Journal of Cancer Therapy
Vol.2 No.2(2011), Article ID:5469,6 pages DOI:10.4236/jct.2011.22011
Sonodynamic Antitumor Effect of Benzoporphyrin Derivative Monoacid Ring A on KLN205 Cells
![]()
1Veterinary Teaching Hospital, Graduate School of Veterinary Medicine, Hokkaido University, Sapporo, Japan;
2Laboratory of Veterinary Surgery, School of Veterinary Clinical Sciences, Faculty of Agriculture, Tottori University, Tottori, Japan;
3Laboratory of Veterinary Surgery, Department of Veterinary Clinical Sciences, Graduate School of Veterinary Medicine, Hokkaido University, Sapporo, Japan.
Email: tosaki@muses.tottori-u.ac.jp
Received February 22nd, 2011; revised May 16th, 2011; accepted May 25th, 2011.
Keywords: BPD-MA, Cell Damage, Sonodynamic Therapy, Sonosensitizer, Ultrasound
ABSTRACT
Sonodynamic therapy is a new cancer treatment based on the synergetic effect of ultrasound and a drug. In this study, ultrasonically induced antitumor effects of benzoporphyrin derivative monoacid ring A (BPD-MA) on KLN205 cells were investigated. KLN205 cells were irradiated at an ultrasonic frequency of 3 MHz with 10 μg/ml BPD-MA. The ultrasonically induced cell damage significantly increased as an ultrasonic intensity and ultrasound exposure time increased. Confocal microscopic examination revealed that the irradiated cells were induced chromatin condensation and phosphatidylserine exposure. The synergistic effect of the ultrasound exposure and BPD-MA on the tumor cell adhesion rate was significant.
1. Introduction
Sonodynamic therapy (SDT) is a promising new method for cancer treatment utilizing the synergetic effect of ultrasound and a drug as sonosensitizer [1,2]. Ultrasound has some advantages, which includes an appropriate tissue attenuation coefficient for penetrating and reaching deep-seated tissues while maintaining the ability to focus energy onto a small volume. These unique properties could be available for noninvasive treatment of deepseated tumors compared to electromagnetic modalities, such as laser beams.
It has been reported that ultrasound energy enhanced the cytotoxicity of anticancer drugs [3-5]. However, these anticancer drugs have side effects. Therefore, it is necessary for establishment of anticancer effect to identify the effective sonosensitizer with strong cytotoxicity on tumor cells, few side effects, and high tumor-selectivity. The tumor-localizing porphyrins, which have been used as sensitizers in photodynamic therapy (PDT), have also been evaluated as a sonosensitizer [6]. In contrast to anticancer drugs, porphyrins are nontoxic without laser irradiation or ultrasonication. The side effects in surrounding normal tissues are minimized by the tumoraccumulative property of porphyrins and the geometrical selectivity by localized ultrasonic exposure.
Hematoporphyrin (Hp), aminolevulinic acid, and benzoporphyrin derivative monoacid ring A (BPD-MA) are used as a photosensitizer. Hematoporphyrin, one of the first generation photosensitizer, prolonged cutaneous photosensitivity of 1 - 3 months [7-9]. Benzoporphyrin derivative monoacid ring A (BPD-MA) is one of the second generation photosensitizers. The clearance from the body is more rapid than Hp, and skin photosensitivity lasts for only a few days [10,11]. This short clearance of photosensitizer can be a great advantage in treatment.
To our knowledge, the synergistic effects of ultrasound and BPD-MA on tumor cells were not reported. In this study, ultrasonically induced antitumor effects of BPD-MA on murine lung squamous cell carcinoma (KLN205) cells were investigated, in order to apply SDT with BPD-MA in a clinical setting. To estimate the efficacy of SDT, it was observed the cell adhesion rate and the morphological change of tumor cells following sonication with BPD-MA.
2. Materials and Methods
2.1. Cell Culture
KLN205 cells were obtained from the Institute of Development, Aging and Cancer, Tohoku University (Miyagi, Japan). KLN205 cells were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA, USA), penicillin (100 IU/ml), and streptomycin (0.1 mg/ml) (Wako, Osaka, Japan) in a humidified incubator at 37˚C with a mixture of 5% CO2 and 95% air. The cells were used for assays when they were in logarithmic growth. A total of 3 × 105 cells were cultured in each 35-mm culture dish for 24 h. Culture dishes were divided randomly and evenly into 4 groups (control, ultrasound alone, BPD-MA alone and BPD-MA + ultrasound).
2.2. Photosensitizer
Liposomal BPD-MA was kindly donated by QLT Inc. (Vancouver, BC, Canada) in lyophilized powder form. Liposomal BPD-MA was reconstituted in sterile water (Fuso Pharmaceutical Industries, Ltd., Osaka, Japan) at a concentration of 2 mg/ml.
2.3. Ultrasound Exposure
Figure 1 shows a schematic diagram of the set up used in exposing KLN205 cells in vitro to ultrasound. Culture dishes were placed above a water bag filled with degassed waster. The transducer was placed under a water bag. Each gap between apparatuses was filled with echo gel (ITO physiotherapy & rehabilitation, Tokyo, Japan). Ultrasound generator US-700 (ITO physiotherapy & rehabilitation, Tokyo, Japan) was dual freuquency (1 and 3 MHz). The focus ultrasound transducer is a circular single disk in a diameter of 35 mm and a focal length of 22 mm.
2.4. Examination of the SDT Effect on the Growth of KLN205 Cells
The culture medium in the culture dish was replaced with a fresh culture medium. The cells were incubated with
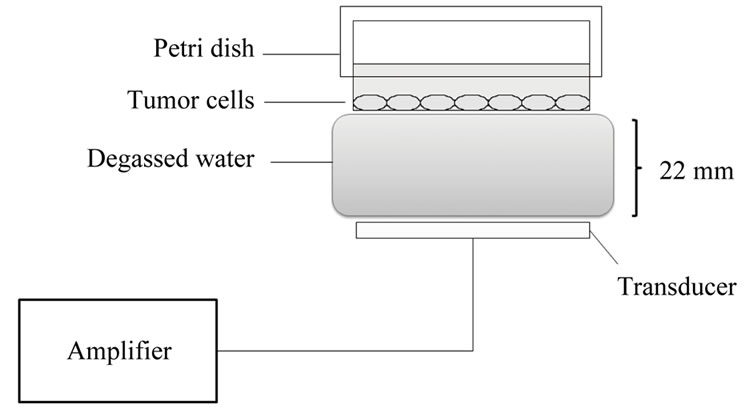
Figure 1. Ultrasonic exposure system. Culture dishes were placed above a water bag filled with degassed waster. The transducer was placed under a water bag. Each gap was filled with echo gel.
BPD-MA at a final concentration of 10 μg/ml for 3 h in the dark. After being washed with a fresh medium, the cells were exposed to ultrasound (except control and BPD-MA alone). To determine the influence of SDT for the cell adhesion ability, the cells were re-incubated at 37˚C for 24 h in the dark. The cell adhesion rate was calculated by using Gimza staining, modified as described [12]. Intact cells were attached to culture dish and stained with staining fluid. Cells that were detached by SDT were defied as dead cells. The area of stained cells on culture dished was counted using commercially available software (Scion Image, Scion Co., Frederick, MD, USA). The cell adhesion rate was calculated using following Equation (1).
Cell adhesion rate (%) = the stained area of treated group/the stained area of control group × 100 (1)
2.5. Confocal Microscopy
The culture medium in the culture dish was replaced with a fresh culture medium. The cells were then incubated with BPD-MA at a final concentration of 10 μg/ml for 3 h. These cells were sonicated with a 3 W/cm2 for 60 sec. (A): untreated cells (Control group), (B): cells with 10 μg/ml BPD-MA alone (BPD-MA group), (C): cells irradiated with US alone (US group), (D): cells irradiated at an ultrasonic intensity of 3 W/cm2 with 10 μg/ml BPD-MA (SDT group).
To assess apoptosis, the cells were stained with 1 mM bisbenzimidazole (Hoechst dye 33342) for 15 min at room temperature. Nuclear morphology was examined using the Nikon ECLIPSE microscope (Nikon, Tokyo, Japan) at 3 h after the sonication. The percentage of apoptotic cells was calculated, all cells from five random microscopic fields at 40 × magnification were counted.
To assess disruption of the membrane phospholipid asymmetry, the cells were stained with Annexin V-FITC Apoptosis Detection Kit (Abcam, Cambridge, MA, USA) under the Nikon ECLIPSE at 1 h after the sonication. After treatment, the cells were washed with Annexin V Binding Buffer. Cells were visualized under a fluorescence microscope following incubation with Annexin V-FITC and propidium iodide (PI).
2.6. Statistics
All results were expressed as means ± standard deviation (SD). The data of respective groups were compared by the Kruskal-Wallis test. A value of p < 0.05 was considered to indicate a statistically significant difference.
3. Results
3.1. Influence of SDT for the Cell Adhesion Ability
Figure 2 shows the cell adhesion rate of KLN205 cells

Figure 2. The cell adhesion rate of KLN205 cells after different ultrasonic frequency and intensity for 60 sec without BPD-MA. Error bars mean ± SD. The percentage of cell adhesion at an ultrasonic frequency of 1 MHz significantly decreased as the ultrasound intensity increased (p < 0.001).
without BPD-MA after 60 sec exposure at an ultrasonic intensity of 1, 2 and 3 W/cm2. The percentage of cell adhesion at an ultrasonic frequency of 1 MHz significantly decreased as the ultrasound intensity increased (p < 0.001). No significant cell damage was observed at an ultrasonic frequency of 3 MHz.
Figure 3 shows the cell adhesion rate of KLN205 cells without BPD-MA for up to 120 sec exposure at an ultrasonic intensity of 3 W/cm2. Significant cell damage was not observed as the exposure time increased. Based on these results, the cytotoxic effect of BPD-MA was estimated under the condition of 60 sec exposure at an ultrasonic frequency of 3 MHz (Figure 4). The percentage of cell adhesion significantly decreased as an ultrasonic intensity increased (p < 0.001). No significant differences between the BPD-MA group and the US group.

Figure 3. The cell adhesion rate of KLN205 cells after different exposure time at an ultrasonic frequency of 3 MHz and intensity of 3 W/cm2 without BPD-MA. Error bars mean ± SD. Significant cell damage was not observed as the exposure time increased.

Figure 4. The cell adhesion rate of KLN205 cells after different ultrasonic intensity-control group: no treatment; US only: ultrasound exposure at an ultrasonic frequency of 3 MHz and intensity of 3 W/cm2; BPD-MA only: with 10 μg/ml BPD-MA. Error bars mean ± SD. The percentage of cell adhesion significantly decreased as an ultrasonic intensity increased (p < 0.001).
Figure 5 shows the cell adhesion rate of KLN205 cells with BPD-MA for up to 120 sec exposure at an ultrasonic frequency of 3 MHz and intensity of 3 W/cm2. Significant cell damage was not observed with 10 μg/ml BPD-MA alone. The ultrasonically induced cell damage increased above 180 sec (data not shown). The synergistic cell damage effect was significant when ultrasound exposure time was above 40 sec (p < 0.01), while there were no obvious cell damage when ultrasound exposure time below 20 sec.
The appropriate sonodynamic cell growth inhibition was obtained at an ultrasonic frequency of 3 MHz and
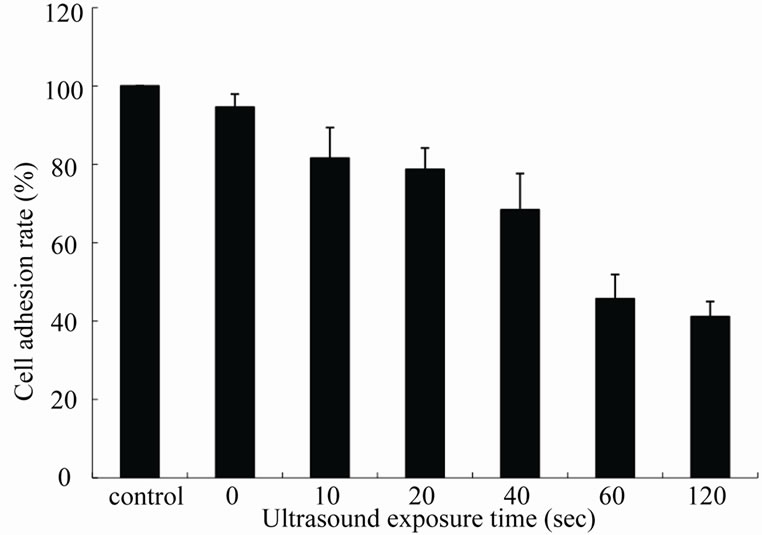
Figure 5. The cell adhesion rate of KLN205 cells after different exposure time at an ultrasonic frequency of 3 MHz and intensity of 3 W/cm2 with BPD-MA. Error bars mean ± SD. The synergistic cell damage effect was significant when ultrasound exposure time was above 40 sec (p < 0.01).
intensity of 3 W/cm2 above 40 sec exposure time with BPD-MA.
3.2. Cell Death Detection
Figure 6 shows Hoechst 33342 staining images in KLN205 cells at 3 h after SDT. Control group (A), BPD-MA group (B) and US group (C) did not show condensation of nuclear chromatins. These results indicated that ultrasound alone did not induce apoptosis. In contrast, almost 24.9% of the cells in the SDT group (D) showed condensation of nuclear chromatins that are characteristic of apoptotic cells.
Figure 7 shows Annexin V-FITC and PI staining images in KLN205 cells at 3 h after SDT. Control group (A), BPD-MA group (B) and US group (C) did not show the binding of Annexin V-FITC to KLN205 cells. These results indicated that ultrasound alone did not induce apoptosis. SDT group (D) showed green fluorescence of Annexin V-FITC in the cell membrane and red fluorescence of PI in the nuclei. These results indicated that SDT induced apoptosis.
4. Discussion
Some sonosensitizers have been evaluated in ultrasound-induced reactions. In vitro, adriamycin and diaziquone statistically decreased the cell survival [4,5]. However, theses anticancer drugs have clinically problems such as cytotoxicity to normal cells and no tumor

Figure 6. Representative photomicrographs of Hoechst dye 33342 labeling of KLN205 cells—(a): Control group; (b): BPD-MA group; (c): US group; (d): SDT group. (a), (b) and (c) do not show condensation of nuclear chromatins. (d) shows condensation of nuclear chromatins. (Scale bar, 20 μm).
Figure 7. Representative photomicrographs of Annexin V-FITC and PI labeling of KLN205 cells—(a): Control group; (b): BPD-MA group; (c): US group; (d): SDT group. (a), (b) and (c) do not show the binding of Annexin V-FITC to KLN205 cells. (d) shows green fluorescence of the cell membrane and red fluorescence of PI in the nuclei. (Scale bar, 20 μm).
selectivity. In addition to anticancer drugs, the tumorlocalized photosentizers have also studied in sonodynamic reactions. In contrast to anticancer drugs, photosensitizers are nontoxic in the absence of ultrasound.
Hematoporphyrin, the first generation photosensitizer, enhanced the synergistic killing of Hepatoma 22 cells in the presence of ultrasound. The results indicated that the ultrasonic intensity of 2 W/cm2, the ultrasound exposure time of 60 sec and the Hp concentration of 100 μg/ml were the best conditions for SDT in vitro [6].
Hematoporphyrin monomethyl ether (HMME), the second generation porphyrin-related photosensitizer, at a concentration of 10 μg/ml and ultrasound (1 MHz) at intensity of 0.5 W/cm2, applied for 2 min, enhanced the cell killing of C6 glioma cells. The growth inhibition rate of C6 glioma cells at 24 h after treatment, in the presence and absence of HMME, were 72.68% and 13.34%, respectively. HMME was found to enhance the cell-amaging effect of ultrasound irradiation [13].
However, these porphyrins prolonged cutaneous photosensitivity. Therefore, these have not been used clinically.
In the many previous reports on SDT, cells were sonicated with suspended [12,13]. However, tumor cells within the solid tumors are connected with each other. Therefore, it needs to evaluate tumor cells with attached to culture dishes. In the present study, we examined whether the application of an ultrasound treatment could influence the cell adhesion ability in KLN205 cells using BPD-MA with fast clearance. At first, the influence of ultrasound was estimated. As shown in Figure 2, KLN205 cells were damaged after 60 sec exposure at an ultrasonic frequency of 1 MHz without BPD-MA, and the cell damage increased as the ultrasound intensity increased. It was considered that ultrasound at a frequency of 1 MHz caused the cell damage. However, KLN205 cells were not damaged after 60 sec exposure at an ultrasonic frequency of 3 MHz without BPD-MA. There was also no significant cell damage as the exposure time increased (Figure 3). It was thought that the ultrasonic frequency of 3 MHz was suitable for SDT.
Significant synergistic cell damage effect was observed in SDT at a frequency of 3 MHz with at a concentration of 10 μg/ml BPD-MA (Figure 4). The cell damage increased as the ultrasound intensity increased. No cell damage was observed with ultrasound alone. The cell damage also increased as the ultrasound exposure time increased (Figure 5). There was obvious enhancement of ultrasonically cell damage with BPD-MA, the enhancement became more significant when ultrasound exposure time was above 40 sec.
Runnels et al. reported that intracellular damage mediated by PDT with BPD-MA could affect cellularextracellular matrix interactions and resulted in loss of FAP formation [14]. Further studies on adhesion molecular changes after SDT are necessary.
Cell death was evaluated by Hoechst 33342 staining and by the binding of Annexin V on externalized phosphatidylserine after BPD-MA sonosensitization. In the Hoechst 33342 staining study, almost 24.9% of the cells showed the condensation of nuclear chromatin at 3 h after SDT with BPD-MA (Figure 6). In the Annexin V staining study, sonicated tumor cells with BPD-MA showed green fluorescence of the cell membrane and red fluorescence of PI in the nuclei. The appearance of phosphatidylserine on the outer leaflet of the plasma membrane is one of the earliest manifestations of this programmed cell death [15,16]. Qui et al. reported that SDT induce changes on the surface of cell membrane in scanning electron microscope examination [17]. In prior studies using this murine model, tumor growth was retarded when tumors were irradiated with LED at 1 min after BPD-MA intratumoral injection [18]. It was considered that sonochemistry or photochemistry activated photosensitizer, which localized near the cell membrane, might affect the change of cell membrane functions. Apoptosis (anoikis) is induced by lack of adhesion or inappropriate adhesion to the extracellular matrix [19]. Therefore, membrane changes induced by SDT might lead to anoikis. Chromatin condensation and phosphatidylserine exposure are also observed resulting from anoikis [20]. Therefore, SDT might induce apoptosis and anoikis, although there is more research to identify the cell death mechanism induced by SDT.
Recently, it was reported that a mechanism for the sonodynamic activation of sonosensitizer might be attributed to the enhancement of active oxygen generation through acoustic cavitation [21,22]. It has been proposed that the sonoluminescent light produced during cavitation is responsible for the photoexcitation of the sensitizer, with subsequent formation of singlet oxygen a known reactive oxygen species (ROS) [23,24]. To confirm the production of the formation of various ROS during SDT, it needs to examine the protective effect of different ROS scavengers on SDT-induced cell damage in the future.
In conclusion, combination of ultrasound and BPD-MA showed synergistic cytotoxic effect of KLN205 cells in vitro. Therefore, it was suggested that BPD-MA was a potential sonosensitizer for sonodynamic therapy. Sonodynamic therapy seems to be a promising cancer treatment since ultrasound can penetrate deep within the tissue and can be focused into a tumor to activate photosensitizer. In future, we plan to conduct in vivo experiments to confirm whether this synergistic cytotoxic effect inhibits tumor growth.
5. Acknowledgements
We thank QLT, Inc. (Vancouver, British Columbia, Canada) for donating BPD-MA. We would like to thank Dr. Mutsumi Inaba for technical support.
REFERENCES
- N. Yumita, R. Nishigaki, K. Umemura and S. Umemura, “Synergistic Effect of Ultrasound and Hematoporphyrin on Sarcoma 180,” Japanese Journal of Cancer Research, Vol. 81, No. 3, March 1990, pp. 304-308.
- N. Yumita, R. Nishigaki, K. Umemura and S. Umemura, “Hematoporphyrin as a Sensitizer of Cell-Damaging Effect of Ultrasound,” Japanese Journal of Cancer Research, Vol. 80, No. 3, March 1989, pp. 219-222.
- I. Rosenthal, J. Z. Sostaric and P. Riesz, “Sonodynamic Therapy—A Review of the Synergistic Effects of Drugs and Ultrasound,” Ultrason Sonochem, Vol. 11, No. 6, September 2004, pp. 349-363.
- A. H. Saad and G. M. Hahn, “Ultrasound-Enhanced effects of Adriamycin against Murine Tumors,” Ultrasound in Medicine and Biology, Vol. 18, No. 8, 1992, pp. 715-723. doi:10.1016/0301-5629(92)90122-Q
- G. H. Harrison, E. K. Alcer-Kubiczek and P. L. Gutierrez, “In Vitro Mechanisms of Chemopotentiation by ToneBurst Ultrasound,” Ultrasound in Medicine and Biology, Vol. 22, No. 3, 1996, pp. 355-362. doi:10.1016/0301-5629(95)02053-5
- Q. Liu, X. Li, L. Xiao, P. Wang, X. Wang and W. Tang, “Sonodynamically Induced Antitumor Effect of Hematoporphyrin on Hepatoma 22,” Ultrasonics Sonochemistry, Vol. 15, No. 6, September 2008, pp. 943-948. doi:10.1016/j.ultsonch.2008.04.001
- T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. J. Peng, “Photodynamic Therapy,” Journal of the National Cancer Institute, Vol. 90, No. 12, June 1998, pp. 889-905. doi:10.1093/jnci/90.12.889
- T. J. Dougherty, “An Update on Photodynamic Therapy Applications,” Journal of Clinical Laser Medicine and Surgery, Vol. 20, No. 1, February 2002, pp. 3-7. doi:10.1089/104454702753474931
- L. Ayaru, S. G. Bown and S. P. Pereira, “Photodynamic Therapy for Pancreatic and Biliary Tract Carcinoma,” International Journal of Gastrointestinal Cancer, Vol. 35, No. 1, 2005, pp. 1-13. doi:10.1385/IJGC:35:1:001
- A. M. Richter, S. Cerruti-Sola, E. D. Sternberg, D. Dolphin and J. G. Levy, “Biodistribution of Tritiated Benzoporphyrin Derivative (3H-BPD-MA), a Newpotent Photosensitizer, in Normal and Tumorbearing Mice,” Journal of Photochemistry and Photobiology B, Vol. 5, No. 2, April 1990, pp. 231-244. doi:10.1016/1011-1344(90)80008-L
- G. I. Stables and D. V. Ash, “Photodynamic Therapy,” Cancer Treatment Reviews, Vol. 21, No. 4, July 1995, pp. 311-323. doi:10.1016/0305-7372(95)90035-7
- D. Huang, K. Okada, K. Comori, E. Itoi and T. Suzuki. “Enhanced Antitumor Activity of Ultrasonic Irradiation in the Presence of New Quinolone Antibiotics in Vitro,” Cancer Science, Vol. 95, No. 10, October 2004, pp. 845-849. doi:10.1111/j.1349-7006.2004.tb02192.x
- J. H. Li, D. Y. Song, Y. G. Xu, Z. Huang and W. Yue, “In Vitro Study of Haematoporphyrin Monomethyl EtherMediated Sonodynamic Effects on C6 Glioma Cells,” Neurological Sciences, Vol. 29, No. 4, September 2008, pp. 229-235. doi:10.1007/s10072-008-0972-8
- J. M. Runnels, N. Chen, B. Ortel, D. Kato and T. Hasan, “BPD-MA-Mediated Photosensitization in Vitro and in Vivo: Cellular Adhesion and β1 Integrin Expression in Ovarian Cancer Cells,” British Journal of Cancer, Vol. 80, No. 7, June 1999, pp. 946-953. doi:10.1038/sj.bjc.6690448
- J. F. Tait, “Imaging of Apoptosis,” Journal of Nuclear Medicine, Vol. 49, No. 10, September 2008, pp. 1573-1576. doi:10.2967/jnumed.108.052803
- M. van Engleland, L. J. Nieland, F. C. Ramaekers, B. Schutte and C. P. Reutelingsperger, “Annexin V-affinity Assay: A Review on an Apoptosis Detection System Based on Phosphatidylserine Exposure,” Cytometry, Vol. 31, No. 1, January 1998, pp. 1-9. doi:10.1002/(SICI)1097-0320(19980101)31:1<1::AID-CYTO1>3.0.CO;2-R
- Q. Liu, X. Wang, P. Wang, H. Qi, K. Zhang and L. Xiao, “Sonodynamic Effects of Protoporphyrin IX Disodium Salt on Isolated Sarcoma 180 Cells,” Ultrasonics, Vol. 45, No. 1-4, July 2006, pp. 56-60. doi:10.1016/j.ultras.2006.06.063
- L. D. Barnes, E. A, Gluliano, J. Ota, L. A. Cohn and C. P. Moore, “The Effect of Photodynamic Therapy on Squamous Cell in a Murine Model: Evaluation of Time Between Intralesional Injection to Laser Irradiation,” The Veterinary Journal, Vol. 180, No. 1, February 2008, pp. 60-65. doi:10.1016/j.tvjl.2007.11.023
- P. Chiarugi and E. Giannoni, “Anoikis: A Necessary Death Program for Anchorage-Dependent Cells,” Biochemical Pharmacology, Vol. 76, No. 11, July 2008, pp. 1352-1364. doi:10.1016/j.bcp.2008.07.023
- G. Melino, R. A. Knight and P. Nicotera, “How Many Ways to Die? How Many Different Models of Cell Death?” Cell Death & Differentiation, Vol. 12, November 2005, pp. 1457-1462. doi:10.1038/sj.cdd.4401781
- S. Umemura, N. Yumita, R. Nishigaki and K. Umemura, “Mechanism of Cell Damage by Ultrasound in Combination with Hematoporphyrin,” Japanese Journal of Cancer Research, Vol. 81, No. 9, September 1990, pp. 962-966.
- N. Yumita, N. Okuyama, K. Sasaki and S. Umemura, “Sonodynamic Therapy on Chemically Induced Mammary Tumor: Pharmacokinetics, Tissue Distribution and Sonodynamically Induced Antitumor Effect of Porfimer Sodium,” Cancer Science, Vol. 95, No. 9, September 2004, pp. 765-769. doi:10.1111/j.1349-7006.2004.tb03259.x
- M. W. Miller, D. L. Miller and A. A. Brayman, “A Review of in Vitro Bioeffects of Inertial Ultrasonic Cavitation from a Mechanistic Perspective,” Ultrasound in Medicine and Biology, Vol. 22, No. 9, 1996, pp. 1131- 1154. doi:10.1016/S0301-5629(96)00089-0
- S. Umemura, N. Yumita and R. Nishigaki, “Enhancement of Ultrasonically Induced Cell Damage by a GalliumPorphyrin Complex, ATX-70,” Japanese Journal of Cancer Research, Vol. 84, No. 5, May 1993, pp. 582-588.

