Chinese Medicine
Vol.4 No.2(2013), Article ID:33028,10 pages DOI:10.4236/cm.2013.42009
The Effects of Ganoderma lucidum on Initial Events Related to the Bacillus Calmette-Guérin Efficacy and Toxicity on High-Risk Uroepithelial Cells: An in Vitro Preliminary Study
1School of Nursing, The Hong Kong Polytechnic University, Hong Kong, China
2Department of Medical Science, Tung Wah College, Hong Kong, China
3Department of Surgery, The Chinese University of Hong Kong, Hong Kong, China
Email: *john.yuen@polyu.edu.hk, dannygohel@twc.edu.hk, ngcf@surgery.cuhk.edu.hk
Copyright © 2013 John Wai-Man Yuen et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 9, 2013; revised April 30, 2013; accepted May 18, 2013
Keywords: Ganoderma lucidum; Bacillus Calmette-Guérin; Bladder Cancer; Uroepithelial Cells; Synergism
ABSTRACT
A novel prophylactic regimen is demanded for preventing bladder cancer recurrence, because of the high side-effect tolls of conventional adjuvant Bacillus Calmette-Guérin (BCG) immunotherapy, in addition to its only moderate efficacy. In vitro and animal studies have demonstrated the anti-cancer properties of a medicinal mushroom called Ganoderma lucidum (GL). In this study, a pre-malignant human uroepithelial cells (HUC-PC) model was utilized to compare the effectiveness between ethanol extract of GL (GLe) and BCG on interleukin-6 (IL-6) secretion and lactate dehydrogenase (LDH) cytotoxicity. Additionally, parameters relevant to the BCG efficacy and safety, including free soluble fibronectin (FN) and cell-surface glycosaminoglycans (GAGs) levels were tested, following the exposure of GLe to the cells. GLe at 100 μg/ml and BCG at 4.8 × 107 CFU were shown to induce equivalent levels of IL-6, suggesting the potential synergism, while the tested concentrations of GLe were non-cytotoxic. During the initial four hours of GLe exposure, the free FN concentrations in harvested media were significantly reduced that might facilitate the binding of BCG for uroepithelial internalization to enhance BCG efficacy. Furthermore, the cell membrane-bound GAGs levels of HUC-PC cells were significant increased in response to GLe to suggest cellular protection from BCG infection. In summary, current findings suggest the potential additive synergism of GLe with the BCG efficacy, as well as its protective effects, and thus reducing the BCG toxicity.
1. Introduction
Ganoderma lucidum (GL), a popular ancient medicinal mushroom ranked as a superior tonic in traditional Chinese medicine, is commonly used for health promotion and longevity. Nowadays, the mushroom is being used by many cancer patients because of its perceived health benefits including immunomodulating properties and antitumorigenicity. In the recent years, our research team and collaborators have focused on bladder cancer, and reported a range of in vitro chemopreventive activities for GL. It was demonstrated that remarkable growth inhibitory effects via G2/M phase cell cycle arrest [1] and apoptosis [2] were exhibited by a defined ethanol extract of GL (GLe) on the pre-malignant human uroepithelial cell line (HUC-PC). The GLe-treated HUC-PC cells were also characterized to have significant oxidative DNA damage [3] and secretion of several cytokines including IL-2, IL-6 and IL-8, [4] altogether suggesting the proinflammatory mechanism for adverse cell eradication. Furthermore, GLe was found to suppress the migration and telomerase activity that induced by a bladder cancer-relevant carcinogen 4-aminobiphenyl [1,2].
The recurrence rate of superficial transitional cell carcinoma (TCC) of bladder remains exceptionally high even with the effective transurethral resection (TUR) technique [5]. Refers to the “field cancerization hypothesis” and “seeding theory”, residual cells at treated and adjacent sites are highly susceptible for mutagenic attacks and can potentially develop into tumors again, and hence powerful chemopreventive agents are demanded for prophylaxis [6]. Bacillus Calmette-Guérin (BCG), is currently the most effective prophylactic agent available and, when introduced intravesically, it triggers a local inflammatory response inside the urinary bladder [7,8]. In response to BCG, host leukocytes infiltrate into the urothelial wall and are responsible for most of the urinary cytokine secretion [9,10]. Evidence has also indicated that in situ lymphocytes are able to eradicate BCG-internalizing tumor cells through specific cell lysis against mycobacterial antigens [11]. However, only certain cytokines, including IL-2, IL-6 and TNF-α, are detectable in a patient’s urine within the first 24 hours upon BCG instillation [12]. Particularly, the two pro-inflammatory cytokines, IL-6 and TNF-α, could also be secreted from various human bladder cancer cell lines [13-15]. Interestingly, well-differentiated bladder tumor cells that are incapable of internalizing BCG were also unable to upregulate IL-6 expression [16]. In contrast, normal urothelial cells and poorly differentiated TCC cells were able to internalize BCG and produce IL-6 [11,16]. Therefore, IL-6 cytokine was considered as an indicative marker for BCG internalization [17]. Given that binding of BCG to the urothelial surface is a pre-requisite for successful internalization [17], the urothelium and mycobacterium are linked through fibronectin (FN) opsonization [18-20]. Formation of FN bridges might facilitate the process of BCG internalization [17-19]. Such linkage induces the expression of the IL-6 gene through NF-κB and AP-1 signal transducers in bladder tumor cells [21]. However, excess free FN was reported to be competitive with each other for the limited binding sites on urothelium and BCG surface, and thus impairing the internalization of BCG and its subsequent responses [22]. Besides its efficacy, BCG instillation also has high side-effect tolls of up to 90% of the patients develop cystitis and haematuria [8]. Patients receiving BCG treatment are taking the risk of systemic mycobacterial infection that could be lethal, although it is rare [23]. In fact, direct adhesion is not required for the process of BCG internalization, where a close-docking distance (70 - 100A) is set by the repellent force between BCG and urothelium [24,25]. The luminal wall of the bladder is protected by the mucosal lining that is covered with negatively charged glycosaminoglycans (GAGs), which keeping away the bacteria and toxins from certain distance of the anionic urothelial mucosa.[17] Thus, free FN concentration and cell surface-bound GAGs are two relevant biomarkers for BCG binding efficiency as well as potential toxicity.
In the present study, the HUC-PC cell model is continuously being utilized, firstly to compare between the effectiveness of GLe and BCG on the cytotoxicity and IL-6 secretion. IL-6 has been suggested to be responsible for the cytotoxicity of BCG on several TCC cell lines [26,27]. Whether BCG and GLe would both be cytotoxic to HUC-PC was determined using the LDH cytotoxicity assay, if they are capable of inducing IL-6 secretion. Secondly, the effects of GLe on extracellular FN and cell surface GAGs are explored. These findings will aid in elucidating whether GLe is a possible candidate to supplement or even replace the BCG immunotherapy.
2. Materials and Methods
2.1. Preparation of GLe and BCG
The active ingredients of RishiMax GLPTM G. lucidum (Pharmanex, Hong Kong) were commercially standardized to 13.5% polysaccharides and 6% triterpenes. Powdered G. lucidum from capsules was re-extracted as previously described [3,4]. GLe was dissolved freshly in absolute ethanol (0.1% v/v) and diluted with culture medium to make a 1000 μg/ml stock solution. The whole vial (dry weight 81 mg) of live attenuated BCG (IMMUCYST®, Aventi, Toronto, Ontario, Canada) was reconstituted with 3 ml of the accompanying diluents to make a suspension containing a minimal dosage of 6.6 × 108 colony forming units (CFU). A 4.8 × 107 CFU BCG stock solution was prepared with culture medium. For assays, working assay media were prepared by further diluting the stock solutions of GLe and BCG into the concentrations of test ranges. Solvent media containing the maximal amount of corresponding solvent, i.e. 0.1% v/v ethanol for GLe and 33% v/v diluents for BCG, were used as controls. Furthermore, GLe and BCG were checked using Limulus Amebocyte Lysate (LAL) endpoint chromogenic kit assay (CAPE CO, E. Falmouth, MA, USA) for lipopolysaccharides (LPS) contamination. GlucashieldTM buffer (CAPE COD) was used to reconstitute pyrochrome to inhibit possible (1,3)-β-D-glucan presented in samples, and thus avoiding potential interference in the assay. Aseptic techniques were strictly applied throughout the procedures.
2.2. Cell Culture for Assays
The HUC-PC cell line was derived in the Department of Human Oncology, University of Wisconsin Medical School, and gifted by Dr. Rao from the University of California, Los Angeles. The cell line was cultured in F12 Ham enriched Dulbecco’s Modified Eagle’s Meium (F12/DMEM purchased from Sigma, St. Louis, MO) with 1% penicillin (10,000 μg/ml) and streptomycin (10,000 mg/ml) and 10% Fetal Bovine Serum (GIBCO BRL Isaland, New York, USA). Logarithmically growing HUC-PC cells were harvested and seeded in 96-well flat-bottle tissue culture plate (Greiner bio-one, Germany) at a concentration of 5 × 104 cells per microtitre well for cytotoxicity, FN and GAGs measurement. In parallel experiments, 1 × 106 cells were also seeded in 100-mm tissue culture dishes (Greiner bio-one, Germany) for IL-6 assay.
2.3. Lactate Dehydrogenase (LDH) Cytotoxicity Assay
Cytotoxicity of GLe and BCG was assayed by measuring LDH released from cells with LDH Cytotoxicity Detection kit (TaKaRa Bio Inc., Shiga, Japan). Following the manufacturer’s instructions, the cells were incubated with assay media containing GLe or BCG for 24 hours in microtitre plate wells (Thermo Labsystems, Franklin, MA). No significant cytotoxicity was observed with the solvent controls. The release of LDH from cells was measured at 490 nm with reference wavelength at 690 nm, using TECAN SPECTRA Fluor Plus microplate reader (TECAN Austria GmbH, Grodig, Austria). Untreated cells were used as low controls to measure the spontaneous LDH release, and Triton X-100 treated cells were used as high controls to measure the maximum releasable LDH activity. No interference was observed from any test substances used in the assay. Cytotoxicity was calculated as a percentage of LDH release with the following formula:

2.4. Enzyme-Linked Immunosorbent Assay (ELISA) for IL-6 Cytokine
Cultured supernatants were collected to measure the IL-6 secretion with the Endogen® Human IL-6 ELISA kit (Pierce Biotechnology Inc, Rockford, USA). The manufacturer’s instructions were followed. Culture medium was used to prepare the standard curve by serial dilutions (ranging from 0 pg/ml to 400 pg/ml). Absorbance of the reaction microplate wells was measured at 450 nm on microplate reader (TECAN, Austria).
2.5. Enzyme Immunoassay (EIA) for Fibronectin Quantitation
Conditioned media were harvested from cell-seeded microtitre plate after four hours (for avoiding cytotoxic effects based on previous findings of apoptosis) of treatment with GLe. The TaKaRa Fibronectin EIA kit (TAKARA Bio Inc., Japan) was used for assay. Following the kit instructions, a 100 μl of sample/standard was added into an ELISA well coated with human anti-fibronectin and incubated for one hour at 37˚C. The microtitre wells were washed four times and then 100 μl of substrate solution was added and incubated for 15 minutes at room temperature. 1 N Sulphuric acid (H2SO4) was added to stop the reaction. Finally, absorbance was read against diluent blank at 450 nm, using TECAN SPECTRA Fluor Plus microplate reader (TECAN, Austria).
2.6. Dimethylmethylene Blue (DMMB) Method for GAGs Quantitation
After four hours of incubation, same as for FN assay, membrane-bound GAGs from HUC-PC cells were extracted by a 0.1 M sodium acetate buffer at pH 5.8 in a microtitre plate overnight, in accordance with previous publication [28] with minor modifications. The supernatant was collected and digested overnight with 20 μl of papain (Merck, UK) at 65˚C. The isolated GAGs were assayed by the DMMB method [29]. A 50 μl of sample/standard was added into each well of a new microtitre plate, which was followed by an addition of 200 μl of working DMMB (Aldrich, USA) reagent. Absorbance of the microtitre wells was read immediately against milliQ blank at 620 nm. GAGs solution (mixture of hyaluronate, chondroitin, sulphate, Keratan sulphate and heparan sulphate) at 0, 4, 8, 16, and 32 μg/ml concentrations was used as standards.
2.7. Statistical Analysis
Each study group was run in triplicate and duplicated samples from each group were measured for each variable. Differences between means were determined using Student’s t-test (GraphPad Prism version 3.0 for Windows, San Diego California, USA). Statistical significance was sought at two tailed P-value of 0.05.
3. Results
3.1. Cytotoxicity and IL-6 Secretion Induced by GLe and BCG
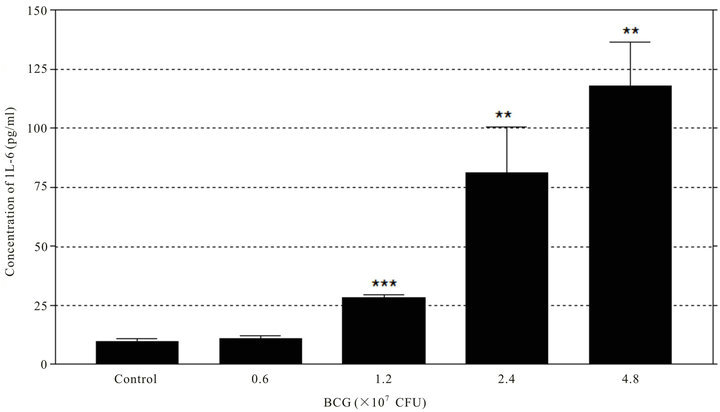
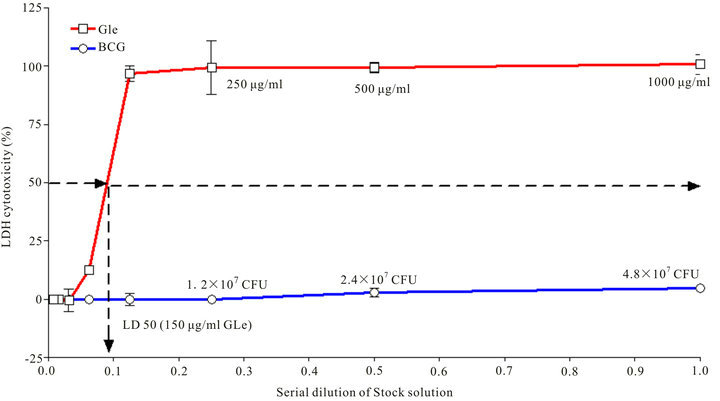
Results indicated that both BCG and GLe were clearly capable of inducing dose-dependent IL-6 secretion in the HUC-PC culture (Figure 1). GLe was shown to be cytotoxic to the HUC-PC cells. No LPS was detected in the β-D-glucan-inhibited fractions of GLe, while approximately 0.4 EU/ml of LPS was detected in BCG at 1.2 × 107 CFU. By serial dilution, 100% ± 12% (Mean ± SEM) of the cells was killed by 250 μg/ml of GLe, and the cytotoxic effects reached a plateau of 100% at concentrations ranged 250 - 1000 μg/ml (Figure 2(a)). LD50 for GLe is between 180 - 190 μg/ml for GLe, the dose-dependence was confirmed by repeating LDH cytotoxicity assay with GLe concentrations at 40, 80, 100 and 200 μg/ml. Results indicated that 100% ± 5% and 13.8% ± 2% (Mean ± SEM) of cells were killed, by 200 μg/ml and 100 μg/ml of GLe respectively, but no cytotoxicity was found at 80
 (a)
(a)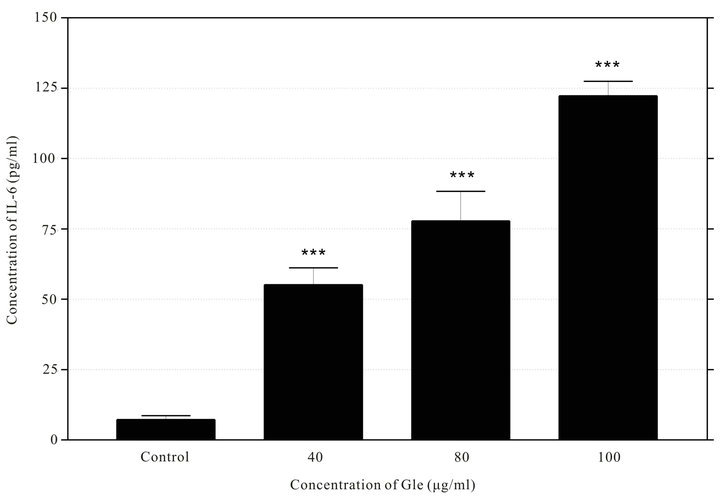 (b)
(b)
Figure 1. Dose-dependent IL-6 secretion was induced by BCG (a) and GLe (b). Culture media were harvested and measured at 24 hours after incubating with BCG at concentrations between 0.6 - 4.8 × 107 CFU while with GLe between 40 - 100 μg/ml (n = 3, error bar: SEM, **P < 0.01; ***P < 0.001).
μg/ml or lower GLe concentration (Figure 2(b)). However, after the 24 hours exposure, no significant cytotoxicity was observed by BCG up to dosage at 4.8 × 107 CFU (Figure 2(a)).
 (a)
(a)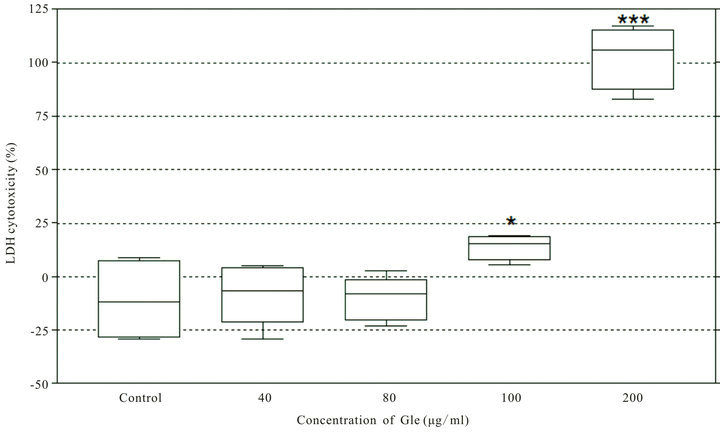 (b)
(b)
Figure 2. Cytotoxicties of BCG and G. lucidum on HUC-PC cells measured by LDH cytotoxicity assay after 24-hour incubation. (a) Serial dilution of GLe and BCG (starting concentrations of 1000 μg/ml and 4.8 × 107 CFU) were incubated with the cells. LD50 (→) was determined as 150 μg/ml for GLe (n = 3, error bar: SEM); (b) Dose-dependent cytotoxic effects of GLe at concentrations 40, 80, 100 and 200 μg/ml (n = 3, error bar: SEM). No cytotoxicity was detected at 40 and 80 μg/ml of GLe (*P < 0.05; ***P < 0.001).
3.2. The Modulation of Extracellular FN and Cell-Surface GAGs by GLe
About 15% of the free FN in the cultured media was significantly (P < 0.01) reduced by GLe at concentrations of 40 - 100 μg/ml (Figure 3(a)). Such reduction may not be the maximum effects of GLe, as it was measured at 4 hours after incubation to avoid the cytotoxic artefact. On
 (a)
(a) (b)
(b)
Figure 3. Culture media were harvested and measured at 4 hours after incubating with GLe (40, 80 and 100 μg/ml), which indicated the GLe-mediated reduction of extracellular FN (n = 3, error bar: SEM, ***P < 0.001; **P < 0.01). Whilst cultured cells were harvested at the same time and treated for measurement, which indicated the elevat of cell surface GAGs (n = 3, error bar: SEM, ***P < 0.001; **P < 0.01).
the other hand, the cell-membrane bound GAGs levels on HUC-PC cells were significantly (P < 0.01) increased (Figure 3(b)).
4. Discussion
In accordance with one of our recent publications [4], GLe has shown to be capable of stimulating IL-6 production in the HUC-PC cells. Current results indicated that the IL-6 secretions induced by GLe at 100 μg/ml and BCG at 4.8 × 107 CFU were almost quantitatively equivalent. This is consistent with reports on the HPV-immortalized Hu35E6E7 HUC cell line [21] and other bladder cancer cells [11,13-16] that IL-6 was commonly up-regulated by BCG. Lipopolysaccharide (LPS), a potent immune-stimulator is found in gram-negative bacteria but also easily being extracted from GL by ethanol [30,31]. However, results of LAL test indicated that unlike the BCG, GLe used in current study was negative for LPS. Thus, the BCG-mediated IL-6 secretion was at least partly owned to its LPS activities while the IL-6 induced by GLe was not. At molecular level, the expression of IL-6 mRNA in the Hu35E6E7 HUC cells was found to be exclusively triggered by BCG through the toll-like receptor (TLR) signaling [21]. NF-κB and AP-1 are the main signaling pathways responsible for IL-6 expression immediately upon BCG stimulation [32]. Coherently, we have reported that GLe also enhanced the p50/p65 NF-κB activity during the immunological events in the HUC-PC cells [4]. In response to BCG, the NF- κB-mediated pathways and thus the IL-6 promoter constructs were triggered through the cross-linking of α5β1 integrin on the surface of human TCC cells and that able to induce cell cycle arrest [26,33]. The idea of “IL-6- activated tumor inhibition” has been proposed earlier [34]. IL-6 of autocrine and recombinant natures were demonstrated to be antiproliferative in TCC cell lines [26, 35-37]. The inhibition of leukaemia cells mediated by GL was also once suggested to be responsible by the increased IL-6 secretion [38,39]. However, the conceptual role of cytotoxic IL-6 is arguable with the fact that normal and low-grade bladder cancer cells which have high capacity of internalizing BCG to induce IL-6 are less efficient being killed by BCG [16]. Cytotoxicity of BCG was shown to be more potent on poorly-differentiated highgrade bladder cancer cells than the low-grade ones [27]. This is further confirmed by the present results where BCG was shown to be non-cytotoxic to the HUCPC cells, at least after 24-hour incubation that IL-6 was significantly induced. In contrast, cytotoxic effects of GLe and IL-6 induction were explicitly demonstrated at 24 hours. Therefore, IL-6 is important to be an inductive marker for BCG internalization rather than its cytotoxicity in BCG-mediated prophylaxis in bladder cancer.
The α5β1 integrin is a classic cellular receptor presented on the malignant urothelium for fibronectin (FN) [26,33]. Expression of α5 and β1 mRNA could be promoted by exogenous and autocrine IL-6, while competive inhibitors of FN inhibit BCG-induced NF-κB signaling pathways [33,40]. Furthermore, autocrine IL-6 enhanced BCG adherence to the 253J TCC cell line through the up-regulation of α5β1 integrin receptor for FN [40]. In the present study, the up-regulation of IL-6 secretion in response to BCG suggested the HUC-PC cells are capable of internalizing BCG, despite further elucidation is needed. FN is an essential adhesion glycoprotein for BCG binding to the surface of urothelium, internalization and production IL-6 [17,41,42]. There are two forms of FN: soluble and surface-bound [43]. Loss of cell surface FN on transformed cells is correlated with acquisition of tumorigenicity [44] and metastatic potential [45]. Such FN losses are mainly due to reduced synthesis, reduced binding and increased degradation rate, and increased FN release into the extracellular matrix [43]. These FN molecules facilitate cell-substrate adhesion, and thus enhance the interaction between the urothelium and extracellular matrix, and ultimately affect the cell morphology, cytoskeletal organization, migration and differentiation [46]. About 90% of urothelial tumor stroma was positive for FN immunohistochemical expression [47]. Expression of extracellular FN is correlated with tumor progression for invasiveness and aggressiveness [47,48]. Blocking of FN attachment sites on TCC cells inhibits the tumor outgrowth in vivo [49]. The diagnostic roles of soluble FN in urine have been proposed and its elevated levels are associated with tumor stage, degree of differentiation, tumor size, multifocal nature or macroscopic appearance [46,50]. Clinical data supported that persistent elevation of urinary FN causes BCG failure after complete TUR [51]. Excess soluble FN, whether from exogenous or autocrine origin, also saturates mycobacterial and cell surface FN receptors simultaneously, and precludes the bridging ability of a single FN molecule, impairing α5β1 integrin/integrin mediated NF-κB signal transduction which is considered to be critical for BCG prophylaxis [22]. Thus, free FN in the culture media is regarded as a key factor for both carcinogenesis of urothelial cells and BCG binding. In contrast, cell surface expression of FN is comparatively less important regarding its roles in bladder chemoprevention. Current findings indicated that free FN was reduced in the culture media of treated HUC-PC cells by GLe.
Furthermore, the expression of GAGs on the HUC-PC cell surface was also increased by GLe. Formerly known as mucopolysaccharides, GAGs are long unbranched polysaccharides, are highly anionic and are often bound to core proteins to become proteoglycans with varying properties of extracellular matrices of tissues [52,53]. GAGs are extremely hydrophilic and trap water at the outer layer of the umbrella urothelium, and this trapped water forms a gel as part of the mucosal barrier that interfaces urine and the bladder wall [54,55]. This provides a protective barrier that becomes highly impermable to any solutes, crystals and even bacteria in urine [54,56]. The disruption of this mucosal permeability is pathologically significant such that interstitial cystitis (IC) occurs [54, 57]. GAGs are also able to repair damaged bladder mucosa [54]. In addition, anti-adherence properties of GAGs have primary innate defence against bacterial attacks [52, 54,56]. Experimental removal of GAGs from the urothelial surface causes a ten-fold higher bacterial adherence [58]. Therefore, the effects of GLe on cell-surface GAGs may strengthen the mucosal barrier of the urothelium, repair the urothelial damage induced by BCG therapy, as well as prevent the side effects of BCG therapy, such as cystitis and infections.
In summary, ethanol extract of GL exerted as a similar activator for IL-6 production as BCG in the HUC-PC cells. IL-6 was the only cytokine selected for measurement because it is the earliest cytokine that can be detected after BCG exposure to urothelial cells and it is also an indicator for successful BCG internalization by these cells. Current results suggested that combinational use of GLe and BCG may exert synergistic effects in several ways (Figure 4): Firstly, the cytotoxic and cytokine secretion can be additive to BCG activities; Secondly, the reduction of free FN can facilitate BCG binding to the
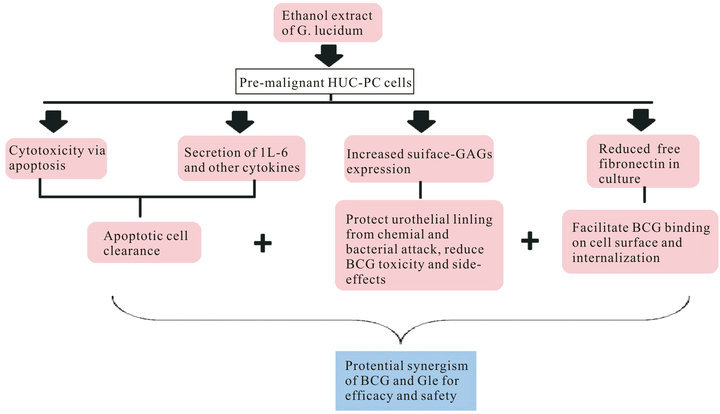
Figure 4. A summary of GLe effects on the HUC-PC cells to deduce the potential synergism between BCG and GLe.
urothelial surface for subsequent internalization and IL-6 secretion, particularly for cells at normal or low grades. Nonetheless, the reduction of free FN, by itself may also suggest being tumor suppressive to inhibit the growth and progression by reducing unnecessary cell-substrate interactions; and thirdly, the increased cell surface GAGs expression provide additional protection from chemical and bacterial attacks, and thus is potential in reducing side-effects caused by BCG. Further experiments are underway to define the synergism of GLe and BCG as well as to investigate the underlying mechanisms. No doubt, the anticancer activities of GLe were demonstrated in the HUC-PC cell model to suggest the associations between IL-6 induction, FN reduction, and BCGGLe synergism were suggested. However, the cause-andeffect mechanism needs to be confirmed by further careful scientific investigation.
5. Acknowledgements
This project was supported by the Research Committee of the Hong Kong Polytechnic University for the postgraduate scholarship (RGH8) and Sir Edward Youde Memorial Fellowship awarded to Dr. John Yuen. The authors are grateful to Dr. J. Y. Rao (UCLA Medical Center, USA) for providing the HUC-PC cell line and professional advice.
REFERENCES
- Q. Y. Lu, Y. S. Jin, Q. Zhang, Z. Zhang, D. Heber, V. L. Go, et al., “Ganoderma lucidum Extracts Inhibit Growth and Induce Actin Polymerization in Bladder Cancer Cells in Vitro,” Cancer Letters, Vol. 216, No. 1, 2004, pp. 9-20. doi:10.1016/j.canlet.2004.06.022
- J. W. Yuen, M. D. Gohel and D. W. Au, “Telomerase-Associated Apoptotic Events by Mushroom Ganoderma Lucidum on Premalignant Human Urothelial Cells,” Nutrition and Cancer, Vol. 60, No. 1, 2008, pp. 109-119. doi:10.1080/01635580701525869
- J. W. Yuen and M. D. Gohel, “The Dual Roles of Ganoderma Antioxidants on Urothelial Cell DNA under Carcinogenic Attack,” Journal of Ethnopharmacology, Vol. 118, No. 2, 2008, pp. 324-330. doi:10.1016/j.jep.2008.05.003
- J. W. Yuen, M. D. Gohel and C. F. Ng, “The Differential Immunological Activities of Ganoderma lucidum on Human Pre-Cancerous Uroepithelial Cells,” Journal of Ethnopharmacology, Vol. 135, No. 1, 2011, pp. 711-718. doi:10.1016/j.jep.2011.04.005
- W. Oosterlinck, “The Management of Superficial Bladder Cancer,” BJU International, Vol. 87, No. 2, 2001, pp. 135- 140. doi:10.1046/j.1464-410x.2001.00948.x
- J. T. Leppert, O. Shvarts, K. Kawaoka, R. Lieberman, A. S. Belldegrun and A. J. Pantuck, “Prevention of Bladder Cancer: A Review,” European Urology, Vol. 49, No. 2, 2006, pp. 226-234. doi:10.1016/j.eururo.2005.12.011
- P. Tyagi, P. C. Wu, M. Chancellor, N. Yoshimura and L. Huang, “Recent Advances in Intravesical Drug/Gene Delivery,” Molecular Pharmaceutics, Vol. 3, No. 4, 2006, pp. 369-379. doi:10.1021/mp060001j
- D. L. Lamm, W. R. McGee and K. Hale, “Bladder Cancer: Current Optimal Intravesical Treatment,” Urologic Nursing, Vol. 25, No. 5, 2005, pp. 323-326, 331-332.
- K. Taniguchi, S. Koga, M. Nishikido, S. Yamashita, T. Sakuragi, H. Kanetake, et al., “Systemic Immune Response after Intravesical Instillation of Bacille Calmette-Guerin (BCG) for Superficial Bladder Cancer,” Clinical & Experimental Immunology, Vol. 115, No. 1, 1999, pp. 131- 135. doi:10.1046/j.1365-2249.1999.00756.x
- M. Sanchez-Carbayo, M. Urrutia, R. Romani, M. Herrero, J. M. Gonzalez de Buitrago and J. A. Navajo, “Serial Urinary IL-2, IL-6, IL-8, TNF Alpha, UBC, CYFRA 21-1 and NMP22 during Follow-Up of Patients with Bladder Cancer Receiving Intravesical BCG,” Anticancer Research, Vol. 21, No. 4B, 2001, pp. 3041-3047.
- J. J. Patard, F. Saint, F. Velotti, C. C. Abbou and D. K. Chopin, “Immune Response Following Intravesical Bacillus Calmette-Guerin Instillations in Superficial Bladder Cancer: A Review,” Urological Research, Vol. 26, No. 3, 1998, pp. 155-159. doi:10.1007/s002400050039
- T. M. de Reijke, E. C. de Boer, K. H. Kurth and D. H. Schamhart, “Urinary Cytokines during Intravesical Bacillus Calmette-Guerin Therapy for Superficial Bladder Cancer: Processing, Stability and Prognostic Value,” Journal of Urology, Vol. 155, No. 2, 1996, pp. 477-482. doi:10.1016/S0022-5347(01)66424-3
- T. M. de Reijke, P. C. Vos, E. C. de Boer, R. F. Bevers, W. H. de Muinck Keizer, K. H. Kurth, et al., “Cytokine Production by the Human Bladder Carcinoma Cell Line T24 in the Presence of Bacillus Calmette-Guerin (BCG),” Urological Research, Vol. 21, No. 5, 1993, pp. 349-352. doi:10.1007/BF00296835
- K. Esuvaranathan, A. B. Alexandroff, M. McIntyre, A. M. Jackson, S. Prescott, G. D. Chisholm, et al., “Interleukin- 6 Production by Bladder Tumors Is Upregulated by BCG Immunotherapy,” Journal of Urology, Vol. 154, No. 2, 1995, pp. 572-575. doi:10.1016/S0022-5347(01)67113-1
- Y. Zhang, R. Mahendran, L. L. Yap, K. Esuvaranathan and H. E. Khoo, “The Signalling Pathway for BCG-Induced Interleukin-6 Production in Human Bladder Cancer Cells,” Biochemical Pharmacology, Vol. 63, No. 2, 2002, pp. 273-282. doi:10.1016/S0006-2952(01)00831-0
- R. F. Bevers, E. C. de Boer, K. H. Kurth and D. H. Schamhart, “BCG-Induced Interleukin-6 Upregulation and BCG Internalization in Well and Poorly Differentiated Human Bladder Cancer Cell Lines,” European Cytokine Network, Vol. 9, No. 2, 1998, pp. 181-186.
- R. F. Bevers, K. H. Kurth and D. H. Schamhart, “Role of Urothelial Cells in BCG Immunotherapy for Superficial Bladder Cancer,” British Journal of Cancer, Vol. 91, No. 4, 2004, pp. 607-612.
- L. R. Kavoussi, E. J. Brown, J. K. Ritchey and T. L. Ratliff, “Fibronectin-Mediated Calmette-Guerin Bacillus Attachment to Murine Bladder Mucosa. Requirement for the Expression of an Antitumor Response,” The Journal of Clinical Investigation, Vol. 85, No. 1, 1990, pp. 62-67. doi:10.1172/JCI114434
- T. L. Ratliff, J. O. Palmer, J. A. McGarr and E. J. Brown, “Intravesical Bacillus Calmette-Guerin Therapy for Murine Bladder Tumors: Initiation of the Response by Fibronectin-Mediated Attachment of Bacillus Calmette-Guerin,” Cancer Research, Vol. 47, 1987, pp. 1762-1766.
- J. Aslanzadeh, E. J. Brown, S. P. Quillin, J. K. Ritchey and T. L. Ratliff, “Characterization of Soluble Fibronectin Binding to Bacille Calmette-Guerin,” Journal of General Microbiology, Vol. 135, No. 10, 1989, pp. 2735-2741.
- J. Miyazaki, K. Kawai, T. Oikawa, A. Johraku, K. Hattori, T. Shimazui, et al., “Uroepithelial Cells Can Directly Respond to Mycobacterium Bovis Bacillus Calmette-Guerin through Toll-Like Receptor Signaling,” BJU International, Vol. 97, No. 4, 2006, pp. 860-864. doi:10.1111/j.1464-410X.2006.06026.x
- G. Zhang, F. Chen, Y. Xu, Y. Cao, S. Crist, A. McKerrow, et al., “Autocrine over Expression of Fibronectin by Human Transitional Carcinoma Cells Impairs Bacillus Calmette-Guerin Adherence and Signaling,” Journal of Urology, Vol. 172, No. 4, 2004, pp. 1496-1500. doi:10.1097/01.ju.0000140193.95528.91
- Z. Akbulut, A. E. Canda, A. F. Atmaca, H. I. Cimen, C. Hasanoglu and M. D. Balbay, “BCG Sepsis Following Inadvertent Intravenous BCG Administration for the Treatment of Bladder Cancer Can Be Effectively Cured with Anti-Tuberculosis Medications,” The New Zealand Medical Journal, Vol. 123, No. 1325, 2010, pp. 72-77.
- D. H. Schamhart, E. C. De Boer, R. Vleeming and K. H. Kurth, “Theoretical and Experimental Evidence on the Use of Glycosaminoglycans in BCG-Mediated Immunotherapy of Superficial Bladder Cancer,” Seminars in Thrombosis and Hemostasis, Vol. 20, No. 3, 1994, pp. 301-309. doi:10.1055/s-2007-1001917
- D. H. Schamhart, E. C. de Boer and K. H. Kurth, “Interaction between Bacteria and the Lumenal Bladder Surface: Modulation by Pentosan Polysulfate, an Experimental and Theoretical Approach with Clinical Implication,” World Journal of Urology, Vol. 12, No. 1, 1994, pp. 27-37. doi:10.1007/BF00182048
- F. Chen, G. Zhang, Y. Iwamoto and W. A. See, “BCG Directly Induces Cell Cycle Arrest in Human Transitional Carcinoma Cell Lines as a Consequence of Integrin Cross-Linking,” BMC Urology, Vol. 5, 2005, p. 8. doi:10.1186/1471-2490-5-8
- Y. Zhang, H. E. Khoo and K. Esuvaranatha, “Effects of Bacillus Calmette-Guerin and Interferon-Alpha-2B on Human Bladder Cancer in Vitro,” International Journal of Cancer, Vol. 71, No. 5, 1997, pp. 851-857. doi:10.1002/(SICI)1097-0215(19970529)71:5<851::AID-IJC25>3.0.CO;2-9
- R. W. Farndale, C. A. Sayers and A. J. Barrett, “A Direct Spectrophotometric Microassay for Sulfated Glycosaminoglycans in Cartilage Cultures,” Connective Tissue Research, Vol. 9, No. 4, 1982, pp. 247-248. doi:10.3109/03008208209160269
- R. W. Farndale, D. J. Buttle and A. J. Barrett, “Improved Quantitation and Discrimination of Sulphated Glycosaminoglycans by Use of Dimethylmethylene Blue,” Biochimica et Biophysica Acta, Vol. 883, No. 2, 1986, pp. 173-177. doi:10.1016/0304-4165(86)90306-5
- W. T. Chung, S. H. Lee, J. D. Kim, Y. S. Park, B. Hwang, S. Y. Lee, et al., “Effect of Mycelial Culture Broth of Ganoderma lucidum on the Growth Characteristics of Human Cell Lines,” Journal of Bioscience and Bioengineering, Vol. 92, No. 6, 2001, pp. 550-555.
- H. S. Chen, Y. F. Tsai, S. Lin, C. C. Lin, K. H. Khoo, C. H. Lin, et al., “Studies on the Immuno-Modulating and Anti-Tumor Activities of Ganoderma lucidum (Reishi) Polysaccharides,” Bioorganic & Medicinal Chemistry, Vol. 12, No. 21, 2004, pp. 5595-5601.
- F. Chen, P. Langenstroer, G. Zhang, Y. Iwamoto and W. A. See, “Androgen Dependent Regulation of Bacillus Calmette-Guerin Induced Interleukin-6 Expression in Human Transitional Carcinoma Cell Lines,” Journal of Urology, Vol. 170, No. 5, 2003, pp. 2009-2013.
- F. Chen, G. Zhang, Y. Iwamoto and W. A. See, “Bacillus Calmette-Guerin Initiates Intracellular Signaling in a Transitional Carcinoma Cell Line by Cross-Linking Alpha 5 Beta 1 Integrin,” Journal of Urology, Vol. 170, No. 2, 2003, pp. 605-610.
- P. Musiani, A. Modesti, M. Giovarelli, F. Cavallo, M. P. Colombo, P. L. Lollini, et al., “Cytokines, Tumour-Cell Death and Immunogenicity: A Question of Choice,” Immunology Today, Vol. 18, No. 1, 1997, pp. 32-36.
- A. Sasaki, S. Kudoh, K. Mori, N. Takahashi and T. Suzuki, “Are BCG Effects against Urinary Bladder Carcinoma Cell Line T24 Correlated with Apoptosis in Vitro?” Urology International, Vol. 59, No. 3, 1997, pp. 142-148.
- E. C. Borden, D. S. Groveman, T. Nasu, C. Reznikoff and G. T. Bryan, “Antiproliferative Activities of Interferons against Human Bladder Carcinoma Cell Lines in Vitro,” The Journal of Urology, Vol. 132, No. 4, 1984, pp. 800- 803.
- A. M. Jackson, A. B. Alexandroff, D. Fleming, S. Prescott, G. D. Chisholm and K. James, “Bacillus CalmetteGuerin (BCG) Organisms Directly Alter the Growth of Bladder Tumour Cells,” International Journal of Oncology, Vol. 5, No. 3, 1994, pp. 697-703.
- International Agency for Research on Cancer, “Monographs on the Evaluation of Carcinogenic Risk of Chemicals,” World Health Organization, Geneva, 1972.
- S. Y. Wang, M. L. Hsu, H. C. Hsu, C. H. Tzeng, S. S. Lee, M. S. Shiao, et al., “The Anti-Tumor Effect of Ganoderma Lucidum Is Mediated by Cytokines Released from Activated Macrophages and T Lymphocytes,” International Journal of Cancer, Vol. 70, No. 6, 1997, pp. 699- 705. doi:10.1002/(SICI)1097-0215(19970317)70:6<699::AID-IJC12>3.0.CO;2-5
- G. J. Zhang, S. A. Crist, A. K. McKerrow, Y. Xu, D. C. Ladehoff and W. A. See, “Autocrine IL-6 Production by Human Transitional Carcinoma Cells Upregulates Expression of the alpha5beta1 Firbonectin Receptor,” The Journal of Urology, Vol. 163, No. 5, 2000, pp. 1553-1559. doi:10.1016/S0022-5347(05)67678-1
- W. Zhao, J. S. Schorey, M. Bong-Mastek, J. Ritchey, E. J. Brown and T. L. Ratliff, “Role of a Bacillus CalmetteGuerin Fibronectin Attachment Protein in BCG-Induced Antitumor Activity,” International Journal of Cancer, Vol. 86, No. 1, 2000, pp. 83-88. doi:10.1002/(SICI)1097-0215(20000401)86:1<83::AID-IJC13>3.0.CO;2-R
- A. Bohle, E. van der Sloot, E. Richter, J. Gerdes, W. G. Wood and J. Gerdes, “Binding to Fibronectin (FN)—A Prerequisite Step? Investigations on the Role of FN in Intravesical BCG Immunotherapy,” Investigative Urology, Vol. 5, 1994, pp. 100-104.
- R. O. Hynes and K. M. Yamada, “Fibronectins: Multifunctional Modular Glycoproteins,” The Journal of Cell Biology, Vol. 95, No. 2, 1982, pp. 369-377. doi:10.1083/jcb.95.2.369
- R. O. Hynes, A. T. Destree, M. E. Perkins and D. D. Wagner, “Cell Surface Fibronectin and Oncogenic Transformation,” Journal of Supramolecular Structure, Vol. 11, No. 1, 1979, pp. 95-104. doi:10.1002/jss.400110110
- H. S. Smith, J. L. Riggs and M. W. Mosesson, “Production of Fibronectin by Human Epithelial Cells in Culture,” Cancer Research, Vol. 39, No. 10, 1979, pp. 4138- 4144.
- N. Mutlu, L. Turkeri and K. Emerk, “Analytical and Clinical Evaluation of a New Urinary Tumor Marker: Bladder Tumor Fibronectin in Diagnosis and Follow-Up of Bladder Cancer,” Clinical Chemistry and Laboratory Medicine, Vol. 41, No. 8, 2003, pp. 1069-1074. doi:10.1515/CCLM.2003.165
- E. Ioachim, M. Michael, N. E. Stavropoulos, E. Kitsiou, M. Salmas and V. Malamou-Mitsi, “A Clinicopathological Study of the Expression of Extracellular Matrix Components in Urothelial Carcinoma,” BJU International, Vol. 95, No. 4, 2005, pp. 655-659. doi:10.1111/j.1464-410X.2005.05357.x
- T. Saito, Y. Tomita, T. Kawasaki, V. Bilim and K. Takahashi, “Subsequent Activation of Mitogen-Activated Protein Kinase after Adhesion of Transitional Cell Cancer Cells to Fibronectin,” Urologia Internationalis, Vol. 69, 2002, pp. 125-128. doi:10.1159/000065561
- A. Bohle, A. Jurczok, P. Ardelt, T. Wulf, A. J. Ulmer, D. Jocham, et al., “Inhibition of Bladder Carcinoma Cell Adhesion by Oligopeptide Combinations in Vitro and in Vivo,” The Journal of Urology, Vol. 167, No. 1, 2002, pp. 357-363. doi:10.1016/S0022-5347(05)65468-7
- V. Menendez, A. Fernandez-Suarez, J. A. Galan, M. Perez and F. Garcia-Lopez, “Diagnosis of Bladder Cancer by Analysis of Urinary Fibronectin,” Urology, Vol. 65, No. 2, 2005, pp. 284-289. doi:10.1016/j.urology.2004.09.028
- M. Laufer, I. Kaver, B. Sela and H. Matzkin, “Elevated Urinary Fibronectin Levels after Transurethral Resection of Bladder Tumour: A Possible Role in Patients Failing Therapy with Bacillus Calmette-Guerin,” BJU Internation, Vol. 84, No. 4, 1999, pp. 428-432. doi:10.1046/j.1464-410x.1999.00208.x
- R. E. Hurst, “Structure, Function, and pathology of Proteoglycans and Glycosaminoglycans in the Urinary Tract,” World Journal of Urology, Vol. 12, No. 1, 1994, pp. 3-10. doi:10.1007/BF00182044
- K. H. Kurth, “Glycosaminoglycans and Proteoglycans in the Urinary Tract,” World Journal of Urology, Vol. 12, No. 1, 1994, p. 2.
- C. L. Parsons, “The Role of the Urinary Epithelium in the Pathogenesis of Interstitial Cystitis/Prostatitis/Urethritis,” Urology, Vol. 69, No. 4, 2007, pp. 9-16. doi:10.1016/j.urology.2006.03.084
- J. N'Dow, N. Jordan, C. N. Robson, D. E. Neal and J. P. Pearson, “The Bladder Does Not Appear to Have a Dynamic Secreted Continuous Mucous Gel Layer,” The Journal of Urology, Vol. 173, No. 6, 2005, pp. 2025-2031. doi:10.1097/01.ju.0000158454.47299.ae
- C. L. Parsons, “A Model for the Function of Glycosaminoglycans in the Urinary Tract,” World Journal of Urology, Vol. 12, No. 1, 1994, pp. 38-42. doi:10.1007/BF00182049
- V. B. Lokeshwar, M. G. Selzer, W. H. Cerwinka, M. F. Gomez, R. R. Kester, D. E. Bejany, et al., “Urinary Uronate and Sulfated Glycosaminoglycan Levels: Markers for Interstitial Cystitis Severity,” The Journal of Urology, 2005, Vol. 174, No. 1, pp. 344-349. doi:10.1097/01.ju.0000161599.69942.2e
- C. L. Parsons, S. G. Mulholland and H. Anwar, “Antibacterial Activity of Bladder Surface Mucin Duplicated by Exogenous Glycosaminoglycan (Heparin),” Infection and Immunity, Vol. 24, No. 2, 1979, pp. 552-557.
NOTES
*Corresponding author.

