Journal of Cancer Therapy
Vol.4 No.5(2013), Article ID:33772,9 pages DOI:10.4236/jct.2013.45106
Bicarbonate Attenuates Irinotecan-Induced Cytotoxicity through Regulation of Both Extracellular and Intracellular pHs in Intestine Cell Line
![]()
1Joint Research Center, Tokyo Medical University Ibaraki Medical Center, Ami, Ibaraki, Japan; 2Department of Internal Medicine, Division of Gastroenterology and Hepatology, Tokyo Medical University Ibaraki Medical Center, Ami, Ibaraki, Japan; 3Department of Respiratory Medicine, Saitama International Medical Center, Saitama Medical University, Hidaka, Saitama, Japan; 4Department of Biochemistry and Molecular Biology, The George Washington University, Washington DC, USA; 5Department of Pharmacology, The George Washington University, Washington DC, USA.
Email: *teruom@tokyo-med.ac.jp
Copyright © 2013 Teruo Miyazaki et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 14th, 2013; revised June 12th, 2013; accepted June 19th, 2013
Keywords: Bicarbonate; SN-38; CPT-11; pHi; Anti-Cancer Drug; Intestine
ABSTRACT
The anti-cancer therapy of irinotecan (CPT-11) is often limited due to severe late-onset diarrhea. Because the higher toxic form of CPT-11/its active metabolite (SN-38) is produced at acidification, the usefulness of oral sodium bicarbonate treatment against the CPT-11/SN-38-induced intestinal injuries and diarrhea has been confirmed. However, the roles of bicarbonate have been suggested to affect not only intestinal pH environment but also intracellular pH and CPT-11/SN-38 dynamics. The present study proposed to clarify the hypothesis in CPT-11/SN-38-exposed colon cell line in various pH conditions adjusted by bicarbonate. HT29 cell pre-exposed to ~1.0 µM SN-38 lactone or carboxylate forms was incubated at different pH adjusted by either bicarbonate or HCl/NaOH. The degrees of SN-38-induced cell injury depended on the higher proportion of the toxic form (lactone) of SN-38 rather than mere pH condition of medium. Apoptosis and cell injury induced by SN-38 were significantly inhibited by bicarbonate in a dose-dependent manner. Intercellular pH acidification induced by SN-38 was significantly prevented by 30 mM bicarbonate. Cell cytotoxicity of SN-38 depended on not only extracellular but also intracellular pH that converts the SN-38 form, while the intracellular acidification was prevented by bicarbonate. The multiple regulations of bicarbonate on both exracellular and intracellular pH would be essential mechanism against intestinal cell injury by CPT-11/SN-38.
1. Introduction
Irinotecan hydrochloride (CPT-11; 7-ethyl-10-[4-(1-piperidino)-1-piperidino] carbonyloxy-camptothecin) is a water-soluble derivative of camptothecin, an alkaloid isolated from Camptotheca acuminata, and presents a wide spectrum of antitumor activity through the inhibition of DNA topoisomerase I [1]. This chemotherapeutic agent has been approved by the FDA for treatment of colorectal cancer in the United States, but has shown clinical responses for many other malignancies such as lung, gastric, pancreatic, cervical, and ovarian cancers as well as leukemia and lymphoma [2-6]. However, this agent has major limitation in the therapeutic use for the patients with cancer due to severe side effect as the lethal delayed diarrhea [7,8] including the marked intestinal injury and the presence of pseudomembranous jejunoileitis [9].
CPT-11 is hydrolyzed to active 7-ethyl-10-hydrocy-camptothecin (SN-38) by hepatic carboxylesterase [10], and SN-38 has at least a 100-fold more potent antitumor effect than CPT-11 [1]. The ability of both CPT-11 and SN-38 relies on pH. Both CPT-11 and SN-38 have a labile a-hydroxy-3-lactone ring, which undergoes reversible hydrolysis at a rate that is mainly pH-dependent [11]. At acidic condition, the lactone ring of CPT-11/SN-38 closes, and this form is called as a lactone form. On the other hand, the lactone ring opens at physiological pH and higher, and yields a carboxylate form. The carboxylate form is a less potent inhibition of the topoisomerase I and has much weaker antitumor activity than its lactone counterpart. Therefore, one of the possible strategies for prevention from the CPT-11-induced side effects might be manipulating the pH-dependent interconversion. Indeed, we and others have previously confirmed the oral bicarbonate (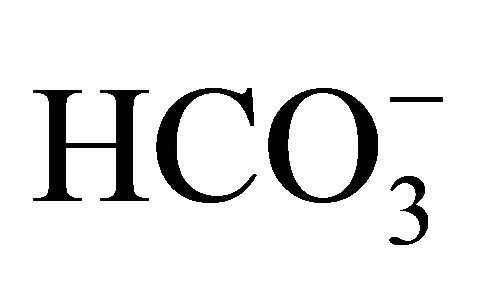 ) administration prior to and/or during CPT-11 treatment avoided acidifications in intestinal lumen and stool, and consequently, could decrease the incidence of side effects including delayed diarrhea, nausea, vomiting, myelotoxicity, and lymphocytopenia in the patients with lung cancer [12], advanced gastrointestinal cancer [13] or colorectal cancer [14], and the experimental animal model [12,15]. Therefore, it has been believed that oral
) administration prior to and/or during CPT-11 treatment avoided acidifications in intestinal lumen and stool, and consequently, could decrease the incidence of side effects including delayed diarrhea, nausea, vomiting, myelotoxicity, and lymphocytopenia in the patients with lung cancer [12], advanced gastrointestinal cancer [13] or colorectal cancer [14], and the experimental animal model [12,15]. Therefore, it has been believed that oral 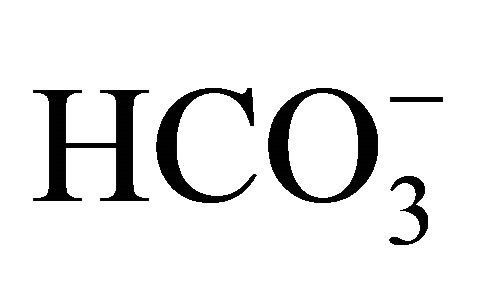 treatment is effectiveness on the attenuation of the side effects in CPT-11 anticancer therapy through the keeping of intestinal alkalization.
treatment is effectiveness on the attenuation of the side effects in CPT-11 anticancer therapy through the keeping of intestinal alkalization.
Additionally, our previous studies also confirmed that there was significant difference in the initial uptake rate of CPT-11/SN-38 by isolated hamster intestinal cells between the respective lactone and carboxyrate forms [9,15]. The uptake rate of the lactone form that is passively transported was higher than the carboxyrate form that is actively absorbed. Therefore, the regulation of ambient pH environment is also important factor for the intracellular uptake of CPT-11/SN-38.
Furthermore, intracellular pH (pHi) is also associated with anti-cancer action of the agent, because it generally influences the cellular proliferation and death [16-19]. Because intracellular acidification is an early event in apoptosis and also essential in genomic DNA destruction [17], it has been also considered as one of the multiplier factors to emphasize the inhibitive action of SN-38 on the topoisomerase I [20]. In addition to the regulation of ambient pH, 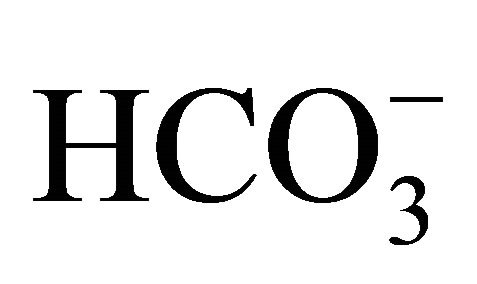 has been reported to act as a regulator of pHi in some cells through Na+-dependent Cl−/
has been reported to act as a regulator of pHi in some cells through Na+-dependent Cl−/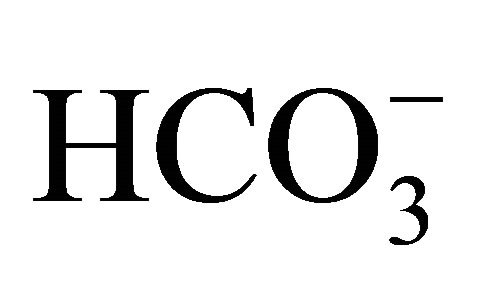 exchange, Na+-independent Cl−/
exchange, Na+-independent Cl−/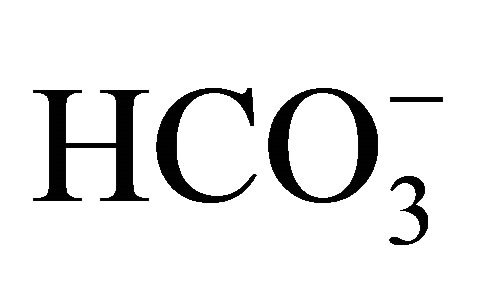 exchange, and Na+-
exchange, and Na+-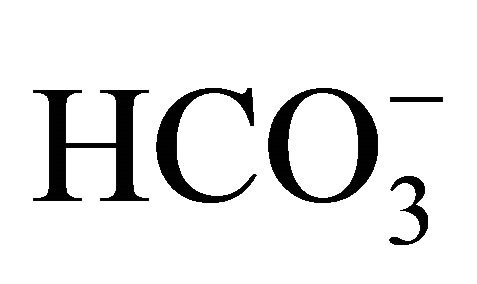 cotransport [21-23]. So, it is suggested that
cotransport [21-23]. So, it is suggested that 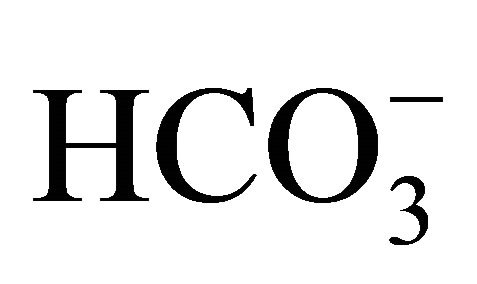 might have another potential protective action against SN-38 through the regulation of the pHi.
might have another potential protective action against SN-38 through the regulation of the pHi.
While the oral 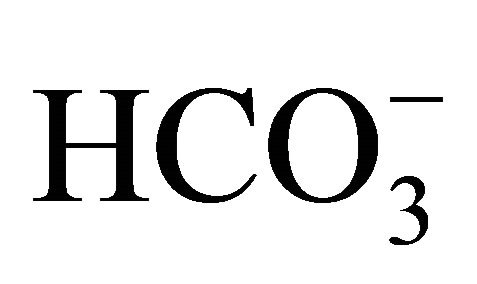 treatment has demonstrated scientific and clinical benefits on CPT-11 treated anticancer therapy, there is few understanding of its action. In the present study, we purposed to clarify the implication of
treatment has demonstrated scientific and clinical benefits on CPT-11 treated anticancer therapy, there is few understanding of its action. In the present study, we purposed to clarify the implication of 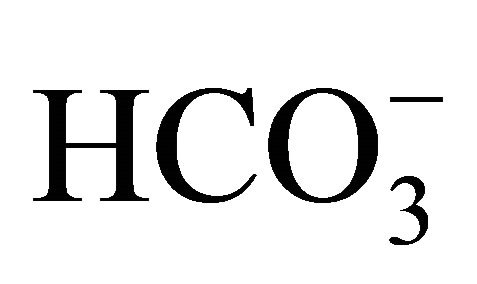 on pH-dependent CPT-11/SN-38 toxicity in the human colon adenocarcinoma HT29 cell line under various culture pH environments adjusted with/without
on pH-dependent CPT-11/SN-38 toxicity in the human colon adenocarcinoma HT29 cell line under various culture pH environments adjusted with/without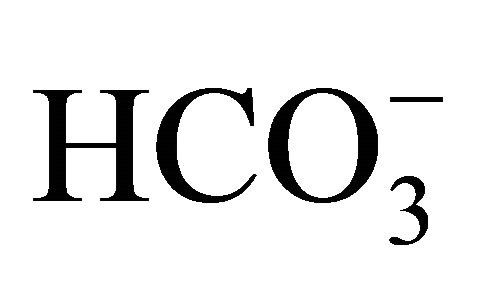 .
.
2. Materials and Methods
2.1. Materials
SN-38 was supplied by Yakult Honsha Co., Ltd. (TokyoJapan) and was 98% - 99% pure as judged by gas-liquid chromatography. Nigericine was purchased from Sigma (St. Louis, MO). Snarf1-AM was from Calbiochem (San Diego, CA). Other chemicals were of the highest purity available. Dulbecco’s Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco®, Invitrogen Corporation (Carlsbad, CA). For the culture conditions, the HT29 cell line (ATCC®, Manassas, VA) was maintained in DMEM supplemented with 10% heat-inactivated FBS, as previously described [9,24, 25]. The HT29 model was selected since we have previously reported a similar respective mechanism of uptake of SN-38 lactone and carboxylate to that reported in isolated intestinal cells [26]. Moreover, HT29 cells allow for long-term studies that are not possible using the isolated enterocyte model.
2.2. Chemical (SN-38) Preparation
SN-38 stock solution was prepared by dissolution with DMSO due to highly hydrophobicity. The SN-38 stock solution was incubated with 50 mM PBS, pH 3.0 or 9.0, overnight at RT, as a 100-fold dilution, to obtain the lactone and carboxylate forms, respectively [27]. At this final concentration, DMSO was confirmed to have no effect on the initial uptake of the agent [26]. SN-38 at concentrations up to 5 µM did not significantly change the pH of the culture medium. The resultant solution was 98% lactone form at pH 3.0 and 97% carboxylate form at pH 9.0 (HPLC determination by Dr. K. Arimori and T. Hidaka, Dept. Pharmacy, Miyazaki Medical School, Miyazaki, Japan). For cell culture, SN-38 was diluted to final concentrations of 0.5 or 1.0 µM in DMEM.
2.3. Effect of Extracellular pH on SN-38-Induced Cytotoxicity
HT29 cells seeded on a 96-well plate as density at 1 × 104 cells/well were cultured for 24 h, and then exposed to either 0 or 0.5 µM SN-38 lactone/carboxylate in DMEM for 2 h that the exposed period does not affect the interconversion of SN-38 [10,28]. The cells were then washed twice with PBS, and further incubated in DMEM without the SN-38 at pH 6.2 or 7.2 set by HCl/NaOH for 96 h. Thereafter, cell viability was measured by the MTT assay, and expressed as a relative ratio to control (0 µM SN-38) in each culture condition.
2.4. The Effect of Bicarbonate-Adjusted pH before and after SN-38 Exposure
HT29 cells seeded on a 24-well plate at a density of 5 × 104 cells/well were cultured in standard condition for 24 h, and then, were incubated with DMEM containing 25 mM HEPES and increasing concentrations (5, 20, 44, 100 mM) of 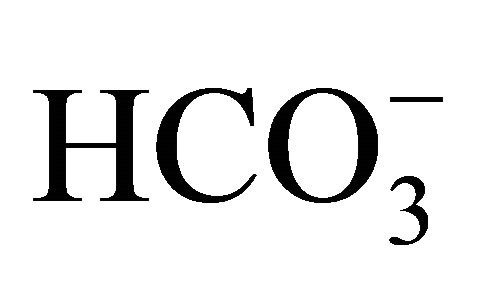 for 2 h prior to SN-38 exposure.
for 2 h prior to SN-38 exposure.
The 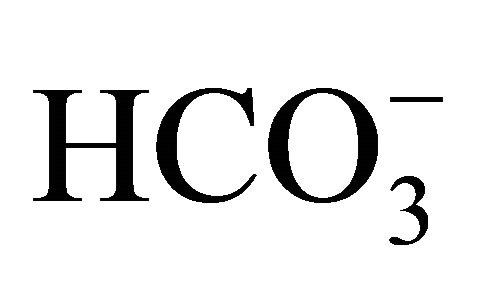 concentrations resulted in the pH adjustment at 7.0, 7.2, 7.4, and 7.8, respectively. The osmolality was maintained constant among the different
concentrations resulted in the pH adjustment at 7.0, 7.2, 7.4, and 7.8, respectively. The osmolality was maintained constant among the different 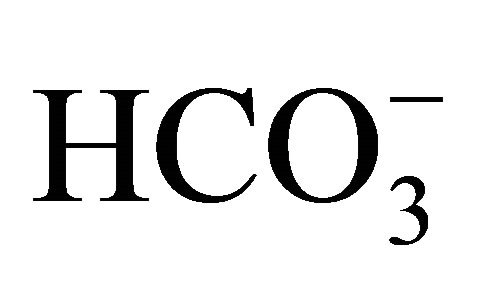 buffers. After the incubation with
buffers. After the incubation with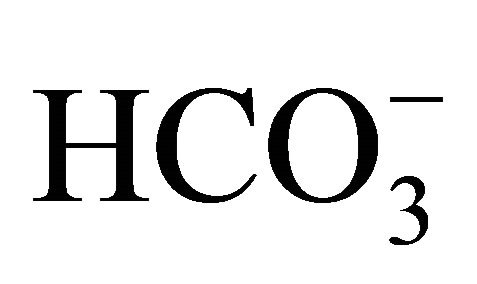 , cell was exposed to either 0.5 or 1.0 µM SN-38 lactone for an additional 2 h. Thereafter, cell was washed twice with PBS, and further incubated for an additional 96 h with the respective
, cell was exposed to either 0.5 or 1.0 µM SN-38 lactone for an additional 2 h. Thereafter, cell was washed twice with PBS, and further incubated for an additional 96 h with the respective 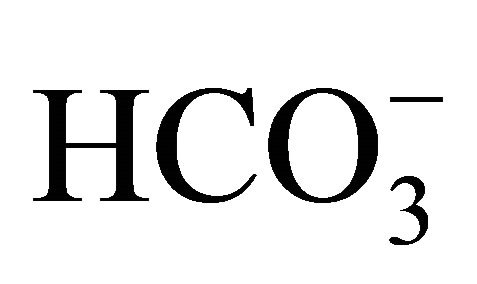 containing culture medium without SN-38. In parallel experiments, HT29 cell was cultured without
containing culture medium without SN-38. In parallel experiments, HT29 cell was cultured without  prior to the SN-38 exposure, and were incubated with the different concentrations of
prior to the SN-38 exposure, and were incubated with the different concentrations of 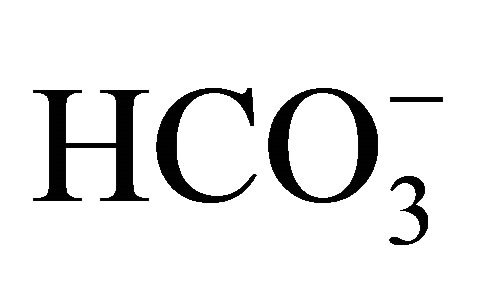 during the additional 96 h exposure.
during the additional 96 h exposure.
2.5. Cell Viability in Various pH Conditions with/without Bicarbonate
HT29 cell cultured under standard condition containing DMEM with 44 mM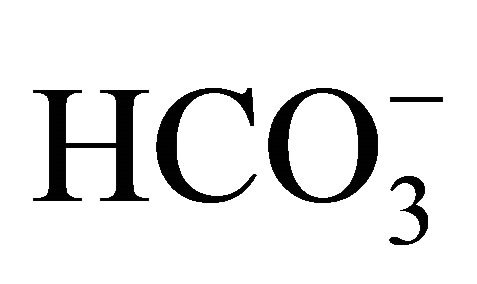 , at pH 7.4, was exposed to either 0 or 1.0 µM SN-38 lactone for 2 h. Thereafter, the washed cell was further incubated for an additional 48 h with 5-set points of pH at 6.8, 7.0, 7.4, 7.8, or 8.2 in culture medium adjusted by either increasing concentrations of
, at pH 7.4, was exposed to either 0 or 1.0 µM SN-38 lactone for 2 h. Thereafter, the washed cell was further incubated for an additional 48 h with 5-set points of pH at 6.8, 7.0, 7.4, 7.8, or 8.2 in culture medium adjusted by either increasing concentrations of 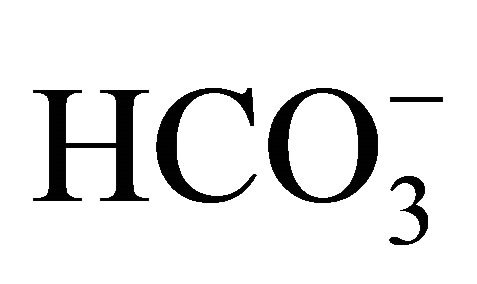 [5, 10, 20, 44, 100 mM, respectively;
[5, 10, 20, 44, 100 mM, respectively; 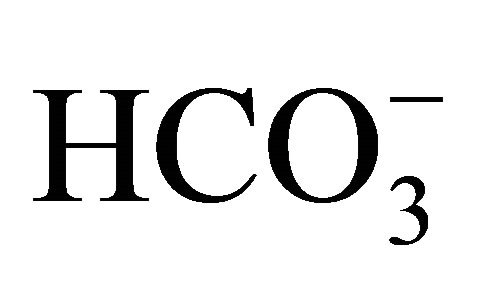 (+)] or NaOH with 5 mM
(+)] or NaOH with 5 mM 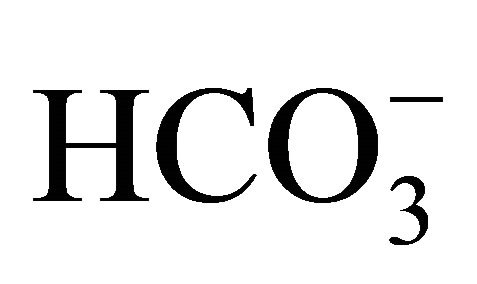 [
[ (−)]. During cell culture, acidification of medium is usually induced due to respiration of cells. Therefore, in the present experiment, the cell was cultured with different media in each condition to maintain pH during culture. DMEM contained 2.5 mM·L-glutamate, 1 mM sodium pyruvate, and 10% FBS, and each culture medium was constituted as shown in Table 1. The constitution of the medium was based on a previous study by Eagle et al. [29] and further modified in the present study. Cell proliferation after culture in these conditions was determined by the MTT assay.
(−)]. During cell culture, acidification of medium is usually induced due to respiration of cells. Therefore, in the present experiment, the cell was cultured with different media in each condition to maintain pH during culture. DMEM contained 2.5 mM·L-glutamate, 1 mM sodium pyruvate, and 10% FBS, and each culture medium was constituted as shown in Table 1. The constitution of the medium was based on a previous study by Eagle et al. [29] and further modified in the present study. Cell proliferation after culture in these conditions was determined by the MTT assay.
For detection of apoptotic cells in the various pH co-
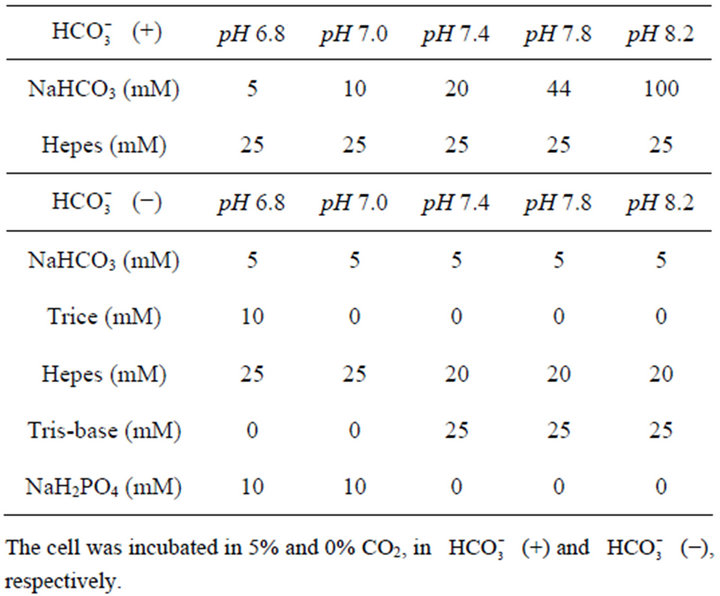
Table 1. Conditions of culture medium at different pHs.
nditions either with or without , following exposure to SN-38 lactone, the cell was incubated with 3-set points of pH at 6.8, 7.0, or 7.8 with increasing
, following exposure to SN-38 lactone, the cell was incubated with 3-set points of pH at 6.8, 7.0, or 7.8 with increasing 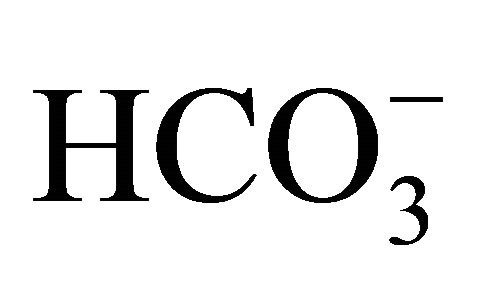 concentrations [
concentrations [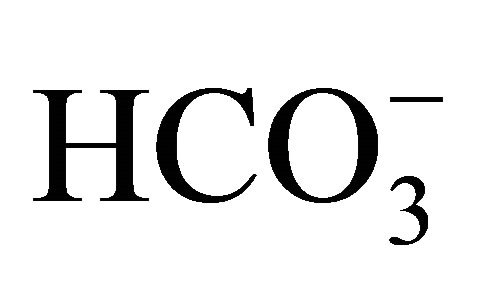 (+)] or without
(+)] or without 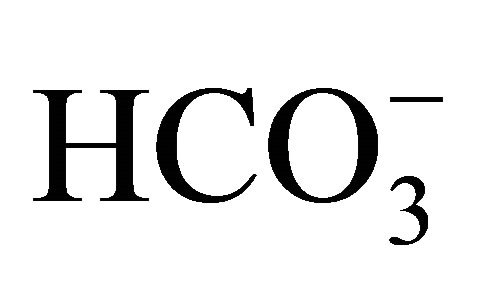 [
[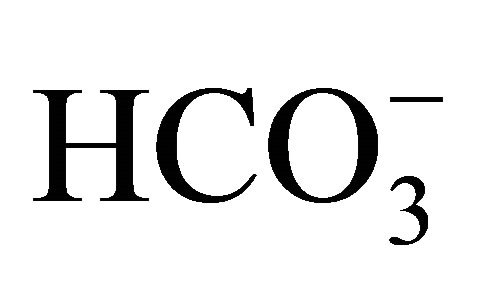 (−)] for an additional 48 h.
(−)] for an additional 48 h.
2.6. Cell Viability Assay
Cell viability was analyzed as cell proliferation and apoptosis by the MTT assay [30] and flow cytometry, respectively. In the MTT assay, following incubation of the cell with the indicated concentration of the agents, the medium was removed and the cell was further incubated with 0.5 mg/mL MTT in medium for 4 h at 37˚C. The blue formazan compound was solubilized with methanol containing 0.01 N HCl. The absorbance was determined at 560 nm, with 670 nm used as a reference in a spectrophotometer. In the flow cytometry, apoptotic HT29 cell was determined by annexin V and propidium iodide (PI) double staining using a commercially available kit (Annexin V-EGFP Apoptosis Detection Kit, BioVision, Inc., Mountain View, CA). Following the respective culture condition, the cell was harvested with trypsin and suspended with annexin V-enhanced green fluorescent protein (EGFP) and PI, and then analyzed by flow cytometry (FacSort, Becton Dickinson, San Jose, CA).
2.7. Intracellular pH Determination
HT29 cell plated at 1 × 106 cells per well was incubated with and without 0.5 µM SN-38 lactone for 2 h in culture medium containing increasing concentrations of 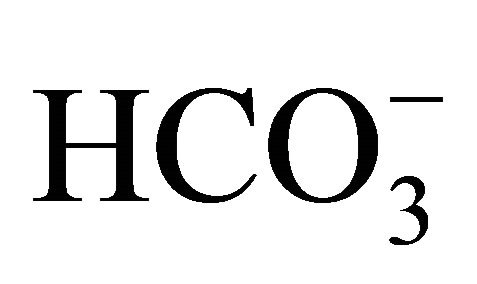 (10 - 58 mM). After removal of SN-38, the cell was maintained under the same culture conditions for an additional 4 h period before being loaded with 5 µM Snarf-1 acetoxymethyl acetate for 20 - 40 min at 37˚C. The cell was then washed, and the respective cellular fluorescence level was determined with the ACAS 570 laser scanner cytometer (Meridian Instruments Inc., Okemos, MI). A pH standard curve (6.8, 7.2, 7.4, 7.6, and 8.0) using high potassium concentration (100 mM) was determined for each series of experiments using either PIPES for buffer pH < 7 or HEPES for buffer pH > 7 and 10 µM nigericin. The cell membrane potassium ionophore nigericin causes the interior of the cells to equilibrate with the external pH of the buffer. The cells were excited with a 514 nm argon laser beam (5 W, Innova 90/5, Coherent) and the 587 nm and 640 nm emissions were simultaneously collected. The fluorescence intensity at each wavelength was detected using a photomultiplier tube. Images were acquired by scanning several fields of cells. Each image consisted of 3000 - 5000 discrete sample points. The ratio of the 640/587 nm emission data was used to determine the pHi. On average, the data were collected from 12 - 20 successive scans.
(10 - 58 mM). After removal of SN-38, the cell was maintained under the same culture conditions for an additional 4 h period before being loaded with 5 µM Snarf-1 acetoxymethyl acetate for 20 - 40 min at 37˚C. The cell was then washed, and the respective cellular fluorescence level was determined with the ACAS 570 laser scanner cytometer (Meridian Instruments Inc., Okemos, MI). A pH standard curve (6.8, 7.2, 7.4, 7.6, and 8.0) using high potassium concentration (100 mM) was determined for each series of experiments using either PIPES for buffer pH < 7 or HEPES for buffer pH > 7 and 10 µM nigericin. The cell membrane potassium ionophore nigericin causes the interior of the cells to equilibrate with the external pH of the buffer. The cells were excited with a 514 nm argon laser beam (5 W, Innova 90/5, Coherent) and the 587 nm and 640 nm emissions were simultaneously collected. The fluorescence intensity at each wavelength was detected using a photomultiplier tube. Images were acquired by scanning several fields of cells. Each image consisted of 3000 - 5000 discrete sample points. The ratio of the 640/587 nm emission data was used to determine the pHi. On average, the data were collected from 12 - 20 successive scans.
2.8. Statistical Analysis
Statistically significant differences were assessed by applying the Student’s unpaired t-test, paired t-test, or analysis of variance (ANOVA) where applicable with an a level of 0.05. Control experiments, using only the vehicle for solubilizing the respective agent, were performed in parallel. The results were expressed as the mean ± SD of the mean based on the number of studies. Each experiment was performed in duplicate or triplicate.
3. Results
3.1. Effect of Extracellular pH on SN-38-Induced Cellular Toxicity
Table 2 shows the cell viability of HT29 cell after exposure to either the SN-38 lactone or carboxylate form in different two pH conditions at 7.4 or 6.2 adjusted by NaOH/HCl. At pH 7.4, cell viability was significantly decreased in the exposure to carboxylate form compared to that in the control, and was further and significantly decreased in the exposure to lactone form compared to that in other conditions. The significantly decreased cell viability in the lactone form rather than the carboxylate form was also observed at pH 6.2. In addition, the viability in lactone form at pH 6.2 was significantly lower than carboxylate form at pH 7.4. Compared between pH 7.4 and 6.2, the toxic level of SN-38 lactone was almost similar. These results are in agreement with the previous study reporting that cell cytotoxicity of SN-38 depended on the form, i.e., lactate or carboxylate forms rather than extracellular pH [15].
3.2. The Difference of Bicarbonate Treatment between before and after SN-38 Exposure at Various pH Conditions in the Cellular Toxicity
Figure 1 presents the cell viability of HT29 cells cult-
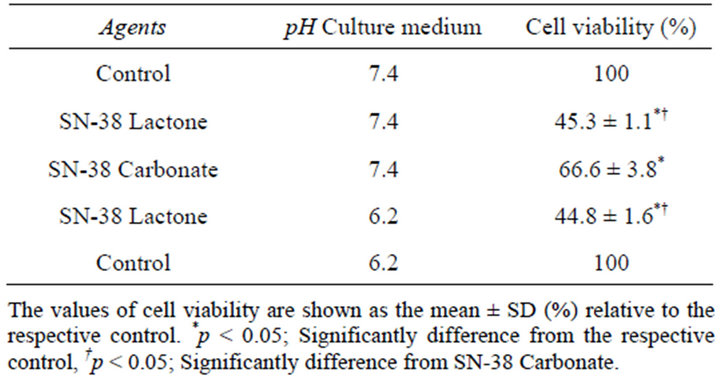
Table 2. Effect of medium pH on SN-38 toxicity.
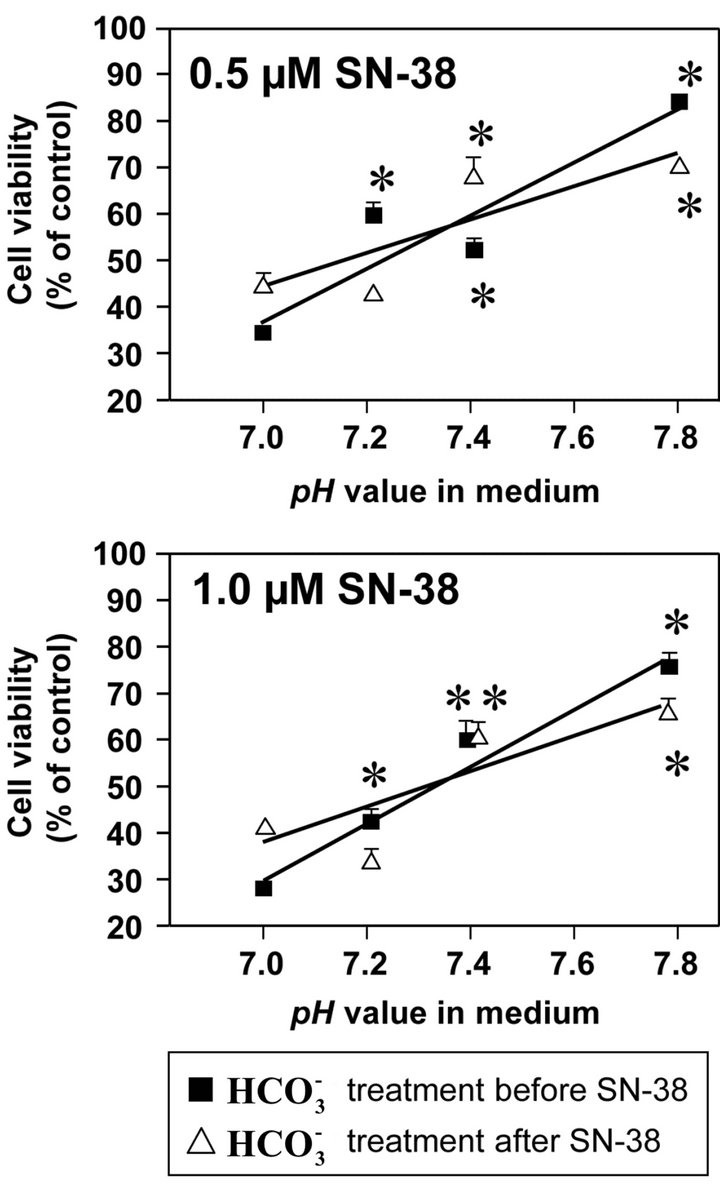
Figure 1. Effect of bicarbonate on SN-38-induced toxicity in HT29 cell. The cell was incubated with SN-38 (0.5 or 1.0 µM) for 2 h and increasing concentrations of bicarbonate (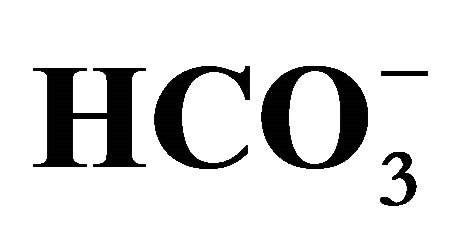 ; 5, 20, 44, and 100 mM) corresponding to pH 7.0, 7.2, 7.4, and 7.8, respectively, either for 2 h before (Closed squares) or after (Opened triangles) the SN-38 exposure. The cell in both conditions was further cultured in the medium with these increasing concentrations of
; 5, 20, 44, and 100 mM) corresponding to pH 7.0, 7.2, 7.4, and 7.8, respectively, either for 2 h before (Closed squares) or after (Opened triangles) the SN-38 exposure. The cell in both conditions was further cultured in the medium with these increasing concentrations of 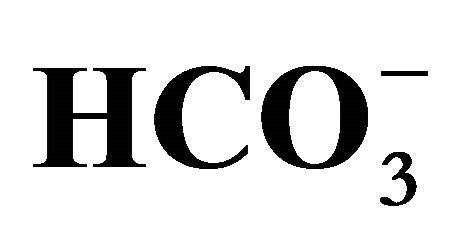 for additional 96 h. Cell viability was determined by the MTT assay. Data are shown as the mean ± SD of the percent of the respective control (0 µM SN-38 treatment). *Significantly different from the respective control determined in the presence of 5 mM bicarbonate, p < 0.05.
for additional 96 h. Cell viability was determined by the MTT assay. Data are shown as the mean ± SD of the percent of the respective control (0 µM SN-38 treatment). *Significantly different from the respective control determined in the presence of 5 mM bicarbonate, p < 0.05.
ured with increasing concentrations of 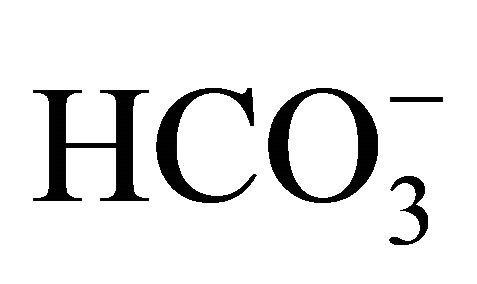 before to or after SN-38 exposures (0.5 or 1.0 µM) for 2 h. With
before to or after SN-38 exposures (0.5 or 1.0 µM) for 2 h. With 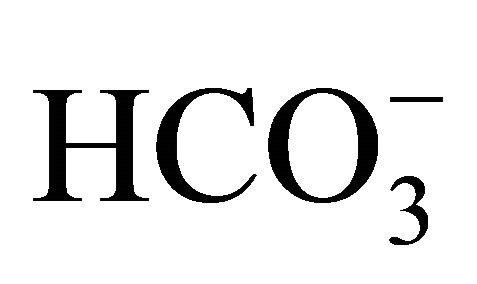 treatment before 0.5 µM SN-38 exposure, cell viability was significantly higher at pH 7.2, 7.4, and 7.8 than at pH 7.0, and at pH 7.8 was the highest. Similarly, with
treatment before 0.5 µM SN-38 exposure, cell viability was significantly higher at pH 7.2, 7.4, and 7.8 than at pH 7.0, and at pH 7.8 was the highest. Similarly, with 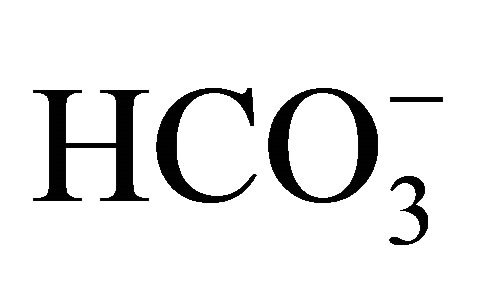 treatment after 0.5 µM SN-38, cell viability at pH 7.4 and 7.8 was significantly higher compared to that at pH 7.0. Moreover, these characteristic patterns of viability seen in the 0.5 µM SN-38 were also observed in the exposure to 1.0 µM SN-38. There was no difference in SN-38-induced toxicity in the respective pH condition between
treatment after 0.5 µM SN-38, cell viability at pH 7.4 and 7.8 was significantly higher compared to that at pH 7.0. Moreover, these characteristic patterns of viability seen in the 0.5 µM SN-38 were also observed in the exposure to 1.0 µM SN-38. There was no difference in SN-38-induced toxicity in the respective pH condition between 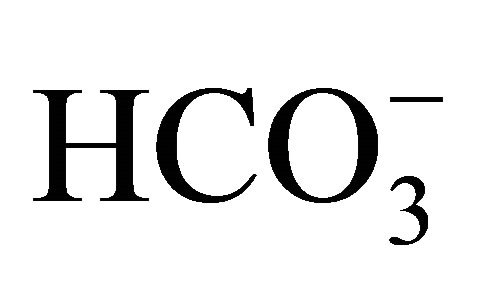 treatment before and after SN-38 exposures. The results indicate that
treatment before and after SN-38 exposures. The results indicate that 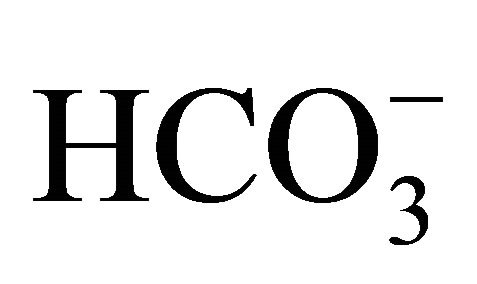 does not affect the conversion of SN-38 in the relatively short 2 h exposure period that is similar to small intestine transit time.
does not affect the conversion of SN-38 in the relatively short 2 h exposure period that is similar to small intestine transit time.
In addition, HT29 cell was incubated with the different  buffers for 4 h before and during 1.0 µM SN-38 lactone exposure. The cell was then washed and incubated in fresh DMEM containing 44 mM
buffers for 4 h before and during 1.0 µM SN-38 lactone exposure. The cell was then washed and incubated in fresh DMEM containing 44 mM  (pH 7.4) for 96 h. Because the result showed that SN-38-induced toxicity was around 50% under all the different conditions (data not shown), it implied that
(pH 7.4) for 96 h. Because the result showed that SN-38-induced toxicity was around 50% under all the different conditions (data not shown), it implied that 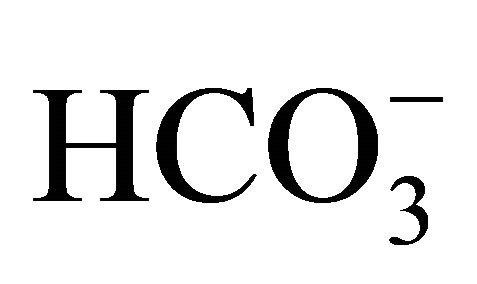 could not modify the influence of SN-38 on cell viability.
could not modify the influence of SN-38 on cell viability.
3.3. Effect of Bicarbonate-Adjusted pH on Cell Viability
Figure 2(a) shows the viability of HT29 cell treated with various pH conditions (6.8 - 8.2) adjusted by increasing concentrations of 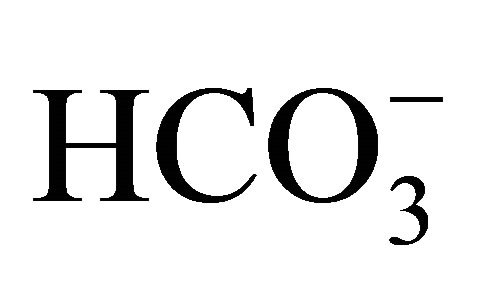 or NaOH for 48 h following a 2 h SN-38 exposure. In the
or NaOH for 48 h following a 2 h SN-38 exposure. In the 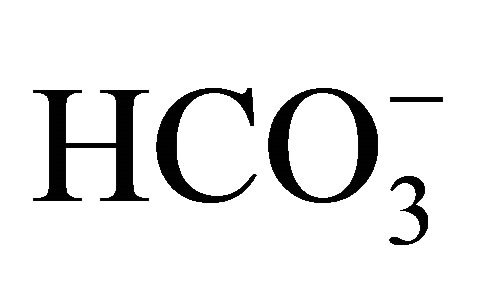 treatment, the cell viability was significantly enhanced at pH 7.4, 7.8, and 8.2 compared to that at pH 6.8. On the other hand, there was no difference in the viability of HT29 cell treated without
treatment, the cell viability was significantly enhanced at pH 7.4, 7.8, and 8.2 compared to that at pH 6.8. On the other hand, there was no difference in the viability of HT29 cell treated without 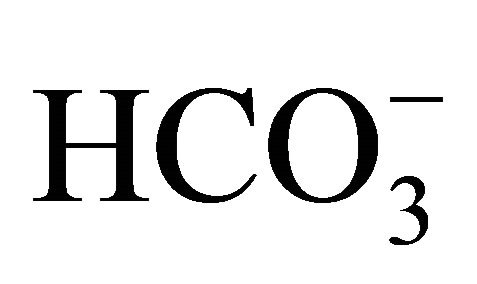 among these pH conditions. In these experiments, the pH in the medium was almost perfectly maintained even after cell culture, and there was no difference in pH after culture between control and SN-38 exposure in both experiments (Figure 2(b)).
among these pH conditions. In these experiments, the pH in the medium was almost perfectly maintained even after cell culture, and there was no difference in pH after culture between control and SN-38 exposure in both experiments (Figure 2(b)).
Figure 3 shows the flow cytometry image (Figure 3(a)) and the percentage of apoptotic cells analyzed by flow cytometry (Figure 3(b)) in the different pH conditions (48 h) with or without 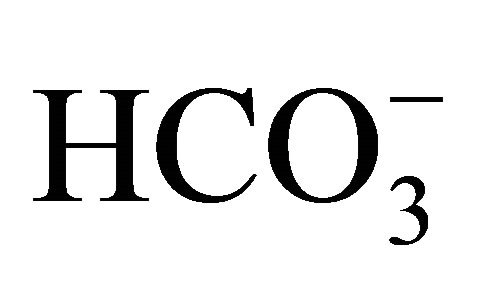 following a 2 h exposure to 1 µM SN-38 lactone. In the control, the per centage of necrotic cells (double positive for annexin V-EGFP and PI) was higher at pH 6.8 with 5 mM
following a 2 h exposure to 1 µM SN-38 lactone. In the control, the per centage of necrotic cells (double positive for annexin V-EGFP and PI) was higher at pH 6.8 with 5 mM  than at pH 7.8 with 44 mM
than at pH 7.8 with 44 mM 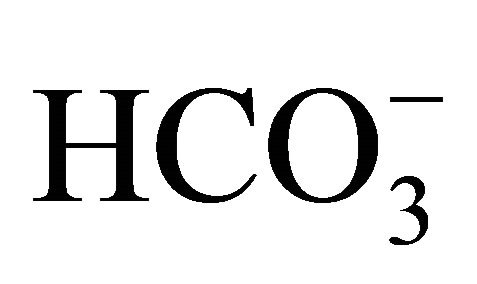 (Figure 3(a)). The necrosis at pH 6.8 was further aggravated by exposure to SN-38. Furthermore, the percentage of apoptotic cells (annexin V-EGFP positive) was increased. In the SN-38-exposed cells, the numbers of apoptotic and necrotic cells were lower at pH 7.8 than at pH 6.8. In the control cells, apoptotic cells were around 10% in all 3-set pH conditions despite of
(Figure 3(a)). The necrosis at pH 6.8 was further aggravated by exposure to SN-38. Furthermore, the percentage of apoptotic cells (annexin V-EGFP positive) was increased. In the SN-38-exposed cells, the numbers of apoptotic and necrotic cells were lower at pH 7.8 than at pH 6.8. In the control cells, apoptotic cells were around 10% in all 3-set pH conditions despite of 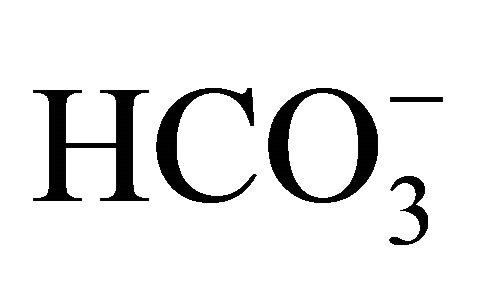 treatment (Figure 3(b)). In case of SN-38-exposed cells, the apoptosis was enhanced by around 40% in all three pH conditions of
treatment (Figure 3(b)). In case of SN-38-exposed cells, the apoptosis was enhanced by around 40% in all three pH conditions of 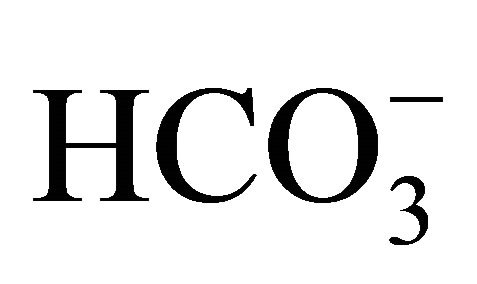 (−). On the other hand, the SN-38-induced apoptosis was significantly inhibited at pH 7.0 and 7.8 with increasing
(−). On the other hand, the SN-38-induced apoptosis was significantly inhibited at pH 7.0 and 7.8 with increasing 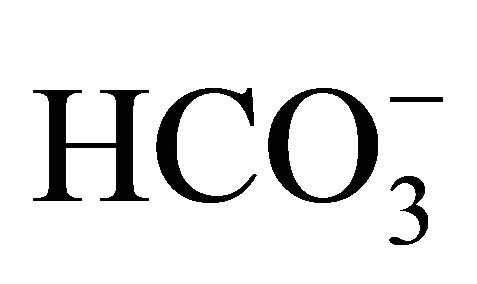 concentrations compared to that at pH 6.8. These results show that higher pH conditions adjusted by
concentrations compared to that at pH 6.8. These results show that higher pH conditions adjusted by 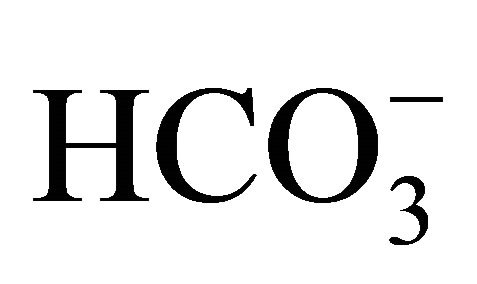 could inhibit the cellular toxicity induced by SN-38.
could inhibit the cellular toxicity induced by SN-38.
3.4. Effect of Bicarbonate on pHi Alternation Induced by SN-38
The level of pHi was determined in the presence of either 10 or 30 mM  following SN-38 (Figure 4). In the 10 mM
following SN-38 (Figure 4). In the 10 mM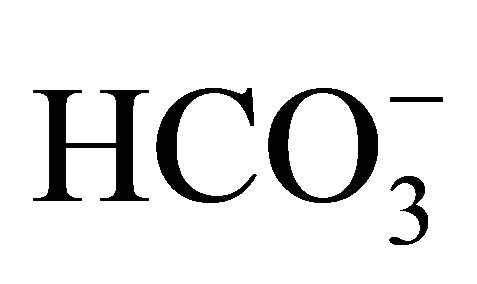 , pHi level in SN-38-exposed cells was significantly decreased compared to that in the control. On the other hand, there was no significant difference in the pHi between the control and SN-38-exposed cells in the presence of 30 mM
, pHi level in SN-38-exposed cells was significantly decreased compared to that in the control. On the other hand, there was no significant difference in the pHi between the control and SN-38-exposed cells in the presence of 30 mM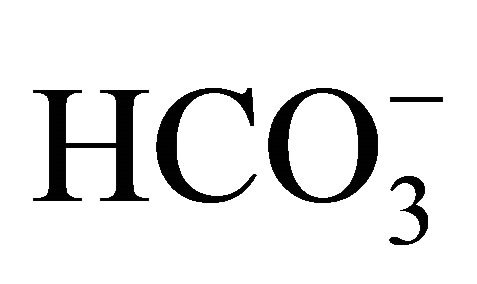 . Similar results
. Similar results
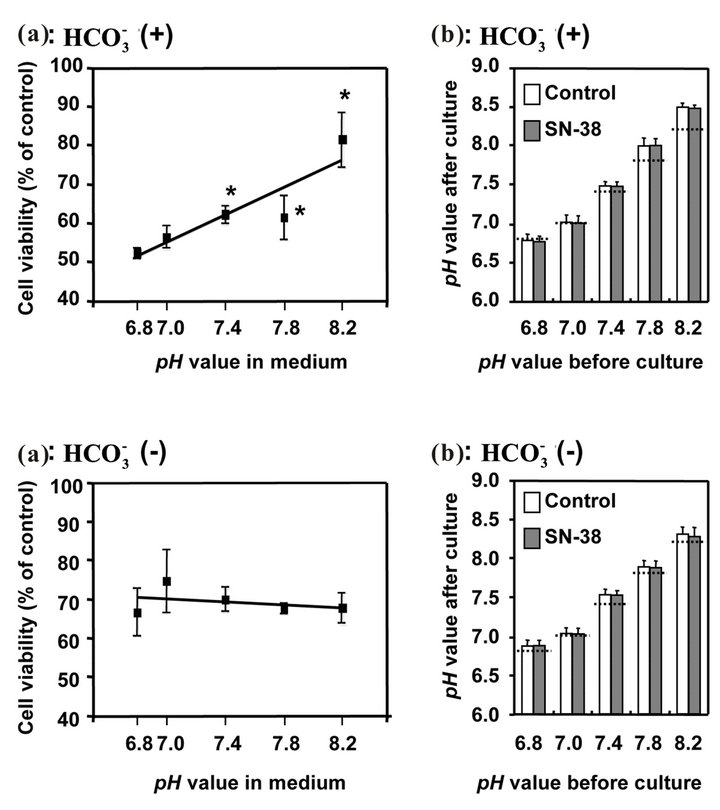
Figure 2. Effect of bicarbonate-adjusted pH on cell viability (a) and pH values in medium after cell culture (b). The cells incubated with/without 1.0 µM SN-38 for 2 h were cultured with pH set media at 5 points (6.8, 7.0, 7.4, 7.8, 8.2) adjusted by either different concentrations (5, 10, 20, 44, 100 mM, respectively) of bicarbonate [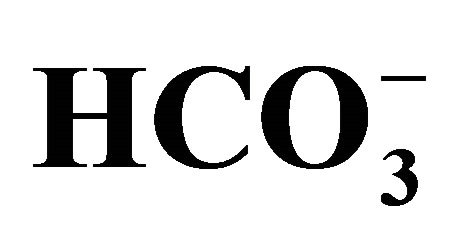 (+)] or NaOH [
(+)] or NaOH [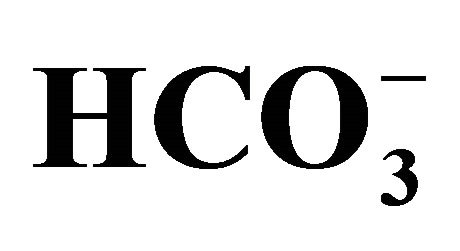 (-)]. (a) Cell viability evaluated by MTT assay was shown as the percent of the respective control (0 µM SN-38). *p < 0.05; Significantly different from pH 6.8; (b) The 5-set points of culture media adjusted by either increasing concentrations of bicarbonate [
(-)]. (a) Cell viability evaluated by MTT assay was shown as the percent of the respective control (0 µM SN-38). *p < 0.05; Significantly different from pH 6.8; (b) The 5-set points of culture media adjusted by either increasing concentrations of bicarbonate [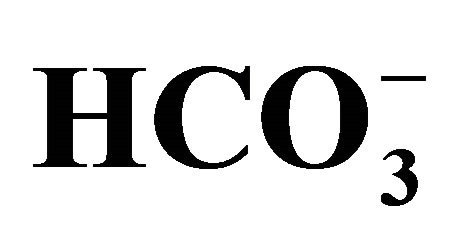 (+)] or NaOH [
(+)] or NaOH [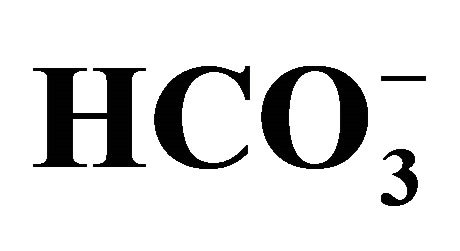 (−)] were measured after the cell culture. The dotted lines on each column show the set-pH values before the cell culture. The pH in each culture medium was measured using a standard pH meter in triplicate from three samples immediately after culture.
(−)] were measured after the cell culture. The dotted lines on each column show the set-pH values before the cell culture. The pH in each culture medium was measured using a standard pH meter in triplicate from three samples immediately after culture.
were obtained with a higher concentration of 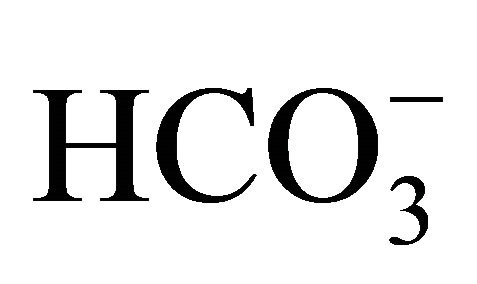 (58 mM) as those obtained with 30 mM
(58 mM) as those obtained with 30 mM 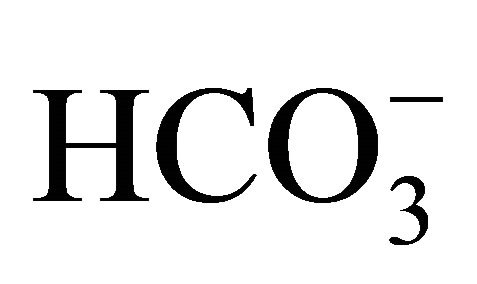 (no data shown). The results indicate that higher concentrations of
(no data shown). The results indicate that higher concentrations of 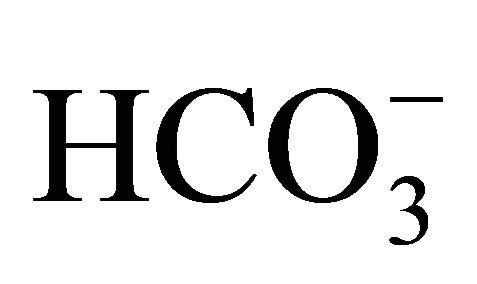 could prevent intracellular acidification caused by SN-38.
could prevent intracellular acidification caused by SN-38.
4. Discussion
Diarrhea is one of the lethal major side effects in the cancer patients receiving CPT-11 [7,31]. We and others have previously demonstrated that intestinal alkalization by 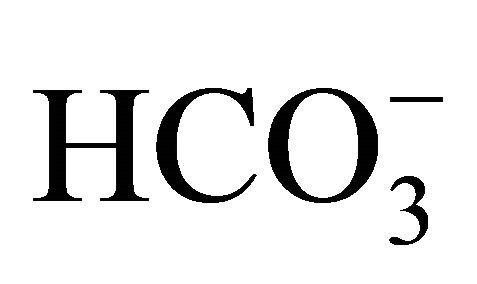 could reduce CPT-11/SN-38-induced cell injuries and diarrhea in both in vitro and in vivo studies [12,13,15]. These studies were based on the role of
could reduce CPT-11/SN-38-induced cell injuries and diarrhea in both in vitro and in vivo studies [12,13,15]. These studies were based on the role of 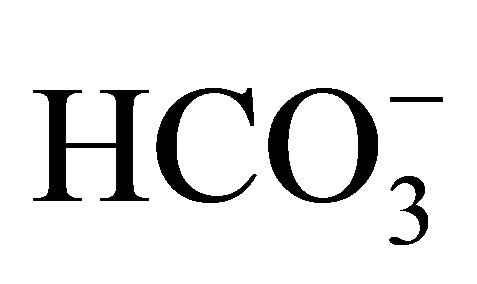 on intestinal pH regulation because of the pHdependent cytotoxicity of CPT-11/SN-38 [1,9,10,12,14, 15,28,32,33]. In addition to the regulation of pH in the
on intestinal pH regulation because of the pHdependent cytotoxicity of CPT-11/SN-38 [1,9,10,12,14, 15,28,32,33]. In addition to the regulation of pH in the

Figure 3. Effect of bicarbonate-adjusted pH on apoptosis. The cell following exposure to SN-38 was incubated for an additional 48 h in 3-set pH conditions (6.8, 7.0, or 7.8) either in 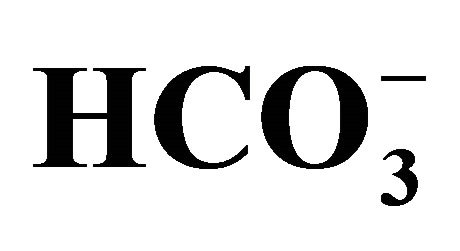 (+) or
(+) or 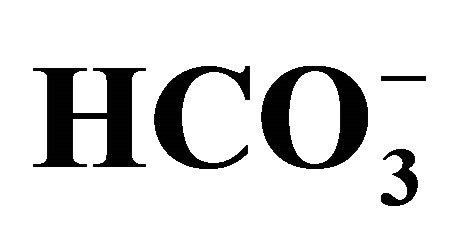 (−) medium. See the legend of Figure 2 for the bicarbonate concentrations that were used to set the pH in the medium. Figure 3(a) shows the images of flow cytometry in the cells exposed to either 0 or 1.0 µM SN-38 lactone in
(−) medium. See the legend of Figure 2 for the bicarbonate concentrations that were used to set the pH in the medium. Figure 3(a) shows the images of flow cytometry in the cells exposed to either 0 or 1.0 µM SN-38 lactone in 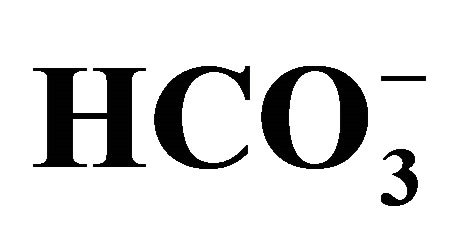 (+) conditions set at pH 6.8 or 7.8. The apoptotic cells were expressed as the percentage of cells stained with annexin V-EGFP positive and PI negative to the total numbers of cells, and shown as the mean ± SD in Figure 3(b).
(+) conditions set at pH 6.8 or 7.8. The apoptotic cells were expressed as the percentage of cells stained with annexin V-EGFP positive and PI negative to the total numbers of cells, and shown as the mean ± SD in Figure 3(b).
intestinal lumen, we suggested that 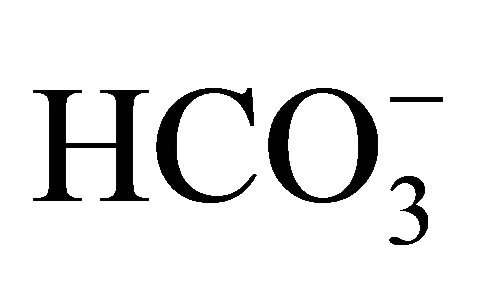 might play other roles on cytoprotection against CPT-11/SN-38 through influence on pHi environment. Palissot et al. have reported that the CPT-11/SN-38-induced apoptosis was less in the multidrug-resistant HL60-Vinc leukemic cells that could keep pHi higher, while apoptosis was much in drug-sensitive HL60 cell which intercellular acidification was induced following CPT-11/SN-38 treatment [34]. Therefore, the regulation of pHi level would be associated with the sensitive to CPT-11/SN-38 [18].
might play other roles on cytoprotection against CPT-11/SN-38 through influence on pHi environment. Palissot et al. have reported that the CPT-11/SN-38-induced apoptosis was less in the multidrug-resistant HL60-Vinc leukemic cells that could keep pHi higher, while apoptosis was much in drug-sensitive HL60 cell which intercellular acidification was induced following CPT-11/SN-38 treatment [34]. Therefore, the regulation of pHi level would be associated with the sensitive to CPT-11/SN-38 [18].
Accordingly, we designed a series of experiments in the large intestine cell line to test the hypothesis that the 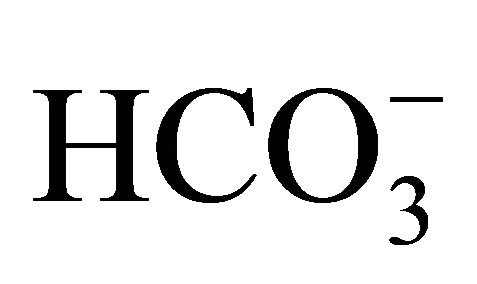 might influence on not only extracellular pH but also pHi environments, and consequently, disturb the intracellular conversion of SN-38 from the less toxic carboxylate form to the more toxic lactone form [9,35]. In the result, the cytotoxicity induced by SN-38 lactone
might influence on not only extracellular pH but also pHi environments, and consequently, disturb the intracellular conversion of SN-38 from the less toxic carboxylate form to the more toxic lactone form [9,35]. In the result, the cytotoxicity induced by SN-38 lactone
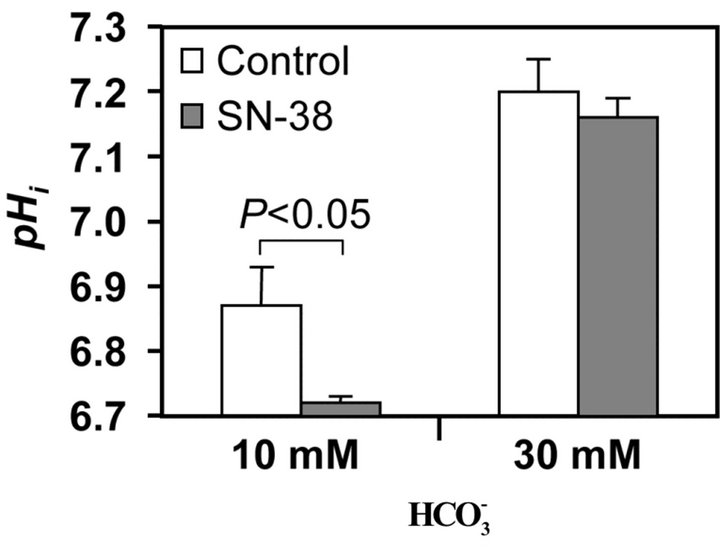
Figure 4. Effect of bicarbonate on SN-38-induced change in intracellular pH. Changes in pHi was assessed following incubation of the HT29 cell in the presence and absence of 0.5 µM SN-38 for 2 h and in a culture medium containing either 10 or 30 mM bicarbonate. The pHi was determined 4 h after removal of SN-38.
form was attenuated in a dose-dependent matter of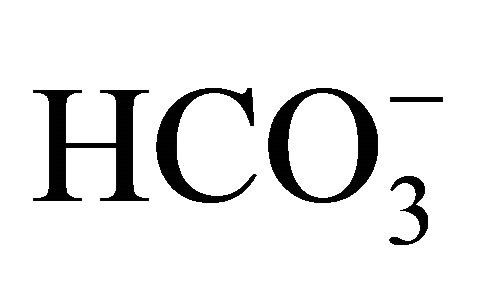 , but not by the respective pH condition merely adjusted by NaOH/HCl with low concentration of
, but not by the respective pH condition merely adjusted by NaOH/HCl with low concentration of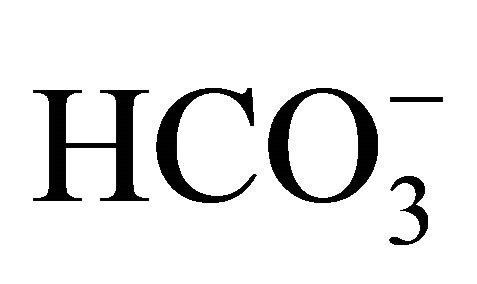 . Although the
. Although the 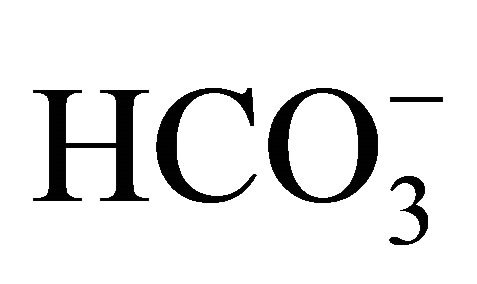 itself changes medium pH, the result implies that the cell damages induced by SN-38 would depend on not only ambient pH environment because the exposed time to SN-38 was only 2 hours that is too short to cause interconversion of SN-38 forms, and therefore,
itself changes medium pH, the result implies that the cell damages induced by SN-38 would depend on not only ambient pH environment because the exposed time to SN-38 was only 2 hours that is too short to cause interconversion of SN-38 forms, and therefore, 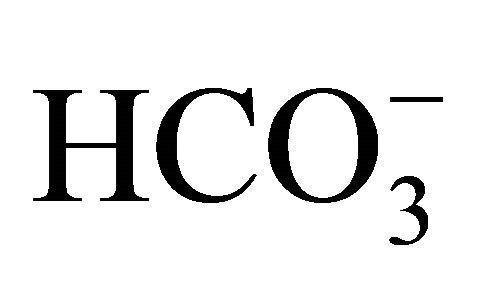 might play other roles on the protection against SN-38. One of the possibilities is that
might play other roles on the protection against SN-38. One of the possibilities is that 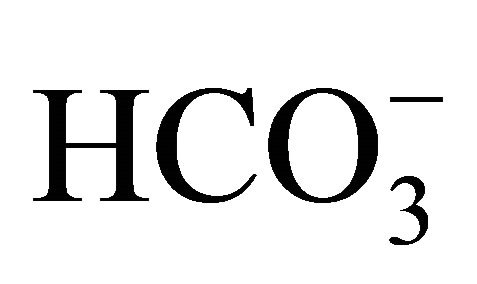 would be uptaked into cells, and then, would affect the pHi. Indeed, the present study confirmed that a higher concentration of
would be uptaked into cells, and then, would affect the pHi. Indeed, the present study confirmed that a higher concentration of 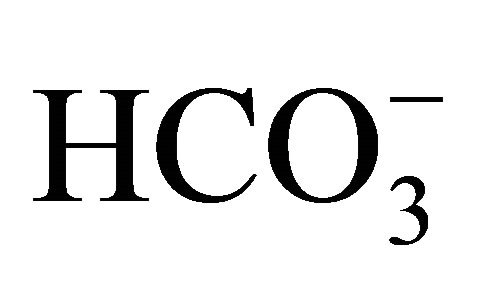 (30 mM) could inhibit SN-38-induced intracellular acidification (Figure 4).
(30 mM) could inhibit SN-38-induced intracellular acidification (Figure 4).
Furthermore, cellular acidification becomes a trigger in the early phase of apoptosis because of activation of endonucleases, and thus, regulation of pHi is critically important for tumor development [21]. The present study showed that the SN-38 itself has the action to cause intercellular acidification in the intestinal cell. Besides the inhibition of DNA topoisomerase I, it means that intercellular SN-38 also behaves as a trigger of apoptosis. Therefore, the regulation of pHi should be further important for attenuation of the side effects on CPT-11/ SN-38 therapy. In addition to the effect of 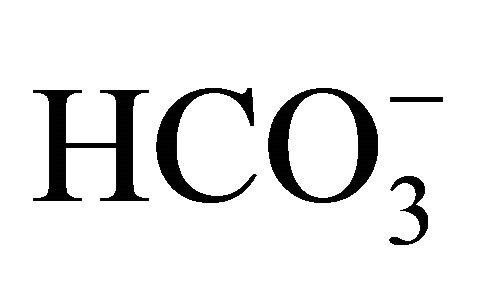 on SN-38-induced intercellular acidification, Flow Cytometry assay showed that apoptosis following SN-38 exposure was significantly decreased by increasing concentrations of
on SN-38-induced intercellular acidification, Flow Cytometry assay showed that apoptosis following SN-38 exposure was significantly decreased by increasing concentrations of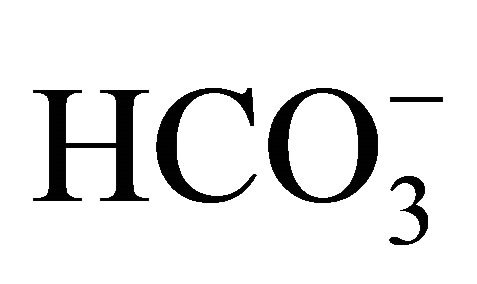 , but not in the respective pH level adjusted by NaOH/HCl. These findings implied that the regulation of pHi by
, but not in the respective pH level adjusted by NaOH/HCl. These findings implied that the regulation of pHi by  is effectiveness on the prevention of SN-38 induced apoptosis in intestinal cells.
is effectiveness on the prevention of SN-38 induced apoptosis in intestinal cells.
In addition to the interconversion of CPT-11/SN-38, pH environment is also associated with other characteristics of the agents. Gabr et al. have reported in L1210 leukemia cells that the uptake of radiolabeled CPT-11 was more rapidly at pH 6.2 than at physiological pH 7.4, and the intercellular retention was predominant at pH 6.2 [36]. Similarly, we have previously reported that the uptake of SN-38 into HT29 cells was significantly increased at ambient lower pH [9]. In this point, 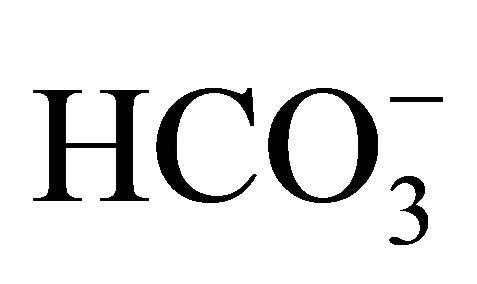 is indirectly contributed on the cellular uptake of SN-38 by regulation of ambient pH level.
is indirectly contributed on the cellular uptake of SN-38 by regulation of ambient pH level.
In conclusion, the present study confirmed that 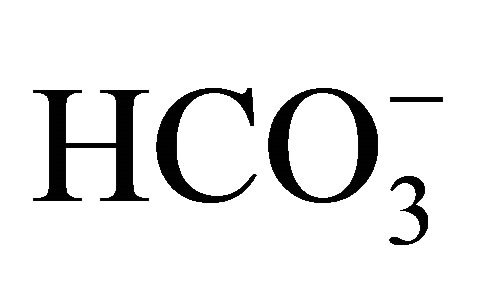 could keep alkalization/neutralization in not only extracellular but also intracellular pH environments, and consequently, cytotoxicity in large intestine cells would be attenuated through the reductions of interconversion to more toxic form and cellular uptake of SN-38. These results would certify the multiple roles of
could keep alkalization/neutralization in not only extracellular but also intracellular pH environments, and consequently, cytotoxicity in large intestine cells would be attenuated through the reductions of interconversion to more toxic form and cellular uptake of SN-38. These results would certify the multiple roles of 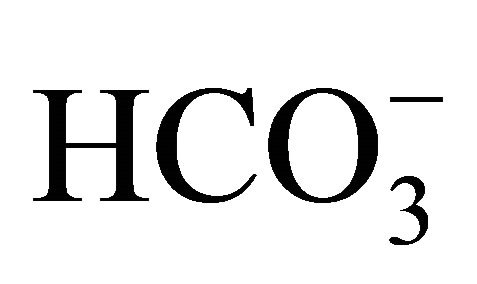 on the attenuation of side effects; cell injuries and diarrhea, in CPT-11/SN-38 antitumor therapy.
on the attenuation of side effects; cell injuries and diarrhea, in CPT-11/SN-38 antitumor therapy.
5. Acknowledgements
We are grateful for the expert advises from the late Dr. Bernard Bouscarel who was associate professor in the Department of Biochemistry and Molecular Biology of the George Washington University, Washington DC, USA. This study was supported in part by the National Institutes of Health Grant; DK-56108, Daiichi Pharmaceutical Co. Ltd., (Tokyo, Japan), Yakult Honsha Co. Ltd., (Tokyo, Japan), Mitsubishi Welpharma Co., Ltd. (Osaka, Japan), and the Elaine H. Snyder Cancer Research Award. This work was presented, in part, at the annual meetings of the American Gastroenterology Association in May 2002 and of the American Association for Cancer Research in April 2010. We thank Mr. Amit Kashyap and Miss Ivy Akid for their technical assistance.
REFERENCES
- Y. Kawato, M. Aonuma, Y. Hirota, H. Kuga and K. Sato, “Intracellular Roles of SN-38, a Metabolite of the Camptothecin Derivative CPT-11, in the Antitumor Effect of CPT-11,” Cancer Research, Vol. 51, No. 16, 1991, pp. 4187-4191.
- S. Kudoh, Y. Fujiwara, Y. Takada, H. Yamamoto, A. Kinoshita, Y. Ariyoshi, K. Furuse, M. Fukuoka and West Japan Lung Cancer Group, “Phase II Study of Irinotecan Combined with Cisplatin in Patients with Previously Untreated Small-Cell Lung Cancer,” Journal of Clinical Oncology, Vol. 16, No. 3, 1998, pp. 1068-1074.
- W. P. Irvin, F. V. Price, H. Bailey, M. Gelder, R. Rosenbluth, H. J. Durivage and R. K. Potkul, “A Phase II Study of Irinotecan (CPT-11) in Patients with Advanced Squamous Cell Carcinoma of the Cervix,” Cancer, Vol. 82, No. 2, 1998, pp. 328-333. doi:10.1002/(SICI)1097-0142(19980115)82:2<334::AID-CNCR13>3.0.CO;2-#
- C. F. Verschraegen, T. Levy, A. P. Kudelka, E. Llerena, K. Ende, R. S. Freedman, C. L. Edwards, M. Hord, M. Steger, A. L. Kaplan, D. Kieback, A. Fishman and J. J. Kavanagh, “Phase II Study of Irinotecan in Prior Chemotherapy-Treated Squamous Cell Carcinoma of the Cervix,” Journal of Clinical Oncology, Vol. 15, No. 2, 1997, pp. 625-631.
- Y. Shimizu, S. Umezawa and K. Hasumi, “Successful Treatment of Clear Cell Adenocarcinoma of the Ovary (OCCA) with a Combination of CPT-11 and Mitomycin C,” Gan to Kagaku Ryoho, Vol. 23, No. 5, 1996, pp. 587- 593.
- H. C. Pitot, D. B. Wender, M. J. O’Connell, G. Schroeder, R. M. Goldberg, J. Rubin, J. A. Mailliard, J. A. Knost, C. Ghosh, R. J. Kirschling, R. Levitt and H. E. Windschitl, “Phase II Trial of Irinotecan in Patients with Metastatic Colorectal Carcinoma,” Journal of Clinical Oncology, Vol. 15, No. 8, 1997, pp. 2910-2919.
- D. J. Sargent, D. Niedzwiecki, M. J. O’Connell and R. L. Schilsky, “Recommendation for Caution with Irinotecan, Fluorouracil, and Leucovorin for Colorectal Cancer,” The New England Journal of Medicine, Vol. 345, No. 2, 2001, pp. 144-145. doi:10.1056/NEJM200107123450213
- L. Saltz, “Irinotecan-Based Combinations for the Adjuvant Treatment of Stage III Colon Cancer,” Oncology (Williston Park), Vol. 14, No. 12, 2000, pp. 47-50.
- K. Kobayashi, B. Bouscarel, Y. Matsuzaki, S. Ceryak, S. Kudoh and H. Fromm, “pH-Dependent Uptake of Irinotecan and Its Active Metabolite, SN-38, by Intestinal Cells,” International Journal of Cancer, Vol. 83, No. 4, 1999, pp. 491-496. doi:10.1002/(SICI)1097-0215(19991112)83:4<491::AID-IJC10>3.3.CO;2-D
- L. P. Rivory, M. R. Bowles, J. Robert and S. M. Pond, “Conversion of Irinotecan (CPT-11) to Its Active Metabolite, 7-Ethyl-10-hydroxycamptothecin (SN-38), by Human Liver Carboxylesterase,” Biochemical Pharmacology, Vol. 52, No. 7, 1996, pp. 1103-1111. doi:10.1016/0006-2952(96)00457-1
- J. Fassberg and V. J. Stella, “A Kinetic and Mechanistic Study of the Hydrolysis of Camptothecin and Some Analogues,” Journal of Pharmaceutical Sciences, Vol. 81, No. 7, 1992, pp. 676-684. doi:10.1002/jps.2600810718
- Y. Takeda, K. Kobayashi, Y. Akiyama, T. Soma, S. Handa, S. Kudoh and K. Kudo, “Prevention of Irinotecan (CPT-11)-Induced Diarrhea by Oral Alkalization Combined with Control of Defecation in Cancer Patients,” International Journal of Cancer, Vol. 92, No. 2, 2001, pp. 269-275. doi:10.1002/1097-0215(200102)9999:9999<::AID-IJC1179>3.0.CO;2-3
- V. Valenti Moreno, J. Brunet Vidal, H. Manzano Alemany, A. Salud Salvia, M. Llobera Serentill, I. Cabezas Montero, S. Servitja Tormo, E. Sopena Bert and J. Guma Padro, “Prevention of Irinotecan Associated Diarrhea by Intestinal Alkalization. A Pilot Study in Gastrointestinal Cancer Patients,” Clinical and Translational Oncology, Vol. 8, No. 3, 2006, pp. 208-212. doi:10.1007/s12094-006-0012-1
- T. Tamura, K. Yasutake, H. Nishisaki, T. Nakashima, K. Horita, S. Hirohata, A. Ishii, K. Hamano, N. Aoyama, D. Shirasaka, T. Kamigaki and M. Kasuga, “Prevention of Irinotecan-Induced Diarrhea by Oral Sodium Bicarbonate and Influence on Pharmacokinetics,” Oncology, Vol. 67, No. 5-6, 2004, pp. 327-337. doi:10.1159/000082915
- T. Ikegami, L. Ha, K. Arimori, P. Latham, K. Kobayashi, S. Ceryak, Y. Matsuzaki and B. Bouscarel, “Intestinal Alkalization as a Possible Preventive Mechanism in Irinotecan (CPT-11)-Induced Diarrhea,” Cancer Research, Vol. 62, No. 1, 2002, pp. 179-187.
- R. Belhoussine, H. Morjani, R. Gillet, V. Palissot and M. Manfait, “Two Distinct Modes of Oncoprotein Expression during Apoptosis Resistance in Vincristine and Daunorubicin Multidrug-Resistant HL60 Cells,” Advances in Experimental Medicine and Biology, Vol. 457, 1999, pp. 365-381. doi:10.1007/978-1-4615-4811-9_39
- R. A. Gottlieb, J. Nordberg, E. Skowronski and B. M. Babior, “Apoptosis Induced in Jurkat Cells by Several Agents Is Preceded by Intracellular Acidification,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 93, No. 2, 1996, pp. 654-658. doi:10.1073/pnas.93.2.654
- A. K. Larsen, A. E. Escargueil and A. Skladanowski, “Resistance Mechanisms Associated with Altered Intracellular Distribution of Anticancer Agents,” Pharmacology & Therapeutics, Vol. 85, No. 3, 2000, pp. 217-229. doi:10.1016/S0163-7258(99)00073-X
- K. H. Sit, B. H. Bay and K. P. Wong, “Effect of Genistein, a Tyrosine-Specific Protein Kinase Inhibitor, on Cell Rounding by Ph Upshifting,” In Vitro Cellular & Developmental Biology—Animal, Vol. 29A, No. 5, 1993, pp. 395-402. doi:10.1007/BF02633988
- J. F. Goossens, J. P. Henichart, L. Dassonneville, M. Facompre and C. Bailly, “Relation between Intracellular Acidification and Camptothecin-Induced Apoptosis in Leukemia Cells,” European Journal of Pharmaceutical Sciences, Vol. 10, No. 2, 2000, pp. 125-131. doi:10.1016/S0928-0987(99)00091-3
- H. Izumi, T. Torigoe, H. Ishiguchi, H. Uramoto, Y. Yoshida, M. Tanabe, T. Ise, T. Murakami, T. Yoshida, M. Nomoto and K. Kohno, “Cellular pH Regulators: Potentially Promising Molecular Targets for Cancer Chemotherapy,” Cancer Treatment Reviews, Vol. 29, No. 6, 2003, pp. 541-549. doi:10.1016/S0305-7372(03)00106-3
- R. D. Vaughan-Jones and K. W. Spitzer, “Role of Bicarbonate in the Regulation of Intracellular pH in the Mammalian Ventricular Myocyte,” Biochemistry and Cell Biology, Vol. 80, No. 5, 2002, pp. 579-596. doi:10.1139/o02-157
- D. Gleeson, N. D. Smith and J. L. Boyer, “BicarbonateDependent and -Independent Intracellular pH Regulatory Mechanisms in Rat Hepatocytes. Evidence for Na+-
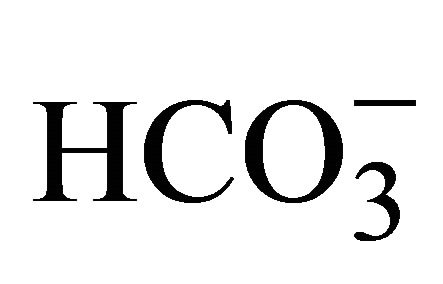 Cotransport,” Journal of Clinical Investigation, Vol. 84, No. 1, 1989, pp. 312-321. doi:10.1172/JCI114156
Cotransport,” Journal of Clinical Investigation, Vol. 84, No. 1, 1989, pp. 312-321. doi:10.1172/JCI114156 - B. Bouscarel, C. Cortinovis, C. Carpene, J. C. Murat and H. Paris, “Alpha 2-Adrenoceptors in the HT 29 Human Colon Adenocarcinoma Cell Line: Characterization with [3H]clonidine; Effects on Cyclic AMP Accumulation,” European Journal of Pharmacology, Vol. 107, No. 2, 1985, pp. 223-231. doi:10.1016/0014-2999(85)90062-7
- H. Paris, B. Bouscarel, C. Cortinovis and J. C. Murat, “Growth-Related Variation of Alpha 2-Adrenergic Receptivity in the HT 29 Adenocarcinoma Cell-Line from Human Colon,” FEBS Letters, Vol. 184, No. 1, 1985, pp. 82-86. doi:10.1016/0014-5793(85)80658-X
- K. Kobayashi, B. Bouscarel, Y. Matsuzaki, S. Ceryak and H. Fromm, “Uptake Mechanism of Irinotecan (CPT-11) and Its Metabolite (SN-38) by Hamster Intestinal Cells,” Gastroenterology, Vol. 114, Supplement 1, 1998, p. A626. doi:10.1016/S0016-5085(98)82560-2
- X. Y. Chu, Y. Kato, K. Niinuma, K. I. Sudo, H. Hakusui and Y. Sugiyama, “Multispecific Organic Anion Transporter Is Responsible for the Biliary Excretion of the Camptothecin Derivative Irinotecan and Its Metabolites in Rats,” Journal of Pharmacology and Experimental Therapeutic, Vol. 281, No. 1, 1997, pp. 304-314.
- K. Akimoto, A. Kawai and K. Ohya, “Kinetic Studies of the Hydrolysis and Lactonization of Camptothecin and Its Derivatives, CPT-11 and SN-38, in Aqueous Solution,” Chemical and Pharmaceutical Bulletin (Tokyo), Vol. 42, No. 10, 1994, pp. 2135-2138. doi:10.1248/cpb.42.2135
- H. Eagle, “Buffer Combinations for Mammalian Cell Culture,” Science, Vol. 174, No. 4008, 1971, pp. 500-503. doi:10.1126/science.174.4008.500
- F. Denizot and R. Lang, “Rapid Colorimetric Assay for Cell Growth and Survival. Modifications to the Tetrazolium Dye Procedure Giving Improved Sensitivity and Reliability,” Journal of Immunological Methods, Vol. 89, No. 2, 1986, pp. 271-277. doi:10.1016/0022-1759(86)90368-6
- P. Rougier, R. Bugat, J. Y. Douillard, S. Culine, E. Suc, P. Brunet, Y. Becouarn, M. Ychou, M. Marty, J. M. Extra, J. Bonneterre, A. Adenis, J. F. Seitz, G. Ganem, M. Namer, T. Conroy, S. Negrier, Y. Merrouche, F. Burki, M. Mousseau, P. Herait and M. Mahjoubi, “Phase II Study of Irinotecan in the Treatment of Advanced Colorectal Cancer in Chemotherapy-Naive Patients and Patients Pretreated with Fluorouracil-Based Chemotherapy,” Journal of Clinical Oncology, Vol. 15, No. 1, 1997, pp. 251-260.
- J. L. Flowers, R. M. Hoffman, T. A. Driscoll, M. E. Wall, M. C. Wani, G. Manikumar, H. S. Friedman, M. Dewhirst, O. M. Colvin and D. J. Adams, “The Activity of Camptothecin Analogues Is Enhanced in Histocultures of Human Tumors and Human Tumor Xenografts by Modulation of Extracellular pH,” Cancer Chemotherapy and Pharmacology, Vol. 52, No. 3, 2003, pp. 253-261. doi:10.1007/s00280-003-0635-7
- L. P. Rivory, E. Chatelut, P. Canal, A. Mathieu-Boue and J. Robert, “Kinetics of the in Vivo Interconversion of the Carboxylate and Lactone Forms of Irinotecan (CPT-11) and of Its Metabolite SN-38 in Patients,” Cancer Research, Vol. 54, No. 24, 1994, pp. 6330-6333.
- V. Palissot, R. Belhoussine, Y. Carpentier, S. Sebille, H. Morjani, M. Manfait and J. Dufer, “Resistance to Apoptosis Induced by Topoisomerase I Inhibitors in MultidrugResistant HL60 Leukemic Cells,” Biochemical and Biophysical Research Communications, Vol. 245, No. 3, 1998, pp. 918-922. doi:10.1006/bbrc.1998.8550
- Y. Kawato, M. Sekiguchi, K. Akahane, Y. Tsutomi, Y. Hirota, H. Kuga, W. Suzuki, H. Hakusui and K. Sato, “Inhibitory Activity of Camptothecin Derivatives against Acetylcholinesterase in Dogs and Their Binding Activity to Acetylcholine Receptors in Rats,” Journal of Pharmacy and Pharmacology, Vol. 45, No. 5, 1993, pp. 444- 448. doi:10.1111/j.2042-7158.1993.tb05573.x
- A. Gabr, A. Kuin, M. Aalders, H. El-Gawly and L. A. Smets, “Cellular Pharmacokinetics and Cytotoxicity of Camptothecin and Topotecan at Normal and Acidic pH,” Cancer Research, Vol. 57, No. 21, 1997, pp. 4811-4816.
NOTES
*Corresponding author.

