Natural Science
Vol.5 No.12A(2013), Article ID:41498,5 pages DOI:10.4236/ns.2013.512A003
Role of mycorrhiza to reduce heavy metal stress
![]()
1Department of Microbiology, University of Haripur, Haripur, Pakistan; *Corresponding Author: asma_baano@yahoo.com
2Medical Laboratory Technology Department, University of Haripur, Haripur, Pakistan
Copyright © 2013 Syeda Asma Bano, Darima Ashfaq. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2013 are reserved for SCIRP and the owner of the intellectual property Syeda Asma Bano, Darima Ashfaq. All Copyright © 2013 are guarded by law and by SCIRP as a guardian.
Received 9 October 2013; revised 9 November 2013; accepted 16 November 2013
Keywords: Mycorrhiza, Heavy Metal Stress; Phytoremediation; AM Fungi; Antioxidant Enzymes
ABSTRACT
Plants have a system of antioxidant enzymes, which helps to alleviate the effects of various types of stresses. Heavy metals like Cadmium and lead are tolerable for plants to certain extent. The antioxidant enzymes do not function properly at higher concentrations of Cadmium, lead and some other heavy metals. The activities of antioxidant enzymes are reduced due to reactive oxygen species produced as a result of heavy metal stress. The catalase activity was directly inhibited by O2− (Kono and Fridovich, 1982). These ROS are O2−, H2O2, and -OH which can react with many other biomolecules. Several metallic ions are produced by radical displacement reactions. These metallic ions inhibit the activity of antioxidant enzymes. Hence, enzymic antioxidant defense system of plants is affected and adversely inhibits plant growth and productivity. Mycorrhizal fungi are important in phytostabilization of toxic heavy metals. Plants having mycorrhizal association accumulate metallic pollutants by storing these heavy metals in Vesicles as well as in fungal hyphae in their roots, hence these metallic pollutants are immobilized and do not inhibit the growth and uptake of phosphorus and some other micronutrients. Mycorrhizal fungi also release various organic acids which increase the solubilisation of insoluble phosphate compounds present in soil. The unavailable forms of phosphorus are converted into available forms as a result of organic acids produced by fungi. AM fungi release glomalins that are certain metal sorble glycoproteins which increase the immobilization of toxic metals. Another protein is metallothionine released by certain AM fungi, which also reduces the heavy metal toxicity in soil. Mycorrhizal fungi also induce resistance in plants against pathogens, drought and salinity stress. Investigation on heavy metal stress resistant genes in mycorrhizal plants can be very helpful for phytoremediation. This review focuses on the use of AM fungi for phytoremediation.
1. INTRODUCTION
The high concentration of heavy metals adversely affects plant growth and development. Soil micro-organisms are also disturbed due to the presence of various pollutants in soil. Several physiological, biochemical and molecular processes are disturbed as a result of heavy metal stress in soil. Plant growth is inhibited and these heavy metals result in various defects like low seed germination, turgor loss, chlorosis, necrosis, senescence and ultimately plant death. Photosynthesis is also decreased as a result of heavy metal stress effects. The increased levels of antioxidant enzymes like Catalase, Superoxide dismutase and peroxidise were observed under heavy metal stress conditions [1]. Among heavy metals, Cadmium and lead cause the biological toxicity [2]. Heavy metals like Cadmium and lead affect several physiological and biochemical processes of plants [2-4]. There is an excessive accumulation of reactive oxygen species and methylglyoxyl due to which peroxidation of lipids, oxidation of proteins, inactivation of enzymes and DNA damage occur, sometimes DNA reacts with other cell constituents. Pesticides, fertilizers and metal contaminated sewage are major causes of heavy metal deposition in soil. Burning of fossil fuels has increased these heavy metals in soil. The concentration of Cd, Pb and Zn has increased rapidly in soil over the past two centuries [5]. Different plant species have evolved different mechanisms to tolerate heavy metal stress. These mechanisms involve inhibition of uptake and transport, immobilization, role of plasma membrane to expel these heavy metals, stress proteins induction, salicylic acid synthesis, nitric oxide and pro and polyamine synthesis etc. Some plants can accumulate a large portion of heavy metals in their cell wall. It was indicated that metallothionins and Ferritins protect plants against oxidative stress caused by excessive heavy metals in soil [6]. Certain Cadmium thiolate complexes are present in soil which is harmful for plants and microorganisms. These chelated metals accumulate in cell walls of plants. These may also be deposited in the vacuoles of plants [7,8]. There are also some reports of expression of genes in AM plants encoding proteins metallothionein, 90 kD heat shock protein, Glutathione-S-transferase in response to metallic stress. This indicates that proteins of these expressed genes may help in the immobilization of toxic heavy metals in plant rhizosphere.
Chemical, physical and biological methods are used to remove toxic metals from soil. Among biological methods, Mycorrhizal fungi can play a role in bioremediation of heavy metal pollution in soil [9]. Increased presymbiotic hyphal extension, Sporulation, and spore germination were observed in G. intraradices under high Cd and Zn concentrations [10]. AM fungi reduce heavy metal toxicity by metabolizing these metals. Metallothionines like polypeptides are known to cause Cd and Cu detoxification in AM fungal cells. Presymbiotic hyphal extension was also observed in an Al tolerant strain of Gigaspora gigantia [11]. Hyphal extension was also observed in Glomus species with moderately elevated concentration of Zn [12]. Mostly ectomycorrhizal and Ericoid mycorrhizal fungi enhance tolerance of host plants [13- 15]. Endomycorrhizal association is also involved in the bioaccumulation and immobilization of toxic heavy metals present in soil. Mycorrhiza is an association between fungi and the roots of higher plants. Fungus enters in plant roots and develops hyphae, arbuscules and vesicles. Transport of nutrients especially phosphorus occurs as a result of this association. Some of the heavy metals like Cadmium and lead interfere with many physiological and biochemical processes in plants like photosynthesis, respiration, nitrogen and protein metabolism etc. Similarly, Zn also affects plants when it is present in high concentration in soil. The effects of these toxic metals can be reduced by using suitable mycorrhizal fungi as an inoculum in the heavy metal contaminated areas. Successful Arbuscular Mycorrhizal association can be explained by finding fungal colonization (arbuscules, vesicles and hyphae) in plant roots (Figure 1). Plants inoculated with mycorrhizal fungi in a metal contaminated area are healthier as compared to uninoculated plants of metals polluted areas. Some mycorrhizal fungi are also affected
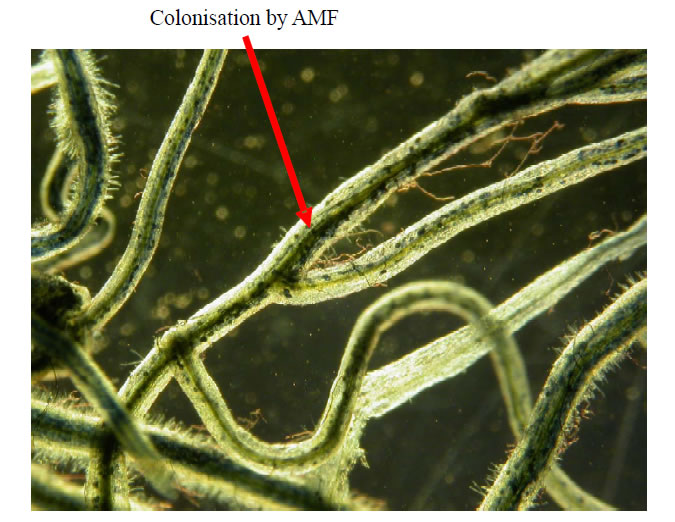
Figure 1. Fungal colonization in plant roots indicating arbuscularmycorrhizal association.
due to the presence of heavy metals in soil. Some reports indicate that the symbiotic mycelial expansion and sporulation are affected due to the presence of heavy metals in soil [10].
Some of the strains of Mycorrhizal fungi can tolerate heavy metal stress, among which, Glomus intraradices, Glomus mosseae and some other species of Glomus are important. Hence selection of heavy metal stress tolerant strains of mycorrhizal fungi is an important step to get healthy plants in metal polluted lands.
2. MYCORRHIZAL ASSOCIATION IN HEAVY METAL CONTAMINATED SOIL
AM fungal colonization was observed in highly contaminated soil [16,17]. The presence of Glomus mosseae was also reported in heavy metal contaminated site by some researchers [18]. The external mycelium of certain AM fungi produce a type of protein called Glycoprotein (Glomalin), which has heavy metal binding sites [19-22]. Heavy metals accumulate at these binding sites. The antioxidant level is also increased as a result of association of AM fungi with plants [2,23]. Various Glomus species are important in mycorrhizal association. Some fungal strains were isolated in the past which were resistant to heavy metal contamination. Many mycorrhizal fungi overcome the stress of heavy metal contamination [24, 25], which was shown by studies of different researchers [12,24,26]. Tolerance level of heavy metals varies among different fungal groups. Fungal colonization is reduced if less tolerant strains are participating in mycorrhizal association. The inhibition of mycorrhizal colonization was indicated as a result of the presence of Cu and Cd in soil [12,24,26,27]. This explains the blocking of certain physiological and biochemical processes in mycorrhizal fungi and plant. The mechanism of mycorrhizal signalling events for a mutual dialogue between plant and fungi can explain the enhancement or inhibition of fungal colonization due to the presence of different heavy metals. This mutual flow of information has been depicted by Figure 2.
Many plants produce root exudates containing strigolactones and flavonoids which attract mycorrhizal fungi. Fungi also produce lipochitooligosacharide signals which are perceived by plants. In some mycorrhizal associations this exchange of signals is not disturbed by the excessive presence of certain heavy metals like Zinc. This means that certain mycorrhizal fungi are tolerant to the high concentration of some metals eg. Zinc. Many heavy metals are immobilized due to the binding capacity of fungal hyphae to metals. The translocation of toxic heavy metals to plants is reduced due to this binding capacity [28,29]. Pine trees inoculated with Pisolithus tinctorius showed high tolerance to heavy metals. Zinc tolerant strains of Suillus bovinus develop resistance in Pinus sylvestris. The immobilization of some heavy metals due to mycorrhizal association may possibly be due to slight increase in pH in the mycorrhizosphere. Many heavy metals are immobilized due to this change in pH under conditions of high concentrations of certain metals. The beneficial effects of mycorrhiza against plant’s heavy metal uptake may be associated with heavy metal solubility caused by changes in soil pH.
Heavy metals are mostly accumulated in fungal hyphae as well as in arbuscules. A significant absorption of Zinc to the mycelium of arbuscular mycorrhizal fungi was observed by using several Glomus species in association with Clover or Ryegrass [30]. AM fungi release an insoluble Glycoprotein (Glomalin), which can extract Copper, Cadmium and Lead from polluted soil. So 1 g of Glomalin was able to extract 4.3 mg Copper, 0.08 mg Cadmium and 1.12 mg Lead from these metal contaminated soil [31,32]. Gohri and Paszwoski also indicated that toxic metallic compounds are stored in Fungal vesicles [32]. Kaldorf et al. (1999) indicated the immobilization of metals in maize which was inoculated with Glomus species. AM fungi which are present in metals contaminated areas are more tolerant as compared to those growing in clean areas [12,33].
3. MYCORRHIZAL DEVELOPMENT AND PLANT GROWTH
Several studies explain the role of mycorrhiza in the enhancement of plant growth and yield. Plants with mycorrhizal association are healthier as compared to the plants without this relationship. Many plant genes which are involved in mycorrhizal symbiosis contribute in the improvement of nutrient uptake especially phosphorus [34-36]. The mechanisms of increased absorption are both physical and chemical. Mycorrhizal mycelia provide a larger surface area for absorption of water and minerals, hence these can explore a greater volume of soil. The role of mycorrhizal fungi in the acquisition of mineral nutrients, especially phosphorus was explained previously by some Scientists [37]. Smith et al., 1986 demonstrated the increase in growth of Allium Cepa as a result of inoculation with mycorrhizal fungi [38]. Mycorrhizal fungi improve plant growth by enhancing the nutrient uptake under phosphorus limiting conditions.
4. CONCLUSION
Arbuscular mycorrhizal associations play an important role in protecting plants in heavy metal contaminated sites. Plants with mycorrhizal association, growing in heavy metal contaminated areas, are more tolerant as compared to the plants of clean areas. Fungal hyphae can approach the soil which is beyond the approach of plant roots, hence absorption of water and mineral nutrients is enhanced by increasing the exposed absorptive area. Healthy plants are more tolerable to different stress conditions as compared to unhealthy plants. Hence plants having mycorrhizal association show toxic metals accumulation, and immobilization of excessive metals such as copper, zinc, lead and Chromium etc. without disturbing the mobilization of useful micro and macro-nutrients, which can further help to improve the yield of plants.
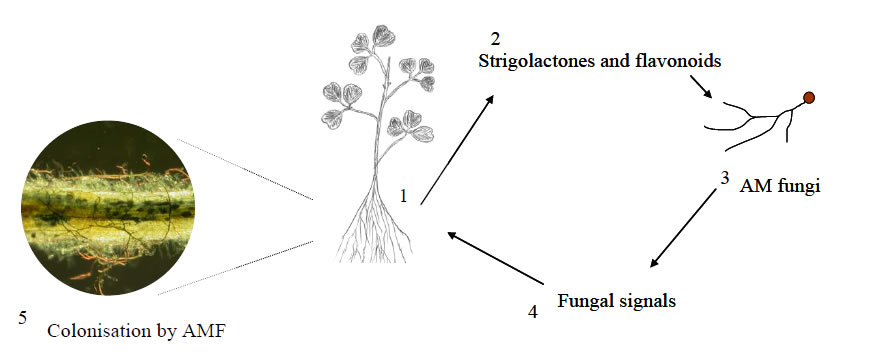
Figure 2. Signal exchange between Plant and mycorrhizal fungi (steps 1-4). Step 5 Mycorrhizal fungal development in plant roots. Step 5 Figure from Michael Schultze.
5. FUTURE PERSPECTIVES
Heavy metal stress tolerant genes from mycorrhizal plants should be transformed in less tolerable plants for better growth and productivity. Efficient transgenic plants can be produced to get agricultural benefits from heavy metal polluted areas. This will prove an important strategy in phytoremediation. Understanding the mechanism involved in uptake of toxic heavy metals and nutrients in plants will further open new areas of phytoremediation research. Identification of new stress resistant genes in mycorrhizal plants and fungi will also broaden the horizon of research related to bioremediation.
REFERENCES
- Garg, N. and Aggarwal, N. (2011) Effects of interactions between cadmium and lead on growth, nitrogen fixation, Phytochelatin, and Glutathione production in mycorrhizal Cajanus cajan L. Mill sp. Journal of Plant Growth Regulators, 30, 286-300. http://dx.doi.org/10.1007/s00344-010-9191-7
- Zhang, L.Z., Wei, N., Wu, Q.X. and Ping, M.L. (2007) Antioxidant response of Cucumis sativus L. to fungicide carbendazin. Pesticide Biochemistry and Physiology, 89, 54-59. http://dx.doi.org/10.1016/j.pestbp.2007.02.007
- Piotrowska, A., Bajguz, A., GodlewskaZylkiewwicz, B. and Zambrzycka, E. (2010) Changes in growth biochemical compounds and antioxidant activity in aquatic plant Wolffia arrhiza (Lemnaceae) exposed to Cadmium and Lead Arch Environment contaminant Toxicology, 58, 594- 604.
- Gupta, D.K., Nicolosa, F.T., Schetinger, M.R.C., Rossato, L.V., Pereira, L.B., Castro, G.Y., Srivastava, S. and Tripathi, R.D. (2009) Antioxidant defense mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. Journal of Hazard Matter, 172, 479-484.
- Candelone, J.P., Hong, S., Pellone, C. and Boutron, C.F. (1995) Post-industrial revolution changes in large-scale atmospheric pollution of the northern hemisphere by heavy metals as documented in central Greenungus Glomus mossaeland snow and ice. Journal of Geophysics Research, 100, 16605-16616. http://dx.doi.org/10.1029/95JD00989
- Fabisiak, J.P., Pearce, L.L., Borisenko, G.G., Tyhurina, Y.Y., Tyurin, V. A., Razzack, J., Lazo, J.S., Pitt, B.R. and Kagan, V.E. (1999) Bifunctional anti/proxidant potential of metallothionein redox signalling of copper binding and release. Antioxidant Redox Signal, 1, 349-364. http://dx.doi.org/10.1089/ars.1999.1.3-349
- Tommasini, R., Vogt, E., Fromenteau, M., Hoertensteiner, S., Matile, P., Amrhein, N. and Martinoia, E. (1998) An ABC transporter of Arabidopsis thaliana has both Glutathione-conjugate and chlorophyll catabolite transport activity. The Plant Journal, 13, 773-780. http://dx.doi.org/10.1046/j.1365-313X.1998.00076.x
- Rea, P. (1999) MRP subfamily ABC transporters from plants and yeasts. Journal of Experimental Botany, 50, 895-913.
- Leyval, C.K., Turnau, K. and Haselwandter, K. (1997) Effect of heavy metal pollution on mycorrhizal colonization and function: physiological, ecological and applied aspects. Mycorrhiza, 7, 3139-3153. http://dx.doi.org/10.1007/s005720050174
- Pawlowska, T.E., Douds, D.D. and Charvat, I. (1999) In vitro propagation and life cycle of the arbuscular mycorrhizal fungus Glomus etunicatum. Mycological Research, 103, 1549-1556. http://dx.doi.org/10.1017/S0953756299008801
- Bartolome-Esteban, H. and Schenck, N.C. (1994) Spore germination and hyphal growth of arbuscular mycorrhizal fungi in relation to soil aluminium saturation. Mycologia, 86, 217-226. http://dx.doi.org/10.2307/3760640
- Weissenhorn, I., Leyval, C. and Berthelin, J. (1993) Cdtolerant arbuscular mycorrhizal (AM) fungi from heavymetal polluted soils. Plant Soil, 157, 247-256. http://dx.doi.org/10.1007/BF00011053
- Bradley, R., Burt, A.J. and Read, D.J. (1981) Mycorrhizal infection and resistance to heavy metal toxicity in Calluna vulgaris. Nature (London), 292, 335-337. http://dx.doi.org/10.1038/292335a0
- Jones, M.D. and Hutchinson, T.C. (1988) Nickel toxicity in mycorrhizal birch seedlings infected with Lactarius rufus or Scleroderma flavidum Effects on growth, photosynthesis, respiration and transpiration. New Phytologist, 108, 451-459. http://dx.doi.org/10.1111/j.1469-8137.1988.tb04186.x
- Jones, M.D. and Hutchinson, T.C. (1988b) Nickel toxicity in mycorrhizal birch seedlings infected with Lactarius rufus or Scleroderma flavidum. 2. Uptake of nickel, calcium, magnesium, phosphorus and iron. New Phytologist, 108, 461-470. http://dx.doi.org/10.1111/j.1469-8137.1988.tb04187.x
- Pawlowska, T.E., Blaszkowski, J. and Ruhling, A. (1996) The mycorrhizal status of plants colonizing a calamine spoil mound in southern Poland. Mycorrhiza, 6, 499-505. http://dx.doi.org/10.1007/s005720050154
- Leung, H.M., Ye, Z.H. and Wong, M.H. (2007) Survival strategies of plants associated with arbuscular mycorrhizal fungi ontoxic mine tailings. Chemosphere, 66, 905- 915. http://dx.doi.org/10.1016/j.chemosphere.2006.06.037
- Debiane, D., Garcon, G., Verdin, A., Fontaine, J., Durand, R., Grandmougin-Ferjani, A., Shirali, P. and LouncesHadj Sahraui, A. (2008) In vitro evaluation of the oxidative stress and genotoxic potenitials of anthracene on mycorrhizal Chicory roots. Environmental and Experimental Botany, 64, 120-127. http://dx.doi.org/10.1016/j.envexpbot.2008.04.003
- Vivas, A., Azcon, R., Biro, B., Barea, J. M. and RuizLozano, J.M. (2003) Influence of bacterial strains isolated from lead polluted soil and their interactions with arbuscular mycorrhizae on the growth of Trifolium pertense L. under lead toxicity. Canadian Journal of Microbiology, 49, 577-588. http://dx.doi.org/10.1139/w03-073
- Agely, A.A., Sylvia, D.M. and Ma, L.Q. (2005) Mycorrhizae increases Arsenic uptake by the hyper accumulator Chinese Brake fern (Pteris vittae L.). Journal of Environmental Quality, 34, 2181-2186. http://dx.doi.org/10.2134/jeq2004.0411
- Citterio, S., Prato, N., Fumagalli, P., Aina, R., Massa, N., Santagostino, A., Sgorbati, S. and Berta, G. (2005) The arbuscular mycorrhizal fungus Glomus mossaeae induces growth accumulation changes in Cannabis sativa L. Chemosphere, 59, 21-29. http://dx.doi.org/10.1016/j.chemosphere.2004.10.009
- Trotta, A., Falaschi, P., Cornara, L., Minganti, V., Fusconi, A., Drava, G. and Berta, G. (2006) Arbuscular mycorrhize increase the Arsenic translocation factor in the As hyperaccumulating fern Pteris vittate L. Chemosphere, 65, 74- 81. http://dx.doi.org/10.1016/j.chemosphere.2006.02.048
- Gopi, R., Jaleel, C.A., Sairam, R., Lakshamanam, G.M.A., Gomathinayagam, M. and Paneerselvam, R. (2007) Differential effects of hexaconazole and paclobutrazol on biomass, electrolyte leakage, lipid peroxidation and antioxidant potential of Daucus carota L. Colloids and Surfaces B Biointerfaces, 60, 180-186. http://dx.doi.org/10.1016/j.colsurfb.2007.06.003
- Hildebrandt, U., Regvar, M. and Bothe, H. (2007) Arbscular mycorrhiza and heavy metal tolerance. Phytochemistry, 68, 139-146. http://dx.doi.org/10.1016/j.phytochem.2006.09.023
- Joschim, H.J., Makoi, R. and Ndakidemi, P.A. (2009) The agronomic potential of vesicular-arbuscular mycorrhiza (AM) in cereals-legume mixtures in Africa. African Journal of Microbiological Research, 11, 664-675.
- Kaldorf, M., Kuhn, A.J., Schroder, W.H., Hildebrandt, U. and Bothe, H. (1999) Selective element deposits in maize colonized by a heavy metal tolerance conferring arbuscular mycorrhizalfungus. Journal of Plant Physiology, 154, 718-728. http://dx.doi.org/10.1016/S0176-1617(99)80250-8
- Griffioen, W.A.J. and Ernst, W.H.O. (1989) The role of VA mycorrhiza in the heavy metal tolearance of Agrostis capillaries L. Agriculture Ecosystem Environment, 29, 173-177. http://dx.doi.org/10.1016/0167-8809(90)90272-F
- Brown, M.T. and Wilkins, D.A. (1985) Zinc tolerance of mycorrhizal Betula. New Phytologist, 99, 101-106. http://dx.doi.org/10.1111/j.1469-8137.1985.tb03640.x
- Wasserman, J.L., Mineo, L., Majumdar, S.K. and Vantyne, C. (1987) Detection of heavy metals in oak mycorrhizae of north eastern Pennsylvania forests, using X-ray microanalysis. Canadian Journal of Botany, 65, 2622-2627. http://dx.doi.org/10.1139/b87-353
- Joner, E.J., Briones, R. and Leyval, C. (2000) Metal-binding capacity of arbuscular mycorrhizal mycelium. Plant and Soil, 226, 227-234. http://dx.doi.org/10.1023/A:1026565701391
- Gonzalez-Chavez, M.C., Carillo-Gonzalez, R., Wright, S.F. and Nicholas, K.A. (2004) The role of Glomalin, a protein produced by arbuscular mycorrhizal fungi in sequestering potentially toxic elements. Environmental Pollution, 130, 317-323. http://dx.doi.org/10.1016/j.envpol.2004.01.004
- Gohre, V. and Paszkowski, U. (2006) Contribution of arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta, 223, 1115-1122. http://dx.doi.org/10.1007/s00425-006-0225-0
- Malcova, R., Vosatka, M. and Gryndler, M. (2003) Effects of inoculation with Glomus intraradices on lead uptake by Zea mays L. and Agrostis capillaris L. Applied Soil Ecology, 23, 255-267. http://dx.doi.org/10.1016/S0929-1393(02)00160-9
- Baylis, G.T.S. (1959) Effect of vesicular-arbuscular mycorrhizas on growth of Griselinia littoralis (Cornaceae). New Phytologist, 58, 274-280. http://dx.doi.org/10.1111/j.1469-8137.1959.tb05358.x
- Daft, M.J. and Nicolson, T.H. (1966) Effect of Endogone mycorrhiza on plant growth. New Phytologist, 65, 343- 350. http://dx.doi.org/10.1111/j.1469-8137.1966.tb06370.x
- Gerdemann, J.W. (1964) The effect of mycorrhizas on the growth of maize. Mycologia, 56, 342-349. http://dx.doi.org/10.2307/3756675
- Smith, S.E. and Read, D.J. (2008) Mycorrhizal symbiosis. Academic Press, London.
- Smith, S.E. and Read, D.J. (1997) Mycorrhizal symbiosis. 2nd Edition, Academic Press, San Diego.

