Journal of Cancer Therapy
Vol. 3 No. 4 (2012) , Article ID: 21585 , 8 pages DOI:10.4236/jct.2012.34032
Cystic Tumours of the Pancreas—A Rare and Diverse Type of Tumours
![]()
1Department of Surgery, Haukeland University Hospital, Bergen, Norway; 2Department of Surgical Sciences, University of Bergen, Bergen, Norway.
Email: Dag.Hoem@helse-bergen.no
Received May 18th, 2012; revised June 22nd, 2012; accepted July 5th, 2012
Keywords: Cystic Pancreatic Tumours; Incidentalomas; Clinical Features; Complications; Prognostic Factors; Survival
ABSTRACT
Objective: Increasing incidence of non-inflammatory cystic lesions of the pancreas challenges work-up algorithms, surgery and surveillance. We have reviewed our experience with these tumours in accordance with International Consensus Guidelines and evaluated the outcome. Patients and Methods: From 1999 to 2009, 34 cases referred to Haukeland University Hospital were operated upon; ten were serous (SCN), twenty-one IPMNs, one MCN, and two solid pseudopapillary neoplasms (SPPN). A thorough medical history was supplemented by CT, MRI, and US before each case were discussed in a MDT to decide on the best subsequent care. Results: All SCN and SPPN patients had benign histopathology, and no deaths were reported. Survival for malignant IPMN was, 2 years: 75%, and 5 years: 67%, where after no deaths were registered in the observation period. Presence of jaundice had the highest impact on survival (p = 0.0009), followed by weight loss (p = 0.005) and dilatation of the common bile duct (p = 0.04). In the IPMN group two had pancreatic juice leakage, and reoperation was performed in one. Conclusions: All SCN turned out benign which justify a high threshold for resection unless unacceptable symptoms dominate. For branch duct IPMNs resections of asymptomatic and smaller lesions (<3 cm) should be avoided but kept under surveillance, whereas symptomatic and lager lesions together with main duct IPMNs lodge a substantial malignant potential and should be resected. Symptomatic or large SPPNs can be, or turn, malignant which require resection.
1. Introduction
Pancreatic cancer is a common malignancy worldwide and the prognosis is poor. However, non-inflammatory cystic lesions of the pancreas are increasingly recognized and include subgroups of malignant lesions with considerably better outcome than the conventional pancreatic ductal adenocarcinomas.
The increasing incidence of cystic tumours reflects the backdrop that only a few patients are symptomatic but the availability and ubiquitous use of imaging techniques reveal many lesions. These factors together explain that a considerable number of referred patients have incidentalomas detected by US, CT or MR done for other reasons or found by wild screening. In addition, nearly 50% of patients in an autopsy material lodged cystic pancreatic lesions [1-3]. Asymptomatic patients especially challenge the algorithms of work-up, treatment, and follow-up.
The non-inflammatory cystic lesions are usually trifurcated in serous, mucinous, and solid pseudopapillary neoplasms. Other rare pancreatic cystic tumours exist and have been reported, but have not been found in our material and therefore not further discussed.
Serous cystic neoplasms are defined as benign neoplasms composed of numerous small cysts lined by cuboidal epithelial cells around a central stellate scar. Most are referred as fairly large incidentalomas, whilst a minority suffers from symptomatic lesions [4].
WHO’s classification from 1996, for the first time established the concept of different groups of mucinous cystic lesions of the pancreas, making Mucinous Cystic Neoplasms (MCN) and Intraductal Papillary Mucinous Neoplasm (IPMN) independent entities, and defined the latter as a neoplasm of the main pancreatic duct or side branches, with variable degrees of papillary formation, mucin production, and cystic dilation. The WHO classification has been updated in 2000 and 2010 [5]. This change led to stepwise improved classification, and international consensus guidelines for management surfaced in 2006 [6].
Intraductal papillary mucinous neoplasm (IPMN) is by far the largest group and dichotomized in main duct and branch duct lesions, and can furthermore be divided in gastric, intestinal, pancreatobiliary, and oncocytic subtype [7].
Solid pseudopapillary neoplasms (SPPN) are rare lesions and account for 1% to 2% of all exocrine pancreatic tumours. They are most often encountered in young females, pathogenesis is unknown, and tumour markers like CEA and CA 19-9 are usually negative. Most tumours are benign and most often referred as incidentalomas although malignancy can be seen rarely in advanced cases [8-10].
The aim of this paper is to analyze and report results following work-up and surgery for non-inflammatory cystic lesions of the pancreas at a tertiary referral hospital in Norway for a ten-year period, and explore significant survival factors.
2. Patients and Methods
During the years 1999 to 2009, 34 patients were referred to Department of surgery for non-inflammatory cystic lesions of the pancreas. The study is retrospective and data were retrieved from the patients’ medical records and filed in a database for further analyses.
Work-up was mainly based on CT and MR/MRCP and supplemented with ERCP, EUS and biochemical markers as found indicated. All cases were discussed in multidisciplinary team meetings to decide on subsequent treatment.
Most patients underwent a partial pancreatectomy, either a pylorus preserving pancreaticoduo denectomy (PPPD) or a classic Whipple procedure (WP) with distal gastrectomy. The latter was preferred in cases where an R0- resection could not be achieved by PPPD. Distal pancreatectomy was done in left-sided neoplasms and total pancreatectomy in patients with advanced tumours affecting both the head and body/tail of the pancreas. Tumour classification and pathology assessment was performed according to WHO classifications [5].
Pancreatic juice leakage was defined as amylase-rich fluid collected from drains or wounds on or after postoperative day three [11].
Follow-up was done in accordance with the histopathological assessment in order to monitor patients at risk for recurrent disease in the remnant pancreas.
All patients had a follow-up until death or observation was censored at 31 December 2009. Date of death was obtained from The Death Registry at The Norwegian Institute of Public Health. The project was performed according to the Declaration of Helsinki and approved by the Regional Committees for Medical and Health Research Ethics.
3. Statistical Analyses
The statistical analyses were performed using the software package Statistica 6.0 (StatSoftTM Inc., Tulsa, OK). A Chi-square test was used to compare categorical variables. Non-parametric data were analyzed by Mann-Witney-U-test or Kruskal-Wallis analysis of ranks. Continuous variables where analyzed by Student’s t-test or ANOVA.
Survival was assessed by the Kaplan-Meier method, and log-rank (LR) or Cox-F for test of significance. A p-value ≤ 0.05 was chosen for statistical significance.
4. Results
In this series of 34 cases with cystic pancreatic neoplasms ten were serous (SCN), twenty-one IPMNs, one MCN, and two solid pseudopapillary neoplasms (SPPN).
4.1. Serous Cystic Neoplasms (SCN)
Ten patients (seven females and three men with median age of 77 years (55 - 84), and 72 years (71 - 79) had serous cystic neoplasms. Seven patients with sparse phenotypic features were referred as incidentalomas, whereas two patients had pain, and one had jaundice. Five patients were treated with PPPD and five had a resection of the pancreatic body and tail. Indications for surgery were pain, jaundice, and uncertainty of malignant degeneration in the larger lesions with impression of the stomach.
Median tumour size was 5 cm (1 - 10), median operating time 205 min (66 - 295) and blood loss 400 ml (250 - 3500). There were no postoperative complications, histopathology examination turned out to be benign in all cases, and no deaths were reported in the observation period (12 to 100 months), (Figures 1(1a)-(1d)).
4.2. Mucinous Tumours
The mucinous tumour group is subdivided into Intraductal Papillary Mucinous Neoplasms (IPMN) and Mucinous Cystic Neoplasms (MCN).
Twenty-two patients, 11 men and 11 women, with median age 64 and 67 years, respectively, had mucinous tumours (Table 1). MCN was only diagnosed in one 51 years old woman who had a benign lesion, and is therefore not further discussed.
In the remaining 21 cases of IPMNs, abdominal symptoms occurred significantly more often in malignant than in benign cases (p = 0.02). Jaundice and pain were more frequently found in malignant cases whereas neither weight loss nor anorexia dichotomized our patients significantly (Table 1).
Serum median (range) levels of bilirubin, ALP, and γGT were 12 μmol/l (6 - 572), 242U/l (39 - 1614), and 61U/l (14 - 957), respectively, and breakdown by malignant versus benign lesions turned out to be significant for bilirubin (p = 0.02) and ALP (p = 0.02), but not for γGT.
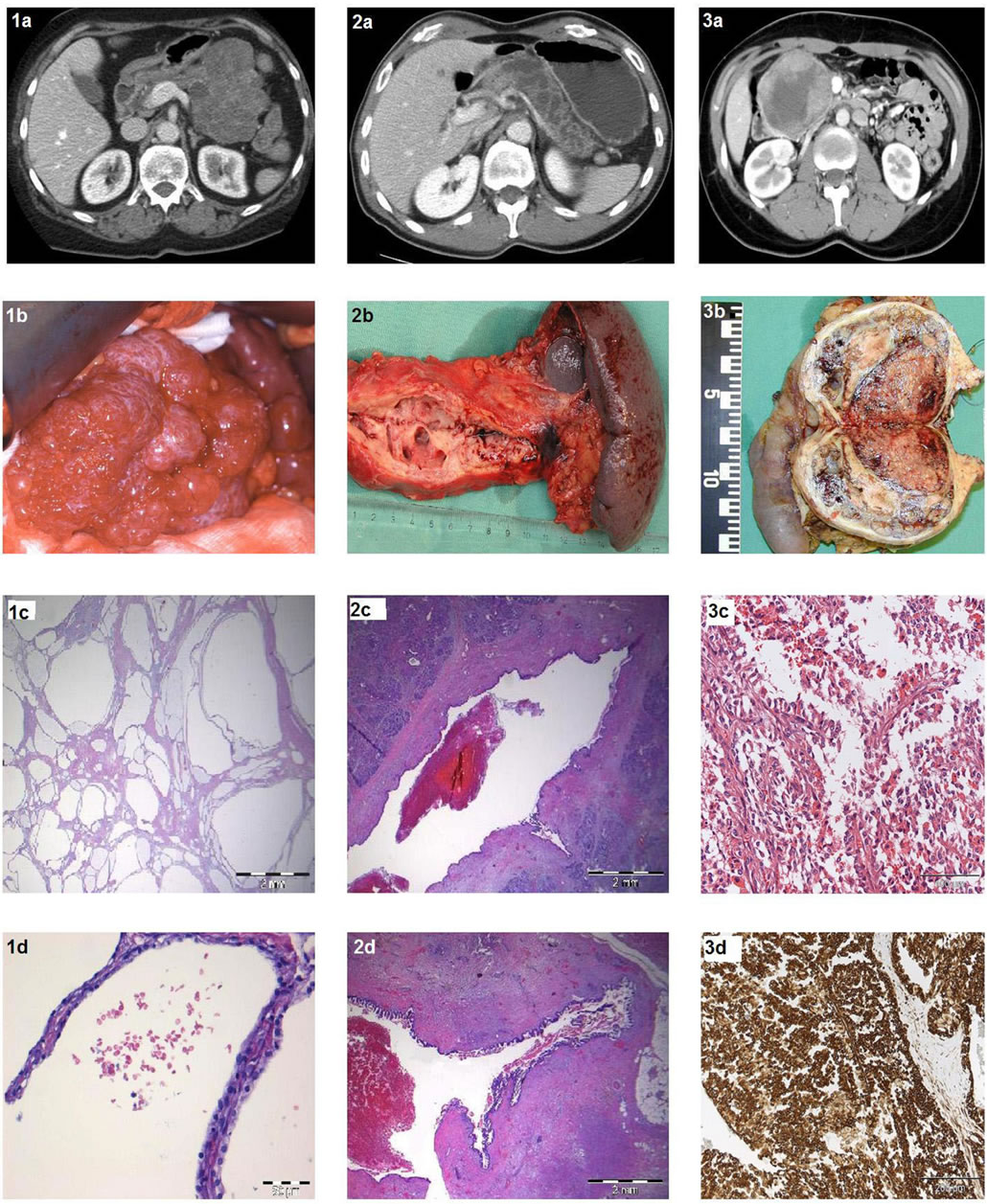
Figure 1. CT, macroand microscopic features of cystic tumours. 1a: Contrast enhanced CT reveal honey-comb features of serous cystic tumour; 1b: Per-operative macroscopic findings of serous cystic tumour in body and tail; 1c: Microscopic overview of serous cystic tumour. HE. Bar = 2 mm; 1d: Cuboid epithelium lining in serous cystic tumour. HE. Bar = 50 μm; 2a: Contrast enhanced CT of IPMN affecting both main and branch ducts in body and tail; 2b: Macroscopic findings of dilated main and branch ducts in resected IPMN specimen; 2c: Microscopic transverse section of part of the main duct. HE. Bar = 2 mm; 2d: Microscopic picture of dilated branch duct that opens into the main duct. HE. Bar = 2 mm; 3a: Contrast enhanced CT of Solid Pseudopapillary Neoplasm in the pancreatic head; 3b: Macroscopic findings in transverse section of SPPN with duodenum attached; 3c: Microscopic illustration of pseudopapillary growth and hyaline globules in SPPN. HE. Bar = 100 μm; 3d: Microscopic immune histology with vimentin staining in SPPN. Bar = 200 μm.
Radiological evaluation (CT or MRCP) of the pancreatic duct dilatation did not reveal significant differences between the malignant and benign IPMNs (p = 0.2). Dilatation of the common bile duct was, however, a significant feature separating the two (p = 0.03).
Median tumour size also differed significantly between malignant 6 cm (2 - 10) and benign cases 2.25 cm (1.2 - 3) (p = 0.02).
Of the 21 IPMNs, 12 turned out to be malignant, leaving nine in the benign group. The IPMN group was bifurcated after assumed origin into main duct or branch duct lesions, although multiple foci throughout the complex duct system must be anticipated to occur in several (mixed) cases. Furthermore, both main duct and branch duct lesions were dichotomized into malignant and benign forms. We found a tendency of more malignant IPMNs to occur in the main duct compared to the branch ducts (p = 0.25) (Table 1). The prevalence of cancer at diagnosis was 70% for main duct lesions and 45% in branch duct cases.
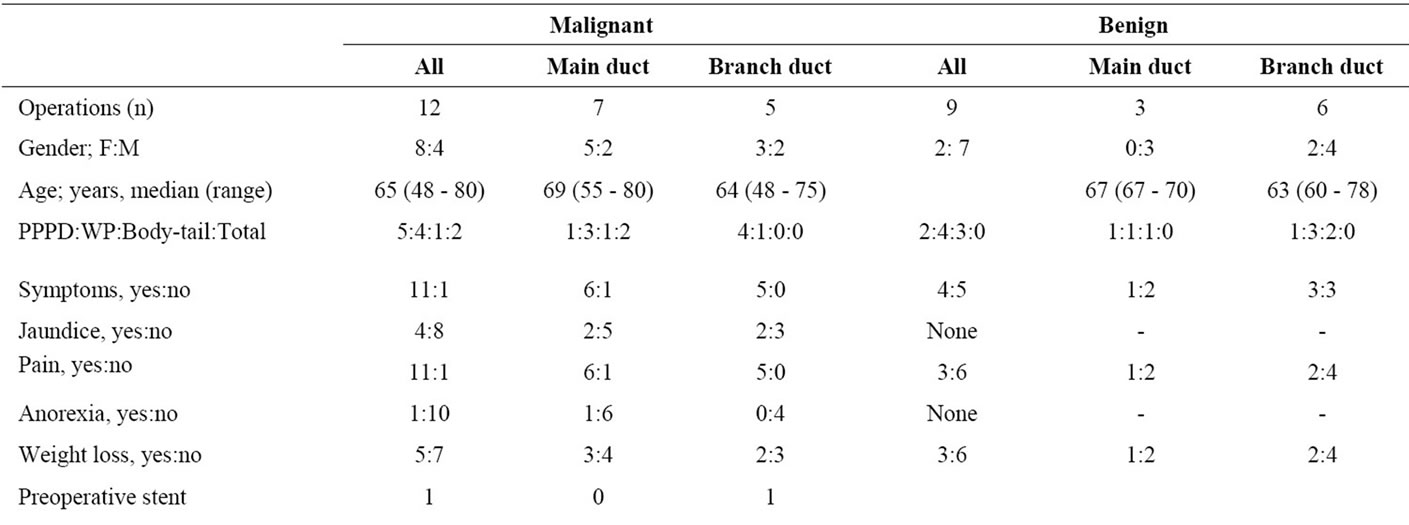
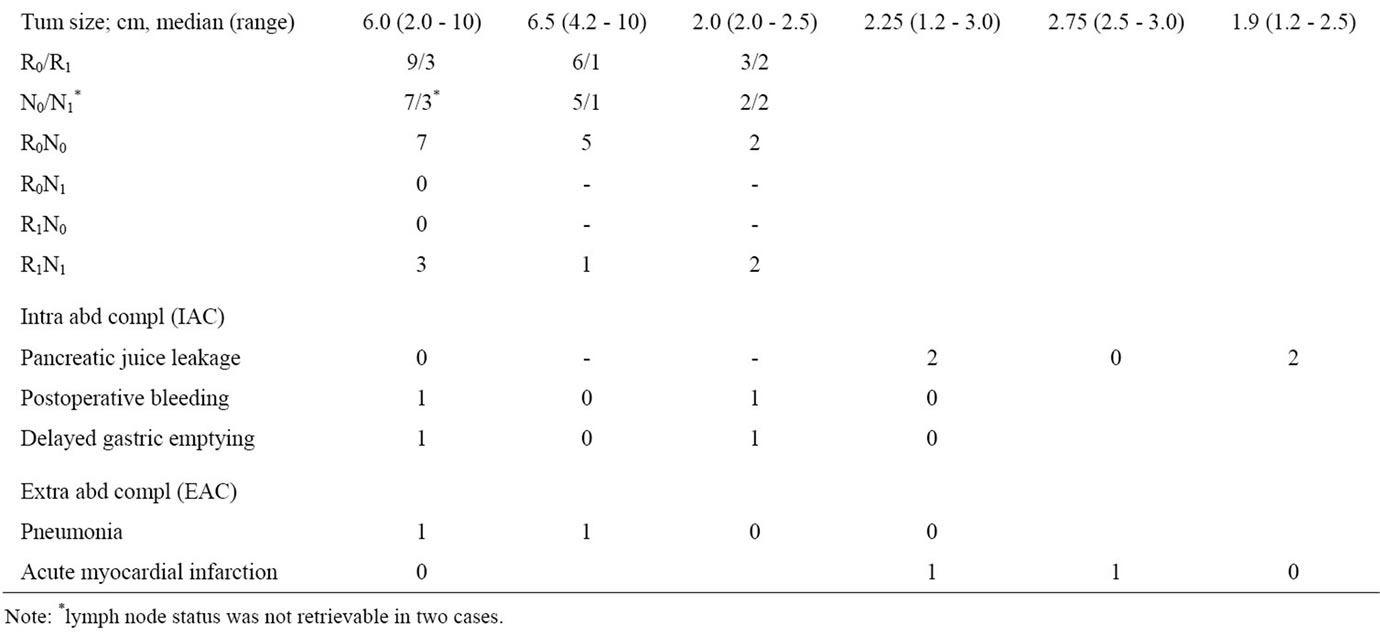
Table 1. Patients operated for intraductal papillary mucinous neoplasms.
4.3. Operative Procedures in the IPMN Group
Pancreatic head resections (PPPD (7) and Whipple (8)) made up 71% of all, whereas the frequencies of body/tailresections (4) and total pancreatectomy (2) were 19%, and 10%, respectively.
The median operating time was 238 min (102 - 455), 272 min (220 - 455) for malignant versus 184 min, (102 - 255) for the benign lesions (p = 0.006). Furthermore, peroperative median blood loss for all was 750 ml (150 - 2500), 1200 ml (400 - 2500) for the malignant versus 500 ml (150 - 1000) for benign lesions (p = 0.01).
4.4. IPMN Tumour Characteristics and RN-Status
Tumour size differed between the resection types, PPPD 2.25 cm (1.2 - 7), Whipple 2.5 cm (1.7 - 10), body/tail 2.5 cm (2.5 - 5), and total pancreatectomies 7.5 cm (6 9), but not in a significant manner. However, malignant tumours were larger than benign tumours, 6 cm (2 - 10) versus 2.25 cm (1.2 - 3), respectively (p = 0.02).
Furthermore, breakdown by tumour location to main duct 6 cm (2.5 - 10 cm) versus branch duct 2 cm (1.2 - 2.5 cm) revealed highly significant differences in tumour size (p = 0.0008).
Due to small numbers, the RN-status of 12 malignant mucinous tumours was limited to two categories, R0N0 and R1N1, since all R0 cases were free from lymph node metastasis, and on the contrary all R1 cases also had lymph node metastasis. Breakdown of RN-status by main duct and branch duct tumours revealed no significant differences. We found no differences in stage of disease or differentiation in these subgroups.
4.5. Outcome—Complication and Mortality
Six patients in the IPMN group (29%) had postoperative complications. Two had pancreatic juice leakage, and one each of postoperative bleeding, delayed gastric emptying, postoperative pneumonia, and myocardial infarction.
Reoperation was performed in one case suffering pancreatic juice leakage, the rest was treated conservatively, and no one died form complications.
4.6. Survival in the IPMN Group
Significant univariate survival predictors are listed in Table 2.
Survival for malignant mucinous tumours was, 2 years: 75%, and 5 years: 67%, where after no deaths were registered in the observation period.
In the malignant IPMN-group, presence of jaundice or not had the highest significant impact on survival (LR p = 0.0009), trailed by weight loss (LR p = 0.005), and finally dilatation of the common bile duct (Cox’s F-test p = 0.04).
Neither operating time nor blood loss was significantly related to survival. Furthermore, we found no difference in survival between the main duct and branch duct subgroups, but a significant survival difference for R0N0 versus R1N1 cases (LR p = 0.02), (Figure 2). As for survival for various stages of disease and histological differentiation our numbers are too small to perform comparative analyses.
For the whole IPMN group survival was better for those without complications (LR p = 0.02), and in the malignant subgroup survival was close to significantly reduced for those suffering complications (LR p = 0.06).
Finally, in the malignant subgroup, four patients died during the follow-up period.
A single R0N0 case, with a 7 cm primary in the main duct, experienced relapse after three and a half months, and died from liver metastasis ten months later. The remaining three, classified as R1N1 cases, succumbed due to lung metastases.
4.7. Solid Pseudopapillary Neoplasms (SPPN)
Solid PseudoPapillary Neoplasm (SPPN) is also named Frantz’s tumour. This neoplasm is diagnosed incidentally, or otherwise work-up is usually triggered by abdominal pain complaints. SPPNs are rare, usually benign, and were typically diagnosed in only two young females, 22 and 30 years old, respectively. One was referred due to an incidental finding by US and the other because of diffuse abdominal pain. CT scans revealed an eight cm lesion in the pancreatic head and a thirteen cm neoplasm in the body/tail, respectively (Figures 1(3a)-(3d)). They were operated upon by a PPPD and a body/tail resection, and both had an uneventful recovery. So far, they have had relapse free survival for 80 and 110 months.
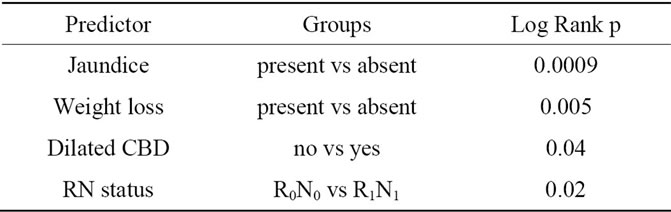
Table 2. Significant survival predictors for malignant IPMNs listed.
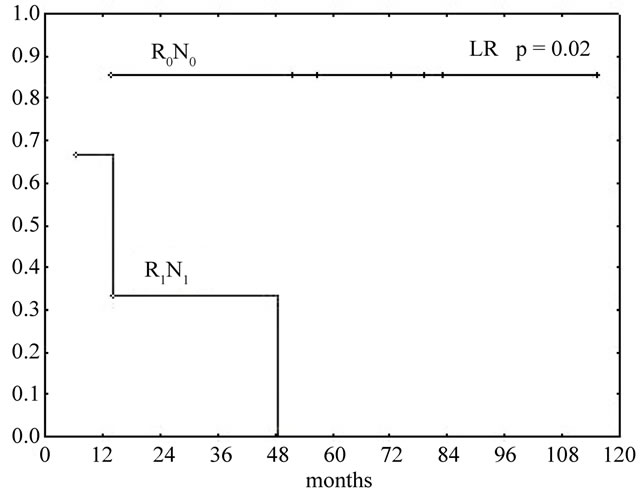
Figure 2. Kaplan-Meyer survival curves of malignant IPMNs. R0N0 vs R1N1. Log Rank p = 0.02.
5. Discussion
5.1. Serous Cystic Neoplasms (SCN)
In accordance with the literature 70% of our SCNs were incidentalomas, and were resected either due to symptoms of pain, weight loss, jaundice, or uncertainty of malignant degeneration at the time of diagnosis in the larger lesions with impression on the stomach.
Asymptomatic patients with classic radiology findings and smaller lesions are suitable for follow-up with reassuring information. Watchful expectation monitors the natural history of the lesions and makes intervention possible on a later stage if needed [4].
5.2. Intraductal Papillary Mucinous Neoplasms (IPMN)
Neither the natural development of this disease entity over a period of time, nor the exact time frame from benign lesions to malignancy is known. However, according to the literature, patients with malignant IPMNs are 5 - 7 years older on the average than those lodging benign IPMNs [12,13]. Moreover, anatomic site of the lesion in the main duct is associated with malignant transformation and cancer prevalence in approximately 70% of the patients, whereas branch duct lesions are associated with malignancy in approximately 25% of operated cases [14].
The uncertainty of malignant potential calls for a meticulous work-up that should include a thorough medical history focusing on discrete symptoms easily overseen. Moderate weight-loss and abdominal discomfort often discreetly appear ahead of jaundice and pain. In our material symptom presence itself (p = 0.02), jaundice (p = 0.04), and pain (p = 0.009) were found significantly more often in malignant cases (Table 1).
Pre-operative biochemical tests can be supplemented by tumour markers as KRAS mutations and CEA in cyst fluid, but sensitivity and specificity levels of the latter, at approximately 67% and 79%, respectively, calls for cautious interpretation as all tests might come across as negative even in malignant cases [15]. Considerable scepticism to cyst fluid CEA levels and DNA analysis predicttion capabilities of malignancy was also voiced in accordance with results in the PANDA study [16]. However, upcoming tests like interleukin 1b (IL 1b) in cyst fluid seems promising in predicting the risk of malignancy, but further validation in clinical practice is needed [17].
In accordance with the literature, and despite our easily available EUS resources, FNA of cyst fluid was not routinely used. Noteworthy however, we found serum median values of bilirubin (p = 0.02) and ALP (p = 0.02) significantly higher in the malignant cases.
As a consequence of imaging findings, FNAC sampling can seem tempting, but again on should be aware of the marginal utility in surgical decision-making cytology alone holds, as it neither represents, nor give trustworthy estimates of the degree of dysplasia that is known to vary throughout the cystic lesions [18].
With this backdrop, final decision making on treatment is preferably done by a multidisciplinary team, considering all the work-up results of the case, to provide the best subsequent management.
According to the literature, malignancy is verified in 60% - 90% of main duct IPMNs [19,20], which corroborates our finding of cancer prevalence of 70% in this group. We also found 86% symptom presence in malignant versus 33% in benign main duct IPMNs.
All main duct lesions were operated with standard pancreatic resections. High rate of established malignancy when operated and the likelihood of dysplastic lesions to progress, have led to the recommendation that all main duct IPMNs should be resected. Furthermore, the surgical resection should be done at referral centers where per-operative pathology assessment of the resection margins is accessible and guide an extended resection whenever needed [21].
On the contrary, branch duct IPMNs’ all over less malignant character opens a window of opportunity to watchful expectation for asymptomatic smaller lesions (<3 cm), whereas others are considered suited for resection without delay. The latter group consists of symptomatic patients, patients lodging lesions with mural nodules, or lesions larger than 3 cm [6,22].
In our series, all malignant branch duct IPMNs presented with pain despite the fact that all were smaller than 3 cm, underlining the importance of a meticulous medical history interview.
Non-operative management should be based on an established surveillance program, and consider patient age and co-morbidity as well as willingness and ability to go through with it.
Intra abdominal complications (IAC) were registered in four patients, of whom two had pancreatic juice leakage, but only one eventually needed reoperation. This is well within the leakage rate reported in other series of pancreatic resections [23-25].
The main findings in the group of malignant IPMNs were survival at 2 years of 75% and 5 years of 67%. Our series is too small for multivariate analysis. It should, however, be noted that we found no differences in survival in main duct versus branch duct malignant IPMNs. Peroperative standard lymph node dissection is recommended and differences in survival for RxNx groups are depicted in Figure 2 [26]. Although all over survival for patients suffering malignant IPMNs is considerably better than for conventional pancreatic ductal adenocarcinomas, they are not in the clear when it comes to recurrent disease.
In addition to surveillance of non-operated asymptomatic cases, follow-up is advocated for operated cases who may suffer curable recurrences in the pancreatic remnant, and finally also for cases lodging multifocal lesions with immanent potential for malignant transformation [19,27].
5.3. Solid Pseudopapillary Neoplasms (SPPN)
Solid Pseudopapillary Neoplasms accounts for 1% to 2% of all exocrine pancreatic tumours. SPPN should especially be kept in mind during work-up of incidentalomas found in young females. Even though clinical features are discrete or absent and tumour markers are negative, the tumours lodge a malignant potential and should always be referred to a specialist center for resection.
5.4. Conclusions
Patients lodging SCN should not be operated unless worrisome symptoms have surfaced, impression on neighbouring viscera afflicts the patient or malignancy cannot be ruled out.
All main duct IPMNs are recommended resected whereas asymptomatic branch duct lesions, less than 3 cm and without mural nodules can be subjected to watchful expectation of symptoms and up-dated radiology examinations at regular intervals. Supplementary work-up with EUS at specialist centers is recommended and opens the possibility for biochemical analyses following FNA in difficult cases. Patients with SPPN should always be referred to a specialist center for work-up and MDT e-valuation.
REFERENCES
- W. Kimura, H. Nagai, A. Kuroda, et al., “Analysis of Small Cystic Lesions of the Pancreas,” International Journal of Gastrointestinal Cancer, Vol. 18, No. 3, 1995, pp. 197-206.
- C. Fernandez-del Castillo, J. Targarona, S. P. Thayer, et al., “Incidental Pancreatic Cysts: Clinicopathologic Characteristics and Comparison with Symptomatic Patients,” Archives of Surgery, Vol. 138, No. 4, 2003, pp. 427-434. doi:10.1001/archsurg.138.4.427
- W. R. Brugge, G. Y. Lasuwers, D. Sahani, et al., “Cystic Neoplasms of the Pancreas,” The New England Journal of Medicine, Vol. 35, 2004, pp. 1218-1226. doi:10.1056/NEJMra031623
- J. A. Wargo, C. Fernandez-del-Castillo and A. L. Warshaw, “Management of Pancreatic Serous Cystadenomas,” Advances in Surgery, Vol. 43, No. 1, 2009, pp. 23- 34. doi:10.1016/j.yasu.2009.03.001
- F. T. Bosman, F. Carneiro, R. H. Hruban and N. D. Theise, “WHO Classification of Tumours of the Digestive System,” WHO Press, Lyon, 2010, pp. 279-337.
- M. Tanaka, S. Chari, V. Adsay, C. Fernandez-del-Castillo, M. Falconi, M. Shimizu, et al., “International Consensus Guidelines for Management of Intraductal Papillary Mucinous Neoplasms and Mucinous Cystic Neoplasms of the Pancreas,” Pancreatology, Vol. 6, No. 1-2, 2006, pp. 17-32. doi:10.1159/000090023
- T. Furukawa, G. Klöppel, N. Volkan Adsay, J. AlboresSaavedra, N. Fukushima, A. Horii, R. H. Hruban, Y. Kato, D. S. Klimstra, D. S. Longnecker, J. Lüttges, G. J. Offerhaus, M. Shimizu, M. Sunamura, A. Suriawinata, K. Takaori and S. Yonezawa, “Classification of Types of Intraductal Papillary-Mucinous Neoplasm of the Pancreas: A Consensus Study,” Virchows Archiv, Vol. 447, No. 5, 2005, pp. 794-799. doi:10.1007/s00428-005-0039-7
- S. Reddy and C. L. Wolfgang, “Solid Psudopapillary Neoplasms of the Pancreas,” Advances in Surgery, Vol. 43, No. 1, 2009, pp. 269-282. doi:10.1016/j.yasu.2009.02.011
- J. M. Matos, R. Grützmann, N. P. Agaram, H. D. Saeger, H. R. Kumar, K. D. Lillemore, et al., “Solid Pseudopapillary Neoplasms of the Pancreas: A Multi-Institutional Study of 21 Patients,” Journal of Surgical Research, Vol. 157, No. 1, 2009, pp. 137-142. doi:10.1016/j.jss.2009.03.091
- Y. E. Chung, M. J. Kim, J. Y. Choi, J. S. Lim, H. S. Hong, Y. C. Kim, et al., “Differentiation of Benign and Malignant Solid Pseudopapillary Neoplasms of the Pancreas,” Journal of Computer Assisted Tomography, Vol. 33, 2009, pp. 689-694. doi:10.1097/RCT.0b013e31818f2a74
- E. C. Lai, S. H. Lau and W. Y. Lau, “Measures to Prevent Pancreatic Fistula after Pancreatoduodenectomy,” Archives of Surgery, Vol. 144, No. 11, 2009, pp. 1174-1180. doi:10.1001/archsurg.2009.193
- P. Bernard, J. Y. Scoazec, M. Joubert, X. Kahn, J. Le Borgne, F. Berger and C. Partensky, “Intraductal Papillary-Mucinous Tumors of the Pancreas: Predictive Criteria of Malignancy according to Pathological Examination of 53 Cases,” Archives of Surgery, Vol. 137, No. 11, 2002, pp. 1274-1278. doi:10.1001/archsurg.137.11.1274
- K. Yamao, K. Ohashi, T. Nakamura, T. Suzuki, Y. Shimizu, Y. Nakamura, Y. Horibe, A. Yanagisawa, A. Nakao, Y. Nimuara, Y. Naito and T. Hayakawa, “The Prognosis of Intraductal Papillary Mucinous Tumors of the Pancreas,” Hepatogastroenterology, Vol. 47, No. 34, 2000, pp. 1129-1134.
- B. Terris, P. Ponsot, F. Paye, P. Hammel, A. Sauvanet, G. Molas, P. Bernades, J. Belghiti, P. Ruszniewski and J. F. Fléjou, “Intraductal Papillary Mucinous Tumors of the Pancreas Confined to Secondary Ducts Show Less Aggressive Pathologic Features as Compared with Those Involving the Main Pancreatic Duct,” The American Journal of Surgical Pathology, Vol. 24, 2000, pp. 1372-1377. doi:10.1097/00000478-200010000-00006
- J. Sreenarasimhaiah, L. F. Lara, S. F. Jazrawi, C. C. Barnett, S. Tang, “A Comparative Analysis of Pancreas Cyst Fluid CEA and Histology with DNA Mutational Analysis in the Detection of Mucin Producing or Malignant Cysts,” Journal of Pancreas, Vol. 10, No. 2, 2009, pp. 163-168.
- A. Khalid, M. Zahid, S. D. Finkelstein, J. K. LeBlanc, N. Kaushik, N. Ahmad, et al., “Pancreatic Cyst Fluid DNA Analysis in Evaluating Pancreatic Cysts: A Report of the PANDA Study,” Gastrointestinal Endoscopy, Vol. 69, No. 6, 2009, pp. 1095-1102. doi:10.1016/j.gie.2008.07.033
- A. V. Maker, N. Katabi, L. X. Qin, et al., “Cyst Fluid Interleukin-1b (IL1b) Levels Predict the Risk of Carcinoma in Intraductal Papillary Mucinous Neoplasms of the Pancreas,” Clinical Cancer Research, Vol. 17, No. 6, 2011, pp. 1502-1508.
- A. V. Maker, L. S. Lee, C. P. Raut, T. E. Clancy and R. S. Swanson, “Cytology from Pancreatic Cysts Has Marginal Utility in Surgical Decision-Making,” Annals of Surgical Oncology, Vol. 15, No. 11, 2008, pp. 3187-3192. doi:10.1245/s10434-008-0110-0
- R. Salvia, C. Fernández-del Castillo, C. Bassi, S. P. Thayer, M. Falconi, W. Mantovani, P. Pederzoli and A. L. Warshaw, “Main-Duct Intraductal Papillary Micinous Neoplasms of the Pancreas: Clinical Predictors of Malignancy and Long Term Survival Following Resection,” Annals of Surgery, Vol. 239, No. 5, 2004, pp. 678-685. doi:10.1097/01.sla.0000124386.54496.15
- C. M. Schmidt, P. B. White, J. A. Waters, C. T. Yiannoutsos, O. W. Cummings, M. Baker, T. J. Howard, N. J. Zyromski, A. Nakeeb, J. M. DeWitt, F. M. Akisik, S. Sherman, H. A. Pitt and K. D. Lillemoe, “Intraductal Papillary Mucinous Neoplasms: Predictors of Malignant and Invasive Pathology,” Annals of Surgery, Vol. 246, No. 4, 2007, pp. 644-651. doi:10.1097/SLA.0b013e318155a9e5
- A. Couvelard, A. Sauvanet, R. Kianmanesh, P. Hammel, N. Colnot, P. Lévy, P. Ruszniewski, P. Bedossa and J. Belghiti, “Frozen Sectioning of the Pancreatic Cut Surface during Resection of Intraductal Papillary Mucinous Neoplasms of the Pancreas Is Useful and Reliable: A Prospective Evaluation,” Annals of Surgery, Vol. 242, No. 6, 2005, pp. 774-778. doi:10.1097/01.sla.0000188459.99624.a2
- J. R. Rodriguez, R. Salvia, S. Crippa, A. L. Warshaw, C. Bassi, M. Falconi, S. P. Thayer, G. Y. Lauwers, P. Capelli, M. Mino-Kenudson, O. Razo, D. McGrath, P. Pederzoli, C. Fernández-Del Castillo, “Branch-Duct Intraductal Papillary Mucinous Neoplasms: Observations in 145 Patients Who Underwent Resection,” Gastroenterology, Vol. 133, No. 1, 2007, pp. 72-79. doi:10.1053/j.gastro.2007.05.010
- C. Bassi, C. Dervenis, G. Butturini, et al., “Postoperative Pancreatic Fistula: An International Study Group (ISGPF) Definition,” Surgery, Vol. 138, No. 1, 2005, pp. 8-13. doi:10.1016/j.surg.2005.05.001
- O. Kollmar, M. R. Moussavian, M. Bolli, et al., “Pancreatojejunal Leakage after Pancreas Head Resection: Anatomic and Surgeon-Related Factors,” Journal of Gastrointestinal Surgery, Vol. 11, No. 12, 2007, pp. 1699- 1703. doi:10.1007/s11605-007-0258-0
- W. B. Pratt, M. P. Callery and C. M. Vollmer Jr., “Risk Prediction for Development of Pancreatic Fistula Using the ISGPF Classification Scheme,” World Journal of Surgery, Vol. 32, No. 3, 2008, pp. 419-428. doi:10.1007/s00268-007-9388-5
- K. Kobayashi, Y. Sadakari, T. Ohtsuka, S. Takahata, M. Nakamura, K. Mizumoto and M. Taknaka, “Factors in Intraductal Papillary Mucinous Neoplasms of the Pancreas Predictive of Lymph Node Metastasis,” Pancreatology, Vol. 10, No. 6, 2011, pp. 720-725. doi:10.1159/000320709
- K. Nagai, R. Doi, A. Kida, K. Kami, Y. Kawaguchi, T. Ito, et al., “Intraductal Papillary Mucinous Neoplasms of the Pancreas: Clinicopathologic Characteristics and Long Term Follow-Up after Resection,” World Journal of Surgery, Vol. 32, No. 2, 2008, pp. 271-278. doi:10.1007/s00268-007-9281-2

