introduction of HIF antagonists and VEGF blocking agents, used in combination on VEGF vertical pathways. Unfortunately the combination targeted drugs that have been utilized to inhibit HIF and VEGF in vertical pathway have proved to be too toxic in humans [9] . Other approaches such as hypoxia activated drugs or prodrugs also have been challenging, although there have been good responses, with up to double the survival rate reported in some cases of pancreatic cancer when combined with Gemcitabine [3] . The application of targeted therapies with histone deacetylase inhibitors have also proven to be generally too toxic.
Sodium butyrate, a novel HDAC inhibitor, has been shown to inhibit hypoxia induced HIF1α induction, and has inhibited in vitro and in vivo angiogenesis. The study demonstrated that sodium butyrate treatment of endothelial cells down-regulated both HIF1α and VEGF protein levels thereby regulating angiogenesis [29] . This indicates that HDACs are involved in oxygen dependent gene expression and angiogenesis [6] [44] [49] [50] . There are several studies suggesting direct inhibition of HIF by anti-oxidants. Polyphenols which have a strong effect on Epigenome by inhibition of DNMT is our area of therapeutic interest. Quercetin was selected as a strong antioxidant with largest pool of available antineoplastic data [24] [25] . We selected Quercetin as its anti-HIF effects are profound in the presence of hypoxic conditions where the tumor cells are inhibited both at migration as well as invasion levels tested by us in vitro on lung adenocarcinoma, as well as Ovarian cancer cell lines. We also showed that the spheroid formation of cells in 3 dimensional cell culture was significantly inhibited secondary to possible anti HIF effect. Interestingly, the literature is controversial on anti-HIF effects of Quercetin, and in fact some of our studies also showed pro-HIF effects, but this is related to the content of iron and intratumoral hypoxia, as the more hypoxic environment, the more Quercetin exhibits anti-HIF properties [24] [25] .
3. Methods
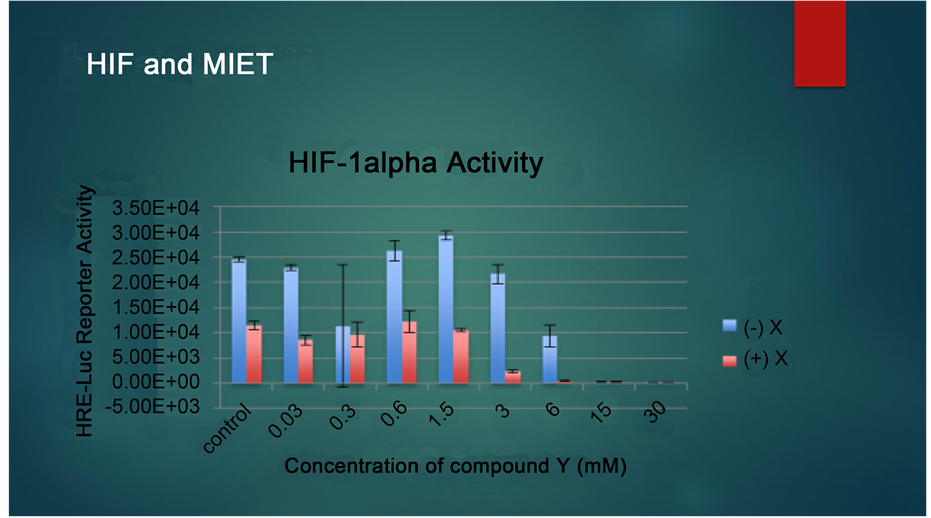
Based on the research above, we combined Quercetin and sodium phenyl butyrate in our patients’ clinical program, aiming at reversing the epimutations that have already occurred in hypoxia related targets, in DNA and histones. Our in vitro finings confirmed the synergistic effects of combination therapy on inhibition of HIF, which was substantially detected in pancreatic cancer cell lines as well as ovarian cancer lines. Our studies showed synergistic effects also when the hepatocellular carcinoma cell lines were pre-treated by quercetin and then SPB was administered (Please see Figure 1 and Figure 2).
We showed a sensitizing effect of Quercetin on cells, when they were pretreated 6 - 24 hours before exposure to SPB. In this experiment, reduction of dose necessary to inhibit HIF was seen up to 50 percent. We correlated these findings with clinical surrogate markers for angiogenesis in case studies treated at our clinic, and the results were consistent. In a case series of patients with advanced solid tumors we treated under the protocol multi-targeted epigenetic therapy (MTET), with application of scientific rationale using SPB and Quercetin in sequential intravenous application. Patients all were informed about their options and signed the appropriate consent forms. The materials were obtained from FDA approved compounding pharmacy. The antineoplastic results were evaluated following serum markers for angiogenesis and circulatory tumor cells (CTC), to correlate with prognosis and survival.
All 17 cases treated had improved overall survival compared historically with SEER data set. There was 100 percent response in their plasma VEGF and CTC. Patients’ quality of life were preserved and improved, documented by improved ECOG scores.
4. Clinical Cases
4.1. Sample Case 1: Metastatic Malignant Melanoma
► 29 year old female with history of melanoma, diagnosed in April 2012 status post biopsy of her right forearm nevus, side margin excision, and graft and LN biopsy. MRI showed disease free status, status post a recurrence in 9 months, with vision problem in January 2013, with multiple brain mets, including Chiasm of Optic nerve, status post 5 cycles of Immunophresis by Dr. Lentz in Germany, Interferon, and Yervoy (5 mg/kg for two rounds), and subsequent Craniotomy by in Australia, and cyberknife of remaining lesion in brain. In February 2013, she had received Sutent.
Figure 1. Drug combinations with Quercetin and Sodium phenyl butyrate.
Figure 2. HIF-1 alpha activity.
► Her molecular profiling of her tumor showed positive NRAS mutations.
► After initial evaluation, immediately she was started on IV epigenetic therapies which she received on daily basis. After ten treatments she expressed improvement in her function and vision. Her ECOG score improved as was her vision. Her labs were repeated which showed following results:
► Neuron Specific Enolase (NSE) dropped from 45 to 9.2 and her interleukin 8 has dropped from 76 to 53, in two weeks, repeated on 4/28/14. LASA dropped from 64 to 27 (measured on /15/14 and 5/5/14 respectively).
► We used Biofocus lab for her circulatory tumor analysis. The test was done PRE and POST epigenetic therapies. She did not receive any chemotherapies, or immune therapies or cytotoxic or targeted therapies during this time. Her CTC showed complete resolution post therapy. Her CT scan was repeated on 6/2/14, which showed mixed response with many lesions in her chest and neck improved in size and activity.
This was a rapid response in an advanced case of RAS positive refractory to immune therapy in melanoma.
Case 1 Figure 1.
Case 1 Figure 2.
4.2. Sample Case 2: Renal Cell Carcinoma
► 67-year-old male with history of a 10 cm renal mass incidentally found in an ultrasound, suspected for malignancy, and scheduled for laparotomy. The surgical biopsy confirmed the RCC.
► He was referred for evaluation and treatment on 8/11/14 and immediately labs were drawn which showed increased plasma VEGF at 248, and interleukin 8 (serum) of 448.4. His D-dimer was also elevated at 0.61. He started the daily IV epigenetic therapies. His labs were repeated after 10 treatments (two weeks), which showed substantial improvements. His VEGF dropped from 248 down to 58 and his Interleukin 8 dropped from 448.4 down to 16.6. Also his initial thrombocytosis as a sign of angiogenesis was resolved with his platelets dropping from 388 to 201. We performed a CTC analysis through Biofocus laboratory before and after the treatments. The results indicated significant reduction of the tumor burden in CTC as well as CTC markers, including CD 20, telomerase, and cytokeratin markers. The results indicate response in the micro-metastatic disease.
► Phenotypical CTC response by MTET:
► Identification of G250 at CTC level (Pre-treatment) correlates with higher invasiveness and aggressive tumor phenotype [43] .
► This suggests the treatment efficacy of MTET, as a TARGETED THERAPY for G250.
► Patient stayed in complete remission to our understanding after 18 months.
Case 2 Figure 1.
Case 2 Figure 2.
4.3. Sample Case 3: Metastatic Pancreatic Cancer
► 64 year old male with history of pancreatic cancer with numerous liver mets, diagnosed after a prolonged course of abdominal pain and weight loss for months. Abdominal CT suggestive of the tumor 2.5 cm × 2.5 cm in pancreatic head along with liver mets. The mass in pancreas was entrapping the vasculature and adjacent areas. He was jaundiced and had lost weight by pounds every day and lost his appetite. He was seeing Stanford oncology and had been given 5 percent chance of response to the recommended therapy, which consisted of neoadjuvant chemotherapy as Whipple procedure was off of the table. The intention of therapy was palliative at best.
► He then decided to refer and be treated at our center. Initial evaluation showed significant pain, jaundice and elevated tumor markers. Immediately he was started on IV daily epigenetic therapies and started to show improvements in his function and ability to eat immediately after the first week. He also had no pain this time and his ECOG score improved.
► We repeated the blood test looking at the tumor markers after 7 treatments. All his tumor markers dropped substantially. His CA 19.9 decreased from 110 to 80, CEA dropped from 6.4 to 6.1, LASA (Lipid associated sialic acid) dropped from 21 to 15 (normalized). His serum Her-2 also dropped from 17.3 to 11.7 (measured on 10/15/14). His PSA which also incidentally was reported to be at 7.7 dropped down to 3.7 (normalized). His CTC wad repeated and showed complete response.
► He then started Gemzar and Abraxane per protocol, in combination with epigenetic therapies, his clinical response continued, with decreased tumor marker CA 19.9 measured on 11/17/14 at 64 from 80. He then was referred for a nanoknife procedure which he completed successfully. He is in complete radiologic remission currently on 6/15/15.
4.4. Sample Case 4: Metastatic Breast Cancer, Renal Cancer, Colon CA and Thyroid CA
► NIH case 53:54 year old post-menopausal female with history of invasive lobular carcinoma of the right breast, ER and PR positive, Her-2 negative, status post progressive disease with stage four, liver and bone mets. Treatments consisted of -2008: Hormonal Blockade with Femara and Zometa and partial mastectomy in March 2009. She further was diagnosed with left renal clear cell adenocarcinoma (4.4 cm in size), status post resection. She also has had her appendix removed which showed mucinous adenocarcinoma, and developed thyroid papillary carcinoma in the interim. (4 types of cancer).
► She was referred due to progressive disease in her thorax and liver to us. She underwent a whole body PET scan and initial laboratory evaluation which confirmed presence of metastatic lesions in all levels in thorax, axillary and cervical chains as well as thyroid with very high SUV activities of 20 and more. Her initial labs on 11/26/2012 revealed increased CA 15.3 and CA 27.29 and CEA, as well as increased circulatory tumor cells at 14. (by Cell Search).
► Immediately she was started on IV epigenetic therapies. She was reevaluated on 12/12/2012 after receiving daily IV therapies for two weeks. Her tumor markers all showed decreased values. Her CA 15.3 dropped from 34 to 28 (normal 25), her CEA dropped from 12.5 to 9.6 and then to 8.5 (normal 4.7). Her CA 27.29 dropped from 43.6 to 34.7 (normalized). Her circulatory tumor cells dropped from 14 to 1 (one), after six treatments between 12/5/2012 and 12/12/2012. Her PET scan on March 1st 2013 confirmed positive response to interval therapy with both decreased size and activity in all thoracic lesions. No new lesion was identified. Abnormal hypermetabolism seen in her prior scan, on 11/30/2012 in multiple thoracic lymph nodes (perivascular, mediastinal, paratracheal, precarinal, hilar) had improved and the nodes appeared smaller (11 × 8 from 15 × 12). The SUV activities dropped from 13.8 to 6.2 and from 12.4 to 5.3.
► She continued the maintenance therapies at our center and her labs indicated that her CTC remained at 1 measured on 3/8/2013. Her CA 15.3 and 27.29 remained at normal level (30 and 38). Her FGF-2 dropped from 10.5 on 4/8/13 to 7.3 on 5/7/13 and further down to 4.8 (normal range) on 5/29/13. This is a marker related to cancer stem cell activity and EMT transition.
► Her re-staging PET scan confirmed significant positive response to the interval therapy suggested by reduction in size and activity of all neck, thoracic, and abdominal lesions. All her tumor activities dropped 70 - 80 percent and 90 percent to normal physiologic activity.
► Metabolic activity of such lesions at the thoracic (hilar, pericardial and perivascular) lymph nodes dropped from 13.8 to 6.2 to 2.8, 12.4 to 5.3 to 3.4, and 10.3 to 6.1 to 3.2. The bony lesion at L4 this time demonstrated no increased activity/physiologic activity as well. This response was obtained without receiving any chemotherapies. Duration of therapy for her to this point was 84 days.
4.5. Sample Case 5: Metastatic Breast Cancer
► 70 year old female with history of right infiltrating lobular carcinoma of breast diagnosed per biopsy in 2/2012. She had chemotherapy in June 2012 for six sessions, consisting of carboplatinum and taxol, Gemzar and Cytoxan, status post progression of disease confirmed by scan and tumor markers. Referred to us for evaluation and treatment on 8/6/2012. Initial evaluation revealed a massive disfiguration of the right breast with a bulky tumor causing ulceration, discharge and pain. Patient mainly complained of shortness of breath secondary to significant increase in the malignant pleural effusion mandating thoracentesis every week, with last drainage of 2100 ml bloody effusion on 8/1/2012. Patient also had severe pitting edema bilaterally in ankles up to the knees.
► Initial labs were drawn which revealed increased tumor marker, CA 15.3 (1322), CA 27.29 (1047.9), as well as VEGF (231) and CA 125 (273.8) and LDH (259), CRP (14).
► Initial staging whole body PET scan on 8/6/2012 confirmed the presence of cancer in bones as well as LNs in the thoracic and abdominal cavity. This scan was compared to the prior PET scan on 2/2012, which showed progression of the disease both in the bones as well as the breast, both in size and activity, translating to lack of response to the IPT chemotherapy regimen. Her pleural fluid chemosensitivity showed resistant tumor cell culture to all chemotherapies tested including Doxorubicin, Taxol, Navelbine, Cytoxan, Cisplatinum, 5-FU and Taxotere.
► Immediately patient decided to start the treatments in our clinic. She was started on the program using Intravenous epigenetic therapies on daily basis, four times a week. After three sessions the pain and discomfort improved, and the pedal edema completely disappeared.
► After 10 days, and seven treatments (8/16/2012) labs were repeated which showed stabilized markers (ex. CA 27.29 1041.3).
► After 16 treatments in three weeks, the CA-15.3 dropped to 1115 from 1351. The CA-27.29 dropped from 1041 to 777.6. The CRP dropped from 16 to 4 and the CA-125 dropped from 326 to 294.4. The VEGF dropped from 135 to 97 (normalized) and LDH dropped from 304 to 267.
► Patient had no longer shortness of breath or pedal edema and the pleural drainage decreased from 2700 to 700 ml. Her VEGF dropped further down to 82 on 10/15/2012. The CA-15.3 decreased to 804, checked on10/1/2012 after receiving 26 treatments in two months. (More than 40 percent decrease.)
► Further her tumor markers, CA-15.3 dropped again this time from 804 to 743, and CA-125 from 294 to 249 and CA-27.298 dropped to 447.5 on 10/15/2012. On 10/31/2012 tumor markers were rechecked. This time, the CA 15.3 dropped to 648.8 and CA 27.29 dropped to 383.1, and CA 125 dropped to 216.2. Her circulatory tumor cells dropped from 7 to 2 and ZERO on 11/16/2012 translating with improved overall survival. (Please see the diagrams.)
► Again on 11/16/2012, CA 15.3 dropped to 512, and CA 27.29 dropped to 358.2 and CA 125 dropped down to 159.8. LDH was normal at 178. Her CA 15.3 dropped again to 480 and CA 125 down to 82 on 12/20/2012. Her natural killer cell activity was measured on 8/6/2012 reported at 4 and re measured on 1/16/2013 and it quadrupled to 17.1. Then it increased to 37 measured on 2/19/2013 after the treatments. Her FGF-2 which is a marker for cancer stem cell activity dropped from 12.9 on 4/9/13 to 8.7 on 5/21/13. (normal range)
► Her pleural malignant drainage completely stopped in April 2013. She accomplished complete pathological response in her pleural fluid dated 9/12/13. This is when she had more than 2700 ml malignant drainage every 5 days, when she started our program in August 2012.
► We restaged her cancer after three months. Her PET scan confirmed her positive response to therapy, in ALL tumor sites, breast, Osseous and soft tissue cancers all shrunk and their activity decreased (up to 70 percent) with small residuals of tumor left. On March first 2013, her restaging scan was repeated and again confirmed positive response to interval therapy with diminished tumor sizes and activity in ALL lesions, lymph nodes, bony lesions as well as breast itself, compared to the prior exam. The PET scan was as close as a complete remission! With SUV activity of lesions all close to the background at less than 2.
► Her treatment course was 108 days.
4.6. Sample Case 6: Metastatic Her 2 Positive Breast CA
► 39-year-old female with history of stage four bilateral infiltrating moderately differentiated ductal carcinoma with angiolymphatic involvement diagnosed in 2009, ER positive, PR negative, Her-2/neu +3.
► Upon diagnosis there was biopsy proven involvement of the adrenals. Patient subsequently was started on Herceptin, carboplatinum and Taxotere, which she completed the six cycles in November 2009 and complete remission was achieved.
► Patient subsequently had mastectomy, and radiation therapy, followed by Herceptin for maintenance and hormonal blockade with tamoxifen/fareston. At this point patient was in complete remission documented on her July 2010 PET scan.
► 11 months later, in June 2011, patient develops seizure and the MRI of brain shows five separate enhancing masses in the brain. The largest was resected in June 2011, and patient received whole brain radiation.
► Patient relapsed again with progression of disease in November 2011, with bony mets, while she was on Tykerb and Xeloda. This time patient is seen at Cancer Treatment Centers of America and advised to start Abraxanxe which she tried for one session and developed severe neuro and hematological toxicity. She opted out of further chemotherapy. She continued to receive Herceptin (Her-2 blocker) and Xgeva, along with Faslodex (hormonal blockade). Notably there were no further options left for her.
► Patient referred to us as a last resort. On 11/27/2012 her initial labs show severe immune suppression by suppressed NK activity, significantly elevated tumor markers and VEGF, and circulatory tumor cells higher than 1000 (per 7.5 ml of blood) by Verdix. (Maximum reading of CTC, is 1000.)
► She immediately started the therapy at our center with IV epigenetic treatments administered on daily basis, five times a week. She started to feel better with increased energy.
► She experienced no toxic side effects, her labs were repeated on 12/10/2012. Her VEGF dropped from 150 (on 11/27/2012) to 75, and her circulatory tumor cells dropped from 1000 to 724. Her LDH also decreased from 755 to 581 and 314 on 12/20/2012, and normalized to 122 on 1/2/2013. Her CEA also dropped from 388 to 327. Her VEGF dropped from 150 to 75 in matter of 14 days after receiving 7 treatments (11/27, and 12/10/2012). Further on 01/16/2012 her circulatory tumor cells dropped again, this time to 359 after 20 treatments, and her VEGF dropped to 24 from 150. Her ECOG performance status improved from 2 to zero (fully active).
► Her whole body scan and brain MRI was repeated in February 2013, which revealed no lesions in the MRI (normal), and resolved pulmonary findings in her PET scan, with remnant bony lesions, stable disease. Her CTC was repeated on 3/5/2013 after receiving 47 treatments and was 56, down from 1000. Her VEGF stayed normal after tapering down the treatments to once a week, and was 112 on 3/18/2013.
► She continued the treatments on twice weekly basis, and her markers continued to drop. Her CEA has dropped from 926 (February 2013) to 239, and further down to 194 (on 5/8/13).
► Her LDH dropped from 504 to 241 and 183, and CA-15.3 dropped from 684 to 311 and then to 294 on 5/8/13. Her CA-27.29 dropped from 881 to 290.5 measured in March 2013, and 285 on 5/2013. Further the markers dropped again on 5/22/13. CEA dropped to 182, CA-15.3 to 232 and CA-27.29 to 258.
► Her CTC was repeated as well on 4/3/13 and it dropped from 1000 when she started down to 5. Then on 5/8/13, the CTC was again repeated and it came down to only one (1).
► Her PET scan was repeated for restaging on 5/14/13 which showed physiologic activities through chest, and no metabolic activity at known bone metastatic lesions seen in prior exam. There was no marrow involvement noted compared to prior MRI findings of marrow enhancement. Duration of treatment for her to this point was 70 days. Further as she was complaining of neck pain, her oncologist referred her for possible spinal involvement by the tumor in June 2013, which the pathology of a herniated disc showed no evidence of malignancy. Also as her PET scan revealed a pulmonary nodule, and suspicious lymphangiomatosis, she was referred for bronchoscopy and the pathology was negative for malignancy in all specimens. She was found to have no residual of disease.
► Further all her markers dropped again this time. CA-15.3 down to 153.3, CA-27.29 down to 182.1, CEA down to 59.1 (please see the graphs below), measured on 1/16/14. Her VEGF also continued to stay in normal range (105 on 1/16/14). Her Her-2 level dropped from 225 to 35, measured respectively on 8/27/13 and 1/16/14.
► Her PET scan on 12/25/13 also confirmed partial response and shrinkage of tumor in all metastatic sites. There were significant improvements in level of activity of all lesions in brain, as well as bones (complete resolution/response) and thyroid. Left mid iliac bone lesion, Right posterior element of L4, S1 joint lesion, adrenal gland lesion, previously seen mass, Left periaortic node activity, thyroid mass, pulmonary large fissure, pleural space effusions, all showed complete resolution. Left cerebellum less intense and smaller (SUV down from 14 to 9).
► Her CTC was re measured on 1/16/14 and it was zero. (From 1000).
4.7. Sample Case 7: Metastatic Colon Cancer
► 63-year-old male with history of stage four colon cancer who has been treated at Stanford oncology and surgical oncology through many therapy modalities including ablation, (microwave) and chemo embolization, and liver resections. He initially has underwent systemic chemo with Xeloda for 3 months upon diagnosis in 2010, and Avastin which he came off in October this year, and subsequently had progressed with CT scan showing new lesions in his liver.
► Started Folfiri, but had severe side effects and stopped after the first dose. He sought our therapy on 11/11/14, and his initial labs showed significantly increased interleukin-8 (plasma at 216, serum at 226), HE 4 at 175, and CEA, along with low IGF-1 and elevated CRP at 58. His CTC showed elevated markers for disseminated cells in the serum measured through purified DNA technique.
► Immediately he was started on IV epigenetic therapies which he received on daily basis, and the labs were repeated after only 10 days (7 treatments). The labs this time, showed stabilized CEA, Decreased CRP to 11, Decreased IL-8 (serum and plasma) down from 226 to 186 and 216 to 155, HE 4 also dropped from 175 to 158.
► The FGF-2 was reported at 11.7 (normal less than 6.5) on 11/11/14 which dropped to 1.9 (measured on 11/21/14).
► On 1/14/15, his circulatory tumor cell analysis was repeated that showed complete eradication of his CTC, post therapy.
Case 7 Figure 1.
Case 7 Figure 2.
4.8. Sample Case 8: Non-Small Cell Lung Cancer (NSCLC)
► 57-year-old female, with oligo metastatic non-small cell lung cancer diagnosed in 2013, status post-surgery (right middle lobectomy), radiation for stage one adenocarcinoma (T1, N0, M0) status post recurrence with solitary pleural base nodule in 9/2013, and status post stereotactic radiation to a paraspinal mass and chemotherapy consisting of four cycles of Carboplatin and Alimta, from November 2013, to January 2014, with progression of disease.
► Despite therapies, she had progressed documented in her PET scan done in January 2015. She was asymptomatic all through the course of treatment except nausea that was resolved. Her LDH was repeated after treatments, and it normalized from 280 down to normal at 216. She did not change her diet and did not receive any other conventional or alternative therapies. Her Circulatory tumor cells were repeated after 15 treatments and showed complete eradication of CTC. (First sample on 1/15/15 and the second on 2/5/15).
► The markers which were positive before starting the treatments were negative after therapy. C MYC and Histone deacetylase markers were normalized. This indicates that MTET therapy targets INCLUDE C MYC and HDAC.
► The PET scan findings showed stable disease in pulmonary lesions, no distant organ involvement or new lesions were reported to interval therapy, documented on restaging scan performed on 2/20/15. Further she achieved near complete response in her imaging, and has maintained such response over 24 months, with single agent erlotinib plus MTET protocol.
► This favors the concept of both independent as well as synergistic antineoplastic effects of MTET with targeted EGFR blockade, in EGFR positive NSCLC.
Case 8 Figure 1.
Case 8 Figure 2.
4.9. Sample Case 9: Pancreatic Cancer
► 75 year old male with history of pancreatic cancer diagnosed in 1/15 after experiencing back pain for 6 months. He had seen many alternative doctors received IV vitamin C and peroxide (3 each) , had seen a surgeon who has indicated that the tumor was too advanced to be removed, (inoperable)
► Referred to us for evaluation and treatments, his last CA 19.9 was reported at 7800, complaining of the back pain was taking Advil daily, along with cannabis oil to relieve the pain, he refused chemotherapies, as it was given only as a palliative option. His labs indicated his CA 19.9 dropped from 7800 down to 5300, status post 10 treatments, which he received on daily basis 5 times a week.
► Other markers for cancer also dropped. His LASA (Lipid Associated Sialic Acid) dropped from 24 to normal (16). His Interleukin-8 (serum) also normalized from 68 to 34. (Measured respectively on 3/2/15 and 3/12/ 15).
► His quality of life improved and he was able to function better and with no pain.
► His Circulatory tumor analysis confirmed the response to the treatments he received in laboratory. Second analysis showed complete resolution of the CTC confirmed post therapy, measured respectively on 3/4/15 and 3/26/15.
Case 9 Figure 1.
Case 9 Figure 2.
4.10. Sample Case 10: Prostate Cancer
► 81-year-old male with history of prostate cancer diagnosed post biopsy, referred by his urologist to us for evaluation and treatment with epigenetic therapies. He had refused hormonal blockade. Initial evaluation revealed increased TGF beta 1, at 7840 on 5/15/15. His PSA was at 40. Immediately he started the treatments on daily basis, which he received through IV.
► He tolerated the therapies well, did not change his diet and after completion of only three treatments his labs were repeated which showed a drop in his TGF-beta-1 down to 6060 on 5/18/15. His PSA as well dropped down to 14 measured on 5/29/15.
► His circulatory tumor cells were analyzed and it showed positive C MYC on 5/6/15 and it was repeated on 5/21/15 and it was negative. (No presence of CTC in blood).
► This is an important result as it indicates that
► C MYC positive CTC respond to this protocol, and through this case we showed that C MYC may for the first time be considered an actionable target.
► The patient continues the treatment at our clinic, with excellent results. (Complete radiological remission).
Case 10 Figure 1.
Case 10 Figure 2.
4.11. Sample Case 11: Metastatic Renal Cell Carcinoma
► 57-year-old female with history of renal cell carcinoma, diagnosed in 2006 and treated with right nephrectomy, since then she had been in remission, until September 2014, where there was a suspicious mass in the contralateral kidney (left side) that was seen in ultrasound, which was monitored till January 2015, where the imaging (U/S and CT) confirmed it’s enlargement to 5 cm.
► Her chest/abd/ pelvis CT in January 2015 showed many metastatic lesions in her lungs/liver/ and pelvic bone, secondary to recurred stage four renal cell carcinoma. It also showed that there was numerous nodules in both lungs. She opted for conventional treatments which was started in March 2015, with sunitinib at 50 mg per day, and local radiation to the pelvic bone where the lesion was found. She then decided to seek alternative therapies as she experienced severe side effects from the Sutent.
► She traveled from Canada where she lived to see us in California on 5/1/15, when she was evaluated and initially found to have extensively high VEGF in her plasma (717) measured on 5/1/15, along with elevated LASA at 22.
► Her blood was also sent to Biofocus to evaluate for circulatory tumor cells and that test came back positive for C MYC as well as G250 and HDAC. Her c DNA also revealed positive genomic alteration s of VHL and RAF-1. She also was experiencing thrombocytopenia (plt of 80) and clinically had stomatitis and very high blood pressure. As part of initial evaluation she was restaged with a whole body PET/CT scan that revealed new lesions in her thyroid, and paraaortic LN. The pelvic lesion was not seen, and other lesions in her kidney and lungs were reported slightly smaller.
► She was started on IV daily epigenetic therapies and the dose of Sutent was decreased to 37.5 mg a day, on schedule of 4/2 on/off therapy. Her stomatitis was resolved and she felt better, her blood pressure stabilized and was reduced. Her platelets were rechecked and it was normalized.
► Her CTC was repeated after the 10 therapies. The CTC assay showed complete resolution of the circulatory tumor cells that were present in the blood. These cells had demonstrated G250 activity as well as Histone deacetylation and DNMT activity as well as C MYC. All these markers COMPLETELY disappeared in the second sample from the patient post therapy. Please see attached.
► Her VEGF was repeated as well and showed a reduction from 717 down to 303 and further down to normal range.
► There were no new lesions in her scan post therapy and her metastatic burden reduced significantly by surrogate markers.
Case 11 Figure 1.
Case 11 Figure 2.
4.12. Sample Case 12: Cervical Carcinoma
► 39-year-old female with history of cervical cancer diagnosed in 2008 status post several rounds of chemotherapies, radiation and surgery starting in January 2009, post remission for 12 months until her first recurrence in 12/09, status post oophorectomy and adjuvant chemotherapies she had in Mayo clinic, she went to remission again until 11/13, status post second recurrence, in right inguinal node, status post second surgery and radiation at Mayo clinic, status post third recurrence in July 2013, status post pelvic exenteration with removal of intestine, bladder, and neoadjuvant chemotherapy in Florida (Miami hospital), status post fourth recurrence in January 2015, with findings in pelvic PET/CT, status post radiation for two weeks and chemotherapies, this time consisting of Gemzar and Avastin which she has been receiving for 9 months, till PET scan in 4/15 shows progression of disease with increased metabolic activity in the pelvic area.
► At this time, her oncologist had stated that she has exhausted all regimens she has for cervical cancer and she is exploring alternative therapies. She referred to us from Spain where she lives and started evaluation and IV epigenetic therapies which she received every day. Her initial labs showed increased plasma VEGF at 166 measured on 5/18/15, along with increased lipid associated sialic acid (LASA) and CA 19.9 at 46. Her Circulatory tumor cell analysis was also positive for presence of CTC.
► Further she received 10 treatments and the labs were repeated. Her VEGF normalized in matter on 10 treatments down to 100 (normalized). Her CA 19.9 also dropped to 40 measured on 6/3/15. CTC was repeated after 10 therapies. The CTC showed complete resolution after the 10 treatments with negative CK 19 (zero) from 312.
► Her tumor size stabilized by the interim therapy.
Case 12 Figure 1.
Case 12 Figure 2.
4.13. Sample Case 13: Ovarian CA
► 75-year-old female with history of Stage IV ovarian cancer that was originally diagnosed in 2009, status post many regimens of chemotherapy, and surgery. The surgical pathology disclosed the papillary serous adenocarcinoma with peritoneal involvements, in June 2009, positive for P53, it was not clear if the endometrial involvement was primary or she had two distinct neoplasms. She initially underwent a 3 cycles of neoadjuvant carbo+ taxol, and 3 cycles of adjuvant therapy in the form of intraperitoneal (IP) as well as oophorectomy, in 2009, then went to remission, for two years or so, until the CT showed a recurrence in liver in 1/12. She was treated with ablation at UCLA in 7/12, status post second remission, and recurrence on 12/12, with CT confirming the progressive disease, in the liver as well as the pelvic lymph nodes. She received Gemzar and Carbo also in 7/13, Since then she had decided to stay off chemo, until she was qualified for a clinical trial for a PARP inhibitor, at UCLA/Fullerton, which she did for 5 cycles, but did not complete due to failure of the therapy, (her HE-4 was reported at 204 in 9/14 and CT showed progressive disease) then she started Doxil from September to December of 2014, and since it failed, started Avastin and Abraxane last received on 4/15 which had caused a drop in CA 125 but she refused further chemotherapies recommended by his oncologist in several steps, such as Topotecan/Alimta, Afinitor, and Abraxane as single agents.
► Upon her arrival her symptoms included sever fatigue, as well as Onycholysis in nails and neuropathy in her fingers. Her initial evaluation revealed a very high level of HE-4 at 301 as well as increased CRP at 29. Further a staging PET/CT was ordered on 6/24/15 which showed significant progression of disease compared to 3/10/15 scan, consisting of mediastinal disease, subpectoral and supraclavicular LNs, extensive hepatic metastasis, left adrenal lesion, extensive lymphadenopathy in peritoneum, retroperitoneal cavity and pelvis, with maximum SUV activities above 20.4.
► Her tumor was evaluated twice through original biopsy as well as recurrent disease subtype analysis through molecular profiling performed by Caris Target Now Lab, Clarity Foundation, and Foundation One laboratories as well as Clarient laboratories, which were consistent with Biofocus findings on CTC, for presence of C MYC mutated cells, as well as positive PI3K activating mutations, and increased PR, PGP, and very high Ki 67 (at 80 percent), and ERBB2 expression, mutated Rb, mutated SOX2, MDR and P53. Her tumor was also sent for Guardant 360 analysis of the ctDNA, which showed PI3KCA overexpressive circulatory DNA presence in the blood.
► She immediately was started on IV epigenetic therapies which she received on daily basis, for two weeks. She did not receive any chemotherapies nor changed her diet or supplements. Her labs were repeated on 7/6/15, and it revealed increased HE-4 still at 384 as well as increased CA 15.3 (135). She improved clinically with less neuropathy and nails grew. Her ECOG also improved. Her circulatory tumor cell assay was repeated and it showed significant decrease in her C MYC EpCAM marker, indicating that the CTC responded to the therapy and there was improved (decreased) tumor burden suggested by the lab.
► This quick response in the CTC markers is exciting and points into possible angiogenic response in the patients with refractory ovarian cancer who have failed other potential therapies.
4.14. Sample Case 14: Endometrial Clear Cell Carcinoma
► 72-year-old female with history of clear cell endometrial cancer diagnosed in 2010, status post-surgery TAH/RSO (bilateral oophorectomy) and status post recurrence with a large (10 × 8 cm) mass in the pelvis, obstructing the right ureter and resulted right hydronephrosis. She was diagnosed in January 2015, documented by a core biopsy performed on 2/13, which was positive for adenocarcinoma (at this time, unknown site of origin) at the omentum (peritoneal carcinomatosis), treated with 6 cycles of adjuvant Carboplatinum and Taxol last one received 4 weeks prior to her admission to our clinic. She also had a remote history of cervical cancer that was treated in 1992 with radiation. Her recent colonoscopy was negative.
► Her main concerns were related to chemotherapy toxicity, including neuropathy and pain, night sweats, cough and dizziness.
► Initial evaluation revealed increased tumor markers of CA 125 at 98 as well as CA 15.3 at 123.2 and HE-4 at 305, and VEGF (at 200). Her blood was sent to Germany (BioFocus Lab) for evaluation of the circulatory tumor cells, and it was positive for CK 19 and telomerase.
► Her tumor tissue molecular profiling showed positive mutations in PI3k as well as ATM gene. Her circulatory DNA was not detectable.
► She immediately was started on IV epigenetic therapies which she received on daily basis. Her symptoms improved just after three days. Her function and ECOG improved. Her tumor markers were repeated after 10 treatments (14 days), as well as her CTC. Her CA 15.3 dropped post therapy down from 123 to 115.8 and her CTC completely disappeared from her blood. (Telomerase and CK 19 negative). Her VEGF also dropped from 200 (measured on 7/10/15) down to 37 (measured on 7/28/15) with therapy.
► Her tumor became operable and was resected/debulked on 1/10/16.
4.15. Sample Case 15: Malignant Melanoma
► 67-year-old female with history of a scalp birth mark that grew to a sizeable mass (2 cm) , with pathology of malignant melanoma diagnosed in 6/18/15 status post-surgical resection, pathology with positive (+) margins, for malignant melanoma, the PET scan confirmed the suspected malignant cervical LNs in right neck, with max SUV activity of 2.8. No other distant organ involved. She further was recommended to remove the residual disease as well as neck lymph node dissection. She saw us for evaluation and therapy. The initial labs indicated normal LDH, as well as S100B. The circulatory tumor cell assay indicated presence of CTC with positive c KIT as well as MART-1. She subsequently started the IV epigenetic therapies which she received on daily basis, for two weeks (10 treatments total).
► She did not change her diet nor received any other therapies.
► Post therapy: The labs were repeated and this time the CTC reported negative, with no indication for presence of C KIT or MART-1 positive cells in liquid biopsy.
► This quick response on epigenetic therapy resulting in complete resolution of micro-metastatic disease and down staging patients from stage four to three, is essentially novel and presents a prove of concept on effectiveness of this therapy. Certainly performing surgical resection of the remaining tumor as well as lymph node dissection would have not been justified when the disease is already spread, and therefore the results of such therapy can potentially change an outcome for this patient from an incurable disease to curable.
► Subsequently, the patient was treated with surgical resection and maintains complete radiological remission for over 12 months at the time of this article.
Case 15 Figure 1.
Case 15 Figure 2.
4.16. Sample Case 16: Metastatic Gastric Cancer
► 58-year-old female with history of gastric poorly differentiated adenocarcinoma who has been treated with Folfox regimen in December 2014, status post good response but an early recurrence in January 2015 and rapid progression since March 2015, she was receiving Taxol and Ramicurimab since June 2015, with no objective response. She is clinically was wasted and has to perform pleural effusions every two to three weeks, Last drew 0.7 liters. She was fatigued and was on wheel chair.
► She was referred by her oncologist to us to receive IV epigenetic therapies in combination with her regimen. Initial evaluation revealed significantly high tumor markers (CEA at 68.4, CA 19.9 at 2759) and elevated He 4 and interleukins 8. Her circulatory DNA was positive for P53 mutation as well as KIT, AR, and CTNNB1, and FGFR2.
► She immediately was started on IV epigenetic therapies which she received for 6 sessions, along with her chemotherapy (unchanged). For the first time, her tumor markers dropped in 10 days. Her CEA dropped from 68 to 49, her CA 19.9 dropped from 2759 to 1764. (Measured on 8/20/15), in ten days. Her HE4 also dropped from 227 down to 198 (measured on 8/10/15 and 8/22/15 respectively). Her HE4 dropped from 227 to 198.
► Her circulatory tumor cells were positive before starting the treatments on 8/12/15. (Positive CK 20), Her Guardant positive for circulatory DNA, alterations; P53, AR, CNNB1, and FGFR2.
► She immediately felt better after the very first treatment, and her pain was gone. Her ECOG score improved 2 scores, and her mood was elevated. Her tumor markers dropped for the first time following the combination therapy. It is noticeable that her first chemo regimen (Taxol and Ramicurimab) was given on 7/22/15 at higher dose (136 and 550), and yet patient was progressing. After combinational therapy, the tumor markers for the first time dropped significantly. CEA which was increased from 4.2 on 1/14/15 to 68, dropped down to 49 in ten days.
► Her Taxol dose was reduced to 110, and ramicurimab reduced to 440, indicating the synergistic effect of MTET protocol with chemotherapy.
4.17. Sample Case 17: Hemangiopericytoma
► 64-year-old white male with advanced hemangiopericytoma, a form of a very rare cancer, resistant to all conventional therapies. He travelled from Oregon to see us. He had a prolonged and unsuccessful course of therapy since his diagnosis in 2009, and was status post-surgery, radiation, ablations and chemotherapy. Unfortunately, there were no approved therapies at his stage for his rare disease. He was referred to, and seen by our oncologist at CCare. The patient fused to see him again, as he was not offered any potentially effective therapy at the time, due to limited options for his advanced disease. He had already failed Temodar and radiation, with lesions present in the kidney (status post ablation at MD Anderson) as well as pancreatic mass that was growing despite all therapies offered by his oncologist.
► Restaging PET scan before starting the treatments have showed that his prior regimen of Avastin had failed, with evidently progressing disease. His tumor molecular profiling at Foundation Laboratory ordered by his oncologist was positive for RAD 50 and STAT mutations, along with PDGFBR.
► Potential targeted therapies could consist of Imatinib to block the PDGFB. Also studies had shown that Kampferol and Quercetin Block the JACK3 and the related IL-4 regulated PDGFB.
► After obtaining appropriate consent forms, he was started on IV epigenetic therapy with Quercetin on daily basis for two weeks. His VEGF was checked post therapy and showed significant reduction (decreased post therapies from 190 to 136), and further normalized (down to 37) during the therapy.
► His labs showed that his other marker, HE-4 was reduced from 303 and stabilized at 223 post therapy.
► During this time, it was found that his tumor molecular profiling showed increased EGFR expression, as well as positive response to anti-folates. We started him on Tarceva as well as Xeloda, (due to lack of TS in his Caris molecular profiling, making him a good candidate for Xeloda), both of which were off label medications for his condition, as there were no approved therapies by the standard of care that could potentially improve his survival at the time.
► Further restaging PET scan revealed progression in his metastatic lesions, mainly in the pancreatic mass, however the metabolic activity of the tumor stabilized. There were also no new lesions reported.
► We further ordered a CT guided diagnostic biopsy on this mass, which confirmed the origin of hemangiopericytoma. Unfortunately, his tumor, described as a form of sarcoma, was very resistant and his treatment options were very limited. Eflornithine has been suggested in the literature to reduce tumor growth, invasion and migration, in neuroblastoma and sarcomas, and studied safely at phase II trials. We discussed this option as it may add to his response. Plaquenil and Pentam have also been studied in sarcoma and as an autophagy inhibitor, and have proved to be effective in literature published in 2014 by Molecular and Cellular Oncology, and these were considered as off label drugs.
► Mayo Clinic studies have not proved cytotoxic therapies to be effective in improving survival, in this group of patients with high toxicity profile, therefore we continued his current regimen of targeted therapies, with adjusted dosing, and Xeloda per his tolerability. He had reported no side effects from the treatments.
► The patient was also suffering from chronic renal failure as a sequel of the treatments received at MD Anderson (tumor ablations in the kidney), which as the result of our therapy, improved significantly with his creatinine decreasing from 1.55 to 1.37.
► Unfortunately, the patient passed away due suspected myocardial infarction at his home, 10 months after his therapy. It is of note that many patients with this rare diagnosis have been reported to suffer from unknown death cause, likely cardiac conditions such as MI or coronary occlusions, demonstrated in the Mayo clinic study [51] . This case presents an angiogenic response to the interim therapy and could suggest further hypothesis generation in combination of such therapy for such rare disease to the current ineffective therapies.
5. Conclusions
Here we discuss the central importance of emerging therapeutic tools in targeting hypoxia by modifying epigenome. We propose a novel approach consisting of combination of epigenetic modifiers in a protocol, both with in vitro and in vivo promising results. Future research is warranted in a large clinical trial studying such combination for advanced cancer.
We understand that non randomized reviews can statistically suffer from selection bias. In this article, we reviewed all patients with positive CTC, (no selection), who were treated with this therapy, to minimize such potential bias. We also understand that the current study analyzes different types of solid tumors, and this could dilute the strength of the study on each type of tumor, however our design is a “basket design for biomarker”, therefore it precludes the traditional disadvantage of tumor type-response analysis.
Cite this paper
M. A. Nezami,Steven Hager,Jessica Garner, (2016) Epigenetic Tumor Response to Hypoxia: An Epimutation Pattern and a Method of Multi Targeted Epigenetic Therapy (MTET). Journal of Cancer Therapy,07,254-269. doi: 10.4236/jct.2016.74027
References
- 1. Thirlwell, C., Schulz, L., Dibra, H. and Beck, S. (2011) Suffocating Cancer: Hypoxia-Associated Epimutations as Targets for Cancer Therapy. Clinical Epigenetics, 3, 9.
http://dx.doi.org/10.1186/1868-7083-3-9 - 2. Anderson, A., Weaver, A., Cummings, P. and Quaranta, V. (2006) Tumor Morphology and Phenotypic Evolution Driven by Selective Pressure from the Microenvironment. Cell, 127, 905-915.
http://dx.doi.org/10.1016/j.cell.2006.09.042 - 3. Yeh, J. and Kim, W. (2015) Targeting Tumor Hypoxia with Hypoxia-Activated Prodrugs. Journal of Clinical Oncology, 1505-1508.
http://dx.doi.org/10.1200/JCO.2014.60.0759 - 4. Ke, Q. and Costa, M. (2006) Hypoxia-Inducible Factor-1 (HIF-1). Molecular Pharmacology, 70, 1469-1480.
http://dx.doi.org/10.1124/mol.106.027029 - 5. Devlin, C., Greco, S., Martelli, F. and Ivan, M. (2011) MiR-210: More than a Silent Player in Hypoxia. IUBMB Life.
http://dx.doi.org/10.1002/iub.427 - 6. Fath, D., Kong, X., Liang, D., Lin, Z., Chou, A., Jiang, Y., et al. (2006) Histone Deacetylase Inhibitors Repress the Transactivation Potential of Hypoxia-Inducible Factors Independently of Direct Acetylation of HIF-α. Journal of Biological Chemistry, 281, 13612-13619.
http://dx.doi.org/10.1074/jbc.M600456200 - 7. Heddleston, J., Li, Z., Lathia, J., Bao, S., Hjelmeland, A. and Rich, J. (2010) Hypoxia Inducible Factors in Cancer Stem Cells. British Journal of Cancer, 102, 789-795.
http://dx.doi.org/10.1038/sj.bjc.6605551 - 8. Harris, A. (2002) Hypoxia—A Key Regulatory Factor in Tumour Growth. Nature Reviews Cancer, 2, 38-47.
http://dx.doi.org/10.1038/nrc704 - 9. Couvelard, A., O’toole, D., Turley, H., Leek, R., Sauvanet, A., Degott, C., et al. (2004) Microvascular Density and Hypoxia-Inducible Factor Pathway in Pancreatic Endocrine Tumours: Negative Correlation of Microvascular Density and VEGF Expression with Tumour Progression. British Journal of Cancer, 94-101.
- 10. Hockel, M. and Vaupel, P. (2001) Tumor Hypoxia: Definitions and Current Clinical, Biologic, and Molecular Aspects. Journal of the National Cancer Institute, 93, 266-276.
http://dx.doi.org/10.1093/jnci/93.4.266 - 11. Varia, M., Calkins-Adams, D., Rinker, L., Kennedy, A., Novotny, D., Fowler, W., et al. (1998) Pimonidazole: A Novel Hypoxia Marker for Complementary Study of Tumor Hypoxia and Cell Proliferation in Cervical Carcinoma. Gynecologic Oncology, 71, 270-277.
http://dx.doi.org/10.1006/gyno.1998.5163 - 12. Pouysségur, J., Dayan, F. and Mazure, N. (2006) Hypoxia Signalling in Cancer and Approaches to Enforce Tumour Regression. Nature, 441, 437-443.
http://dx.doi.org/10.1038/nature04871 - 13. Henze, A. and Acker, T. (2010) Feedback Regulators of Hypoxia-Inducible Factors and Their Role in Cancer Biology. Cell Cycle, 9, 2821-2835.
http://dx.doi.org/10.4161/cc.9.14.12249 - 14. Shahrzad, S., Bertrand, K., Minhas, K. and Coomber, B. (2007) Induction of DNA Hypomethylation by Tumor Hypoxia. Epigenetics, 2, 119-125.
http://dx.doi.org/10.4161/epi.2.2.4613 - 15. Wang, J.-Q. and Wu, K.-J. (2015) Epigenetic Regulation of Epithelial-Mesenchymal Transition by Hypoxia in Cancer: Targets and Therapy. Current Pharmaceutical Design, 21, 1272-1278.
http://dx.doi.org/10.2174/1381612821666141211145610 - 16. Fratelli, M., Goodwin, L., Orom, U., Lombardi, S., Tonelli, R., Mengozzi, M., et al. (2005) Gene Expression Profiling Reveals a Signaling Role of Glutathione in Redox Regulation. Proceedings of the National Academy of Sciences of the United States of America, 102, 13998-14003.
http://dx.doi.org/10.1073/pnas.0504398102 - 17. Watson, J., Watson, C., Mccrohan, A., Woodfine, K., Tosetto, M., Mcdaid, J., et al. (2009) Generation of an Epigenetic Signature by Chronic Hypoxia in Prostate Cells. Human Molecular Genetics, 18, 3594-3604.
http://dx.doi.org/10.1093/hmg/ddp307 - 18. Johnson, A., Denko, N. and Barton, M. (2008) Hypoxia Induces a Novel Signature of Chromatin Modifications and Global Repression of Transcription. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 640, 174-179.
http://dx.doi.org/10.1016/j.mrfmmm.2008.01.001 - 19. Pollard, P., Loenarz, C., Mole, D., Mcdonough, M., Gleadle, J., Schofield, C., et al. (2008) Regulation of Jumonji-Domain-Containing Histone Demethylases by Hypoxia-Inducible Factor (HIF)-1α. Biochemical Journal, 416, 387-394.
http://dx.doi.org/10.1042/BJ20081238 - 20. Jones, P. and Baylin, S. (2007) The Epigenomics of Cancer. Cell, 128, 683-692.
http://dx.doi.org/10.1016/j.cell.2007.01.029 - 21. Gao, P., Zhang, H., Dinavahi, R., Li, F., Xiang, Y., Raman, V., et al. (2007) HIF-Dependent Antitumorigenic Effect of Antioxidants in Vivo. Cancer Cell, 12, 230-238.
http://dx.doi.org/10.1016/j.ccr.2007.08.004 - 22. Cairns, R. and Hill, R.P. (2004) Acute Hypoxia Enhances Spontaneous Lymph Node Metastasis in an Orthotopic Murine Model of Human Cervical Carcinoma. Cancer Research, 64, 2054-2061.
http://dx.doi.org/10.1158/0008-5472.CAN-03-3196 - 23. Xia, X., Lemieux, M., Li, W., Carroll, J., Brown, M., Liu, X., et al. (2009) Integrative Analysis of HIF Binding and Transactivation Reveals Its Role in Maintaining Histone Methylation Homeostasis. Proceedings of the National Academy of Sciences of the United States of America, 106, 4260-4265.
http://dx.doi.org/10.1073/pnas.0810067106 - 24. Lee, D.-H. and Lee, Y.J. (2008) Quercetin Suppresses Hypoxia-Induced Accumulation of Hypoxia-Inducible Factor-1α (HIF-1α) through Inhibiting Protein Synthesis. Journal of Cellular Biochemistry, 105, 546-553.
http://dx.doi.org/10.1002/jcb.21851 - 25. Bach, A., Bender-Sigel, J., Schrenk, D., Flügel, D. and Kietzmann, T. (2010) The Antioxidant Quercetin Inhibits Cellular Proliferation via HIF-1-Dependent Induction of p21WAF. Antioxidants & Redox Signaling, 13, 437-448.
http://dx.doi.org/10.1089/ars.2009.3000 - 26. Semenza, G. (2003) Targeting HIF-1 for Cancer Therapy. Nature Reviews Cancer, 3, 721-732.
http://dx.doi.org/10.1038/nrc1187 - 27. Maxwell, P. (2005) The HIF Pathway in Cancer. Seminars in Cell & Developmental Biology, 16, 523-530.
http://dx.doi.org/10.1016/j.semcdb.2005.03.001 - 28. Gheorghe, C., Mohan, S., Oberg, K. and Longo, L. (2007) Gene Expression Patterns in the Hypoxic Murine Placenta: A Role in Epigenesis? Reproductive Sciences, 14, 223-233.
- 29. Kim, S., Kim, K. and Jeong, J. (2007) Inhibition of Hypoxia-Induced Angiogenesis by Sodium Butyrate, A Histone Deacetylase Inhibitor, through Hypoxia-Inducible Factor-1α Suppression. Oncology Reports, 17, 793-797.
http://dx.doi.org/10.3892/or.17.4.793 - 30. Bao, S., et al. (2006) Stem Cell-Like Glioma Cells Promote Tumor Angiogenesis through Vascular Endothelial Growth Factor. Cancer Research, 66, 7843-7848.
http://dx.doi.org/10.1158/0008-5472.CAN-06-1010 - 31. Pal, A., Srivastava, T., Sharma, M., Mehndiratta, M., Das, P., Sinha, S., et al. (2010) Aberrant Methylation and Associated Transcriptional Mobilization of Alu Elements Contributes to Genomic Instability in Hypoxia. Journal of Cellular and Molecular Medicine, 14, 2646-2654.
http://dx.doi.org/10.1111/j.1582-4934.2009.00792.x - 32. Feinberg, A. and Vogelstein, B. (1983) Hypomethylation Distinguishes Genes of Some Human Cancers from Their Normal Counterparts. Nature, 301, 89-92.
http://dx.doi.org/10.1038/301089a0 - 33. Wang, J., Chen, J., Chang, P., Leblanc, A., Li, D., Abbruzzesse, J., et al. (2009) MicroRNAs in Plasma of Pancreatic Ductal Adenocarcinoma Patients as Novel Blood-Based Biomarkers of Disease. Cancer Prevention Research, 2, 807-813.
http://dx.doi.org/10.1158/1940-6207.CAPR-09-0094 - 34. Irizarry, R., Ladd-Acosta, C., Wen, B., Wu, Z., Montano, C., Onyango, P., et al. (2009) The Human Colon Cancer Methylome Shows Similar Hypo- and Hypermethylation at Conserved Tissue-Specific CpG Island Shores. Nature Genetics, 41, 178-186.
http://dx.doi.org/10.1038/ng.298 - 35. Gee, H., Camps, C., Buffa, F., Patiar, S., Winter, S., Betts, G., et al. (2010) Hsa-miR-210 Is a Marker of Tumor Hypoxia and a Prognostic Factor in Head and Neck Cancer. Cancer, 116, 2148-2158.
http://dx.doi.org/10.1002/cncr.25009 - 36. Kim, S., et al. (2004) Carbonic Anhydrase IX in Early-Stage Non-Small Cell Lung Cancer. Clinical Cancer Research, 10, 7925-7933.
http://dx.doi.org/10.1158/1078-0432.CCR-04-0636 - 37. Louie, E., Nik, S., Chen, J., Schmidt, M., Song, B., Pacson, C., et al. (2010) Identification of a Stem-Like Cell Population by Exposing Metastatic Breast Cancer Cell Lines to Repetitive Cycles of Hypoxia and Reoxygenation. Breast Cancer Research, 12, R94.
http://dx.doi.org/10.1186/bcr2773 - 38. Suzuki, H., Tomida, A. and Tsuruo, T. (2001) Dephosphorylated Hypoxia-Inducible Factor 1α as a Mediator of p53-Dependent Apoptosis during Hypoxia. Oncogene, 20, 5779-5788.
http://dx.doi.org/10.1038/sj.onc.1204742 - 39. Doi, A., Park, I., Wen, B., Murakami, P., Aryee, M., Irizarry, R., et al. (2009) Differential Methylation of Tissue- and Cancer-Specific CpG Island Shores Distinguishes Human Induced Pluripotent Stem Cells, Embryonic Stem Cells and Fibroblasts. Nature Genetics, 41, 1350-1353.
http://dx.doi.org/10.1038/ng.471 - 40. Sadikovic, B., Andrews, J., Carter, D., Robinson, J. and Rodenhiser, D.I. (2007) Genome-Wide H3K9 Histone Acetylation Profiles Are Altered in Benzopyrene-Treated MCF7 Breast Cancer Cells. Journal of Biological Chemistry, 283, 4051-4060.
http://dx.doi.org/10.1074/jbc.M707506200 - 41. Yang, J., Ledaki, I., Turley, H., Gatter, K., Montero, J., Li, J. and Harris, A. (2009) Role of Hypoxia-Inducible Factors in Epigenetic Regulation via Histone Demethylases. Annals of the New York Academy of Sciences, 1177, 185-197.
http://dx.doi.org/10.1111/j.1749-6632.2009.05027.x - 42. Divgi, C.R., Pandit-Taskar, N., Jungbluth, A.A., Reuter, V.E., Gonen, M., Ruan, S., et al. (2007) Preoperative Characterisation of Clear-Cell Renal Carcinoma Using Iodine-124-Labelled Antibody Chimeric G250 (124I-cG250) and PET in Patients with Renal Masses: A Phase I Trial. The Lancet Oncology, 8, 304-310.
http://dx.doi.org/10.1016/S1470-2045(07)70044-X - 43. Abe, T., Toyota, M., Suzuki, H., Murai, M., Akino, K., Ueno, M., et al. (2005) Upregulation of BNIP3 by 5-Aza-2’-Deoxycytidine Sensitizes Pancreatic Cancer Cells to Hypoxia-Mediated Cell Death. Journal of Gastroenterology, 40, 504-510.
http://dx.doi.org/10.1007/s00535-005-1576-1 - 44. Atadja, P. (2010) HDAC Inhibitors and Cancer Therapy. In: Gasser, S.M. and Li, E., Eds., Epigenetics and Disease, Springer, Basel, 175-195.
- 45. Alleman, W., et al. (2004) The in Vitro and in Vivo Effects of Re-Expressing Methylated von Hippel-Lindau Tumor Suppressor Gene in Clear Cell Renal Carcinoma with 5-Aza-2’-Deoxycytidine. Clinical Cancer Research, 10, 7011-7021.
http://dx.doi.org/10.1158/1078-0432.CCR-04-0516 - 46. Kim, W.Y. and Kaelin, W.G. (2004) Role of VHL Gene Mutation in Human Cancer. Journal of Clinical Oncology, 22, 4991-5004.
http://dx.doi.org/10.1200/JCO.2004.05.061 - 47. Gossage, L. and Eisen, T. (2010) Alterations in VHL as Potential Biomarkers in Renal-Cell Carcinoma. Nature Reviews Clinical Oncology, 7, 277-288.
http://dx.doi.org/10.1038/nrclinonc.2010.42 - 48. Polyak, K. and Weinberg, R. (2009) Transitions between Epithelial and Mesenchymal States: Acquisition of Malignant and Stem Cell Traits. Nature Reviews Cancer, 9, 265-273.
http://dx.doi.org/10.1038/nrc2620 - 49. Venugopal, B. and Evans, T. (2011) Developing Histone Deacetylase Inhibitors as Anti-Cancer Therapeutics. Current Medicinal Chemistry, 18, 1658-1671.
http://dx.doi.org/10.2174/092986711795471284 - 50. Chen, S. and Sang, N. (2010) Histone Deacetylase Inhibitors: The Epigenetic Therapeutics That Repress Hypoxia-Inducible Factors. Journal of Biomedicine and Biotechnology, 2010, Article ID: 197946.
- 51. McMaster, M.J., Soule, E.H. and Ivins, J.C. (1975) Hemangiopericytoma: A Clinicopathologic Study and Long-Term Followup of 60 Patients. Cancer, 36, 2232-2244.
http://dx.doi.org/10.1002/cncr.2820360942