Health
Vol.08 No.02(2016), Article ID:63177,12 pages
10.4236/health.2016.82017
The Effect of Circuit Training on Resting Heart Rate Variability, Cardiovascular Disease Risk Factors and Physical Fitness in Healthy Untrained Adults
Adamos Vrachimis1,2, Marios Hadjicharalambous2*, Chris Tyler1
1School of Human & Life Sciences, Roehampton University, London, UK
2Department of Life & Health Sciences, University of Nicosia, Nicosia, Cyprus

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution-NonCommercial International License (CC BY-NC).
http://creativecommons.org/licenses/by-nc/4.0/



Received 24 November 2015; accepted 25 January 2016; published 28 January 2016
ABSTRACT
The purpose of the present study was to examine the effect of circuit training (CT) on resting heart rate variability (HRV) and other cardiovascular disease (CVD) risk factors such as blood lipids and blood glucose and on fitness components. Twenty-four healthy untrained adults (age 26.5 ± 5.1 years; height 1.67 ± 8.4 m; weight 66.8 ± 15.1 kg; 26.3% ± 5.2%; maximum oxygen uptake (VO2max) 48.5 ± 10.0 ml∙kg−1∙min−1) were assigned to either CT (n = 12) involving bodyweight exercises, or control (CON, n = 12) groups. Prior to the start and following the end of the six-week training period, time-, frequency-domain and nonlinear measures of resting HRV, arterial blood pressure, body composition, fasting blood lipids, lipoproteins and glucose, VO2max, upper body muscular endurance (UBME) and abdominal and hip flexor (AHFME), back strength (BS) and handgrip were assessed. None of the resting HRV measures (P > 0.05) were affected by the CT intervention. However, diastolic blood pressure decreased (P = 0.03), lean body weight (P = 0.03) increased, VO2max (P = 0.03), UBME (P < 0.001), AHFME (P = 0.04), and BS (P = 0.03) were significantly higher following CT, whereas the other variables were not influenced by the CT. Six-week of CT involving bodyweight exercises has no significant impact on resting HRV. However, this type of training might decrease the risk for development of CVD by reducing arterial blood pressure and by improving body composition, aerobic capacity, muscular endurance and strength.
Keywords:
Heart Rate Variability, Circuit Training, Healthy Untrained Adults

1. Introduction
According to the World Health Organization (WHO), more people die annually from cardiovascular diseases (CVD) than from any other cause [1] . In particular, an estimated 17.3 million people died from CVD in 2008, representing 30% of all global deaths. Of these 17.3 million deaths, an estimated 7.3 million were due to coronary heart disease and 6.2 million were due to stroke. Almost 23.6 million people are expected to die from cardiovascular diseases in 2030, mainly due to heart disease and stroke [1] . One non-invasive clinical predictor of CVD morbidity and mortality is heart rate variability (HRV) [2] -[4] . HRV is the variation in time between beats [5] and, in particular, the variation in time of the R-R intervals, which is the distance between two R peaks on the QRS complex of an electrocardiography (ECG) wave. Originally, HRV was analyzed by the use of linear methods (time- and frequency-domain) but these means resulted in loss of information on the dynamic patterns used by cardiovascular regulation systems to adjust heart rate (HR) and blood pressure [6] . Nonlinear methods, which were developed recently, may provide additional information on cardiovascular autonomic regulation [6] . Loss of HRV has been associated with increased risk of new cardiac events (angina pectoris, myocardial infarction, or congestive heart failure) [7] , coronary heart disease [8] and mortality of all causes [9] . Concomitantly, increased HRV is linked to improved prognosis and lower CVD mortality [7] . This suggests that HRV could be a valuable tool in predicting future cardiac events, and that any type of exercise intervention that is proven to improve HRV might reduce the risk of such events.
Several studies have reported the positive effect of aerobic exercise training on resting HRV measures. De Meersman [10] found that high-intensity aerobic training increases parasympathetic tone at rest in young athletes. Levy et al. [11] reported the same finding after intensive aerobic training in both healthy older and young men. Whereas, Melanson and Freedson [12] found that moderate-to-vigorous-intensity endurance training induces increases in most time- and frequency-domain measures of HRV in adult males.
Concerning strength training and HRV, studies have produced conflicting results. Carter et al. [13] reported that whole body resistance training does not cause a significant change in sympathetic tone in young subjects. In addition, Van Hoof et al. [14] and Cooke and Carter [15] found that strength training does not affect neural con- trol of neither HR nor blood pressure, and vagal-cardiac control or cardiovagal baroreflex sensitivity, respec- tively. However, Heffernan et al. [16] reported a positive effect in nonlinear dynamics of HR complexity apart from a non-significant effect in spectral measures of HRV after six weeks of resistance training. Tatro et al. [17] found that lower body resistance training can cause a chronic increase in sensitivity and resetting of carotid- cardiac baroreflex in healthy males. Taylor et al. [18] also, reported a hypotensive response and a simultaneous increase in vagal modulation in older adults with hypertension as a result of isometric handgrip training at moderate intensity. To the best of our knowledge, studies investigating the effect of circuit (CT) on all three measures of resting HRV (time-, frequency-domain and nonlinear) do not exist.
CT appears to have multiple benefits on health and fitness, as various studies have shown that it may elicit significant increases in aerobic capacity muscular strength, muscular endurance, lean body weight, and signi- ficant decreases in resting diastolic blood pressure and body fat [19] - [26] . The effect of CT on some other CVD risk factors such as fasting blood glucose, and blood lipids and lipoproteins remain under-investigated.
Since CT has been associated with increases in aerobic capacity and as aerobic training has been shown to increase resting HRV measures, we hypothesized that CT may increase resting HRV measures in healthy un- trained adults aged 18 - 35 years old. In addition, given that CT has been shown to improve various CVD risk factors and fitness components, we tested another hypothesis, that CT improves some other CVD risk factors and fitness components, not yet investigated. The purpose therefore of the present study was to examine the effect of six weeks of CT on a) resting HRV measures, b) blood metabolites and c) fitness components.
2. Materials and Methods
2.1. Subjects
Based on Cohen’s standard effect size for a “large effect” of 0.8 [27] , we estimated a sample size of 24 subjects required to test our hypothesis (1 − β = 0.95, a = 0.05). Subjects were recruited following the distribution of a relevant advertisement leaflets at the University of Nicosia and randomly at two big private health clubs in the city of Nicosia, Cyprus. The subjects voluntarily accepted to participate in the present study. All participants gave their written informed consent to take part in the study, which was approved by the Roehampton University ethics committee. The CT group [n = 12 (10 female, 2 male); age 23.3 ± 3.2 years; height 1.67 ± 8.4 m; weight 67.6 ± 16.7 kg; body fat 27.6% ± 5.1%; maximum oxygen uptake (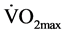 ) 45.1 ± 7.71 ml∙kg−1∙min−1] and the control (CON) group [n = 12 (6 female, 6 male); age 29.8 ± 4.5 years; height 1.68 ± 8.7 m; weight 66.1 ± 14.1 kg; body fat 25.0% ± 5.3%;
) 45.1 ± 7.71 ml∙kg−1∙min−1] and the control (CON) group [n = 12 (6 female, 6 male); age 29.8 ± 4.5 years; height 1.68 ± 8.7 m; weight 66.1 ± 14.1 kg; body fat 25.0% ± 5.3%; 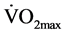 51.7 ± 11.1 ml∙kg−1∙min−1] comprised male and female untrained healthy subjects. They were classified as untrained if they had no background in regular endurance or resistance training or competitive sports for the last six months. Thirty-seven (n = 37) subjects initially participated in the study. However, twelve of them withdrew. Ten withdrew from the CT group; two withdrew right after the pre- training measurements and the other eight withdrew during the training phase. Subjects within the CT group had to attend at least 16 out of 18 sessions in total (90%) in order to include their data in the analysis. One of the thirteen subjects did not complete the minimum number of sessions so that set of data was excluded from the analysis. The remaining two subjects withdrew from the CON group after the first set of measurements. All subjects completed a health history questionnaire in order to confirm their healthy status and the fact that they were normotensive and were not taking any medication, which would alter cardiovascular control [22] .
51.7 ± 11.1 ml∙kg−1∙min−1] comprised male and female untrained healthy subjects. They were classified as untrained if they had no background in regular endurance or resistance training or competitive sports for the last six months. Thirty-seven (n = 37) subjects initially participated in the study. However, twelve of them withdrew. Ten withdrew from the CT group; two withdrew right after the pre- training measurements and the other eight withdrew during the training phase. Subjects within the CT group had to attend at least 16 out of 18 sessions in total (90%) in order to include their data in the analysis. One of the thirteen subjects did not complete the minimum number of sessions so that set of data was excluded from the analysis. The remaining two subjects withdrew from the CON group after the first set of measurements. All subjects completed a health history questionnaire in order to confirm their healthy status and the fact that they were normotensive and were not taking any medication, which would alter cardiovascular control [22] .
2.2. Circuit Training
An induction session took place just before the start of the six-week intervention training, in order to familiarize subjects with the testing equipment and the correct technique of all the exercises. Both the induction and the training sessions were undertaken by a qualified instructor. The subjects were able to train on their own, whenever they could not attend the scheduled supervised sessions by the instructor. Based on previous studies [19] - [26] , CT involved a six-week training program, according to which subjects had to train three times per week. In weeks 1 and 2, 3 and 4, and 5 and 6 they had to complete 1, 2, and 3 circuits per session respectively [20] [22] . In weeks 1, 3 and 5 and weeks 2, 4 and 6 the objective was to complete 15 and 20 repetitions respectively for each exercise [20] [22] . The subjects performed both the concentric and eccentric contraction phase of each exercise in 1 second [21] . Rest between exercises (stations) was the minimum time required for subjects to move from one station to another (<15 seconds), and rest between circuits for weeks 3 - 6 was three minutes of active recovery [21] - [24] .
The subjects were using only their body weight to perform the exercises [26] . The training protocol consisted of the following exercises:
1. Squats;
2. Static lunges;
3. Shoulder bridge;
4. Standing single leg calf raises;
5. Push-ups;
6. Incline bench push-ups;
7. Tricep dips;
8. Crunches;
9. Side crunches;
10. Back raises;
11. Step-ups;
12. Side-to-side jumps over skipping rope.
Female subjects performed push-ups and bench push-ups on their knees, whereas male subjects had to perform these exercises on their toes [28] [29] . If subjects could not complete 15 or 20 repetitions continuously they could rest for a few seconds and then complete the remaining repetitions [28] [29] . During the six-weeks training period, subjects were instructed to refrain from any other type of exercise; all participants included into the statistical analysis comply with this particular instruction.
2.3. Data Collection and Processing
The whole study period lasted for 16 months. During the data collection process, body composition and fitness assessment always followed HRV and blood pressure measurements. Blood samples were collected on a separate day of the same week. The first set of data was collected during the week right before the training commenced and the second set of data was collected during the week right after the end of the training period. All measurements were taken by one investigator to ensure consistency of measurement. A Hosand HR Monitor MC030 (Hosand Technologies S.r.l., Verbania, Italy), was used to record resting HRV data with the subject sitting quietly for 5 minutes. The HR was detected by using two single-use adhesive electrodes, which were applied directly on skin, just below the pectoral muscles and fastened to the HR monitor with snaps. Prior to data collection, subjects rested comfortably in a seated position for 10 minutes.
All measurements were taken in the morning, between the hours of 7 a.m. and 10 a.m., in order to minimize diurnal effects. Subjects were overnight fasted and refrained from any excessive activity and from any caffeine consumption in the morning, prior to reporting to the laboratory. Recordings were analysed using a Hosand MC Software version 1.1.0.25 (Hosand Technologies, Verbania, Italy). Time-, frequency-domain and nonlinear analyses were used to assess HRV in this study. The time-domain measures recorded were: mean HR, standard deviation of R-R intervals (SDRR), number of adjacent R-R intervals more than 50 milliseconds (ms) different (NN50) and proportion of adjacent R-R intervals more than 50ms different (pNN50). The frequency-domain measures, which were determined by spectral analysis using fast Fourier transform, were: low frequency power (LF, 0.04 - 0.15 Hz), high frequency power (HF, 0.15 - 0.4 Hz) and low frequency power to high frequency power ratio (LF/HF). Finally, nonlinear measures were analyzed by using the Poincare plot. The measures recorded were: standard deviation calculated on the vertical axis of the Poincare plot (SD1), standard deviation calculated on the horizontal axis of the Poincare plot (SD2) and the ratio of SD1 to SD2 (SD1/SD2).
An Omron M6 Digital Automatic Blood Pressure Monitor (Omron Healthcare, Kyoto, Japan) was used to measure systolic and diastolic blood pressure. The Omron M6 device has been deemed to be in accordance with the International Protocol criteria and has been recommended for use by adults [30] . Measurements were always taken from the left upper arm in a seated position.
Body weight was measured using a digital weighing scale accurate to the nearest 0.1 kg and height was measured using a measuring pole to the nearest 0.1 cm. Percent body fat was assessed by measuring skinfolds to the nearest 0.5 mm, at four sites of the body using a Harpenden Skinfold Caliper (Baty International, West Sussex, U.K.). The method of skinfolds is considered to be a reasonably accurate tool for measuring subcutaneous fat [31] [32] . A minimum of two measurements was taken at each site (biceps, triceps, subscapular, suprailiac). However, if the difference between the two values was greater than 1 mm, the test was repeated and the two values closer to each other were recorded. The final value recorded was the average of the two values. The body fat percentage was calculated using the following equations:
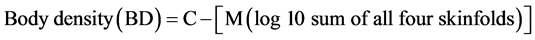 [31] (1)
[31] (1)
The result of the above formula (BD) was used in the Siri equation to calculate body fat percentage.
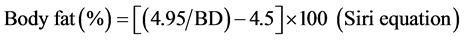 [31] (2)
[31] (2)
Lean body weight was calculated by subtracting fat weight from total body weight.
An estimated value of 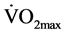 was determined after conducting the Queens College step test. Subjects had to step up and down on a step 16.25-inch high for 3 minutes at a steady pace of 24 steps per minute for male subjects and 22 steps per minute for female subjects. A Wittner 812 K Metronome (Wittner GmbH, Isny, Germany) was used to indicate the appropriate pace and a Polar RS400 HR Monitor (Polar Electro Oy, HQ, Kempele, Finland) was used to record the HR at the end of the 3-minute test. The Queen’s College step test correlation between recovery HR and
was determined after conducting the Queens College step test. Subjects had to step up and down on a step 16.25-inch high for 3 minutes at a steady pace of 24 steps per minute for male subjects and 22 steps per minute for female subjects. A Wittner 812 K Metronome (Wittner GmbH, Isny, Germany) was used to indicate the appropriate pace and a Polar RS400 HR Monitor (Polar Electro Oy, HQ, Kempele, Finland) was used to record the HR at the end of the 3-minute test. The Queen’s College step test correlation between recovery HR and 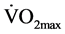 is r = −0.75, and test retest reliability for recovery HR is r = 0.92 [33] .
is r = −0.75, and test retest reliability for recovery HR is r = 0.92 [33] .
A Takei TKK 5402 Digital Back Dynamometer (Takei Scientific Instruments, Tokyo, Japan) was used to measure back strength to the nearest 0.1 kg. Each subject had two attempts and the maximum value was recorded [34] . Handgrip strength was measured to the nearest 0.1 kg by using a Takei TKK 5401 Digital Handgrip Dynamometer (Takei Scientific Instruments, Tokyo, Japan). Measurements were performed twice each with the left and right hands alternately and the mean value of the highest values of the forces of both hands was recorded [34] .
The YMCA Push-up Test was conducted to assess upper body (e.g. arm, shoulder muscular endurance. Male subjects performed the test on their toes, female subjects performed the test on their knees but both kept their hips and back straight [28] [29] .
Abdominal and hip flexor muscular endurance was assessed by using the National Coaching Foundation (NCF) Abdominal Curl Conditioning Test (Coachwise Ltd., Leeds, UK), which is a progressive sit up test. It has been reported that sit-up tests in general have high reliability when measuring abdominal and hip flexor muscular endurance [29] . Subjects were required to perform as many sit ups as possible, keeping in time to the beeps emitted from a NCF Abdominal Curl Conditioning Test audio CD. The total number of sit ups completed correctly and the time from the start of the test until the subject could no longer keep in time with the beeps or when the sit ups were not performed correctly was recorded. Subjects were encouraged during the fitness tests for maximum effort.
Venous blood samples (4 ml) were collected in the morning between 7.00 am and 8.30 am after a 14-hour overnight fast. Following centrifugation, blood samples were analyzed for total cholesterol, HDL, LDL, triglycerides and glucose using an Olympus AU2700 Chemistry Analyzer (Beckman Coulter, Brea, CA, USA) [35] .
2.4. Statistical Analysis
A separate repeated measures ANOVA was used to assess the effect of CT on each of the resting HRV measures and the rest of the variables. The statistical significance was accepted at 5% (P < 0.05). Data were expressed as means ± SD. All data were analyzed using the SPSS Statistical Software Package version 17.0 (SPSS, Inc., Chicago, IL, USA).
3. Results
Several HRV parameters were assessed and their P values are reported separately below and/or in Table 1.
HR (F1,21 = 2.0, P = 0.17), SDRR (F1,21 = 2.1, P = 0.17), NN50 (F1,21 = 0.8, P = 0.38), PNN50 (F1,21 = 0.3, P = 0.59), VLF (F1,2 = 2.8, P = 0.11), LF (F1,21 = 0.03, P = 0.87), HF (F1,21 = 0.7, P = 0.40), LFnu (normalized units) (F1,21 = 0.8, P = 0.39), HFnu (F1,21 = 0.8, P = 0.39), LF/HF (F1,21 = 1.7, P = 0.20), SD1 (F1,21 = 0.7, P = 0.40), SD2 (F1,21 = 2.2, P = 0.16) and the ratio SD1/SD2 (F1,21 = 0.002, P = 0.97) were not affected by training (Table 1). There was a significant group effect in SDRR (F1,21 = 7.0, P = 0.02), LF (F1,21 = 8.4, P = 0.01), SD2 (F1,21 = 7.6, P = 0.01), while there was no significant time effect in any of the measures (P ³ 0.05; Table 1).
Table 1. Means (±SD) for HRV measures.
*Significantly different (P <
Diastolic blood pressure (F1,22 = 5.5, P = 0.03) and lean body weight (F1,22 = 5.2, P = 0.03) were significantly improved in the CT group compared to the CON group, whereas the rest of the variables did not change after training (Table 2). In addition, there was a significant time effect in body fat (F1,22 = 4.7, P = 0.04) but there was no significant group effect in any of the variables (P ³ 0.05).
None of the fasting blood variables changed significantly in the CT group after training, compared to the CON group (P ³ 0.05; Table 3). However, triglycerides (F1,22 = 8.3, P = 0.01), and LDL (F1,19 = 5.4, P = 0.03) were significantly different between groups, while the variables between pre- and post-training measurements did not change (Table 3).
There was a main interaction (time × group) effect in 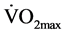 (F1,22 = 5.2, P = 0.03), UBME (F1,22 = 101.3, P < 0.001), AHFME (F1,21 = 5.0, P = 0.04), BS (F1,22 = 5.5, P = 0.03) which all increased significantly with training. However, HS (F1,22 = 0.2, P = 0.65) was not different following the training period (Table 4). UBME significantly also increased over-time comparing between pre- and post-training period in the CT group, (F1,22 = 80.5, P < 0.001). There was no significant group effect in any of the components.
(F1,22 = 5.2, P = 0.03), UBME (F1,22 = 101.3, P < 0.001), AHFME (F1,21 = 5.0, P = 0.04), BS (F1,22 = 5.5, P = 0.03) which all increased significantly with training. However, HS (F1,22 = 0.2, P = 0.65) was not different following the training period (Table 4). UBME significantly also increased over-time comparing between pre- and post-training period in the CT group, (F1,22 = 80.5, P < 0.001). There was no significant group effect in any of the components.
4. Discussion
The primary finding of the present study was that CT, involving bodyweight exercises, significantly reduced diastolic blood pressure, increased lean body weight, aerobic capacity, and upper body, abdominal and hip flexor muscular endurance and back strength. However, CT did not influence resting HRV.
4.1. HRV
To our knowledge, this is the first study to investigate the effect of CT on all resting HRV measures: time-, frequency-domain and nonlinear measures. The non-significant effect on resting HRV is consistent with the find-
Table 2. Means (±SD) for body weight (BW), body fat % (BF%), lean body weight (LBW), systolic blood pressure (SBP) and diastolic BP (DBP).
*Significantly different (P < 0.05). Pre, pre-training; Post, post-training.
Table 3. Means (±SD) for fasting blood triglycerides (TGL), cholesterol (CHL), high density lipoprotein (HDL), low density lipoprotein (LDL) and glucose.
*Significantly different (P < 0.05). Pre, pre-training; Post, post-training.
Table 4. Means (±SD) for maximum oxygen uptake ( , ml∙kg−1∙min−1), upper body (UBME) and abdominal and hip flexor muscular endurance (AHFME), back strength (BS) and handgrip strength (HS).
, ml∙kg−1∙min−1), upper body (UBME) and abdominal and hip flexor muscular endurance (AHFME), back strength (BS) and handgrip strength (HS).
*Significantly different (P < 0.05). Pre, pre-training; Post, post-training. UBME increased over-time comparing between pre- and post-training period in the CT group.
ings reported by previous studies [13] - [16] , with reference to the effect of strength training on the spectral measures of HRV. However, Heffernan et al. [16] also found that the intervention increased HR complexity―a nonlinear method of assessing HR, not used in the present study―and the researchers postulated that this could be because of increased parasympathetic and/or reduced sympathetic cardiac autonomic control. Previous studies have reported that greater reductions in overall HRV and greater elevations in HR after a single bout of acute resistance exercise versus acute endurance exercise may be attributed to greater reductions in cardiac parasympathetic tone [36] . In addition, a reduction in HF spectral power of HRV after an acute resistance exercise bout was found to be related to reduce HR complexity [37] . Despite these findings, Heffernan et al. [16] postulated that these potentially negative acute responses do not appear to transpose to a state of permanence and could lead to positive adaptations after repeated exposure. In addition, Tatro et al. [17] reported a chronic increase in sensitivity and resetting of carotid-cardiac baroreflex in healthy males (32 ± 3 years old) as a result of lower body resistance training. Specifically, the researchers observed an increase in resting HRV that paralleled the increased responsiveness of the vagally mediated carotid-cardiac baroreflex. However, Tatro et al. [17] could not provide a clear interpretation for the increase in parasympathetic cardiac control since they found no significant change in baseline R-R interval (resting HR).
4.2. Arterial Blood Pressure
The most important finding of this study was the decrease in resting diastolic blood pressure following CT training period. These results are in agreement with several previous studies [19] [23] [38] which reported a significant decline in resting diastolic blood pressure but no change in resting systolic blood pressure, as a result of CT intervention. In Hurley et al. [38] study, the resistive training program used was classified as high intensity, however it could be considered as a CT program due to the short rest intervals (<15 seconds) between each exercise. Our findings are strongly supported by the meta-analysis study by Fagard [39] . In his review, Fagard [39] mentions that aerobic power increased by 10.5% in six resistance training study groups in which it was measured, which suggests that the types of resistance exercise used in most protocols comprised an aerobic component to some extent. The circuit format of resistance training used in our study with minimal rest between stations (<15 seconds) could be considered as an aerobic component, which is further supported by the training- induced significant increase in 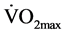 (P = 0.03). It is important to note that Carter et al. [13] and Ray and Carrasco [40] reported that the reduction in both systolic and diastolic was not coupled to resistance and isometric handgrip respectively, exercise-induced decreases of sympathetic neural activity. This finding is consistent with the current results, given that there was not a significant change in low frequency power.
(P = 0.03). It is important to note that Carter et al. [13] and Ray and Carrasco [40] reported that the reduction in both systolic and diastolic was not coupled to resistance and isometric handgrip respectively, exercise-induced decreases of sympathetic neural activity. This finding is consistent with the current results, given that there was not a significant change in low frequency power.
Contrary to the above results, the study by Reid et al. [41] showed no effect of resistance training on resting arterial blood pressure. In this study the rest period between stations in all groups was approximately 10 seconds, which was almost similar to the rest period used in our intervention. Moreover, the researchers observed a significant increase in 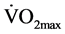 in two of the groups (endurance and strength 2), something also found in the CT group of out study. Although, there was no significant change in resting arterial blood pressure after the training, there was a trend towards an increase in diastolic blood pressure in all four groups. Reid et al. [41] speculated that this was due to the continual use of the Valsavamanoeuvre (expiring against a closed glottis results in an increase in intrathoracic pressure which causes an increase in both systolic and diastolic pressure) which was often observed in subjects of all groups despite admonition. In the current study, subjects were instructed in advance to breathe properly and it was made certain throughout the sessions that they were not using the Valsavamanoeuvre.
in two of the groups (endurance and strength 2), something also found in the CT group of out study. Although, there was no significant change in resting arterial blood pressure after the training, there was a trend towards an increase in diastolic blood pressure in all four groups. Reid et al. [41] speculated that this was due to the continual use of the Valsavamanoeuvre (expiring against a closed glottis results in an increase in intrathoracic pressure which causes an increase in both systolic and diastolic pressure) which was often observed in subjects of all groups despite admonition. In the current study, subjects were instructed in advance to breathe properly and it was made certain throughout the sessions that they were not using the Valsavamanoeuvre.
Aerobic endurance training decreases arterial blood pressure through a reduction of systemic vascular resistance, in which the sympathetic nervous system and the renin-angiotensin system appear to be involved [39] . According to the review by Fagard [39] some studies have addressed the underlying mechanisms responsible for the decrease in blood pressure in response to resistance training, but failed to bring these mechanisms to light. In our study there was no significant change in high and low frequency power, thus no change in cardiac autonomic activity. More research is needed to address the blood pressure-lowering mechanism of resistance training.
4.3. Body Composition
The significant increase in lean body weight and the no change in body fat observed in the current study are in agreement with several previous reports [19] [20] [23] [24] [37] [41] respectively. However, Wilmore et al. [19] and Gettman et al. [20] [22] reported significant reductions in body fat as a result of circuit weight training. Pollock [42] has suggested that exercise programs of duration less than 8 to 10 weeks cause insignificant changes in body composition. The training period in the studies by Wilmore et al. [19] and Gettmanet al. [20] [22] was 10, 12 and 20 weeks respectively. However, in the case of Harris & Holly [23] , Harber et al. [24] , Hurley et al. [38] , Reid et al. [41] and the current study the corresponding duration of the training period was 9, 10, 16, 8 and 6 weeks respectively. In the studies by Harber et al. [24] and Hurley et al. [38] , there would be a significant decrease in body fat since the training period was 10 and 16 weeks respectively. However, the subjects in the study by Harber et al. [24] performed on average two sets for each exercise over the 10 weeks of the program and the subjects in the study by Hurley et al. [38] performed only one set per exercise over the 16-week training period. In contrast, the subjects in the studies by Wilmore et al. [19] and Gettman et al. [20] performed 3 circuits and in the study by Gettman et al. [22] even though the subjects performed 2 circuits, the duration of this study was the longest of all (20 weeks). Consequently, the lower training volume of the studies by Harber et al. [24] and Hurley et al. [38] compared to the studies by Wilmore et al. [19] and Gettman et al. [20] [22] may explain why body fat did not change significantly in those studies.
The non-significant decrease in body fat observed in the current study may be partially explained by the relatively small amounts of total energy expended in exercise over these shorter training periods (<8 - 10 weeks). Wilmore et al. [43] estimated a caloric expenditure of 9.0 kcal×min−1 during a 10-station circuit weight program. Based on these estimates, our subjects expended approximately 90 kcal×session−1 in the first week (total 270 kcal); 110 kcal×session−1 in the second week (total 330 kcal); 205 kcal×session−1 in the third week (total 615 kcal); 225 kcal×session−1 in the fourth week (total 675 kcal); 325 kcal×session−1 in the fifth week (total 975 kcal); 385 kcal×session−1 in the sixth week (total 1155 kcal); or 4000 kcal for the whole 6-week training period. This amount of kcal represents the equivalent loss of approximately 0.520 kg of fat, which is very close to the actual loss of 0.600 kg in body fat mass observed in our study.
4.4. Maximum Oxygen Uptake
The significant increase in 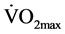 is concordant with the findings of several studies [19] - [21] [23] . Harris & Holly [23] noted that short rest intervals and adequate training stimulus appear to be keys to eliciting changes in aerobic capacity. Rest intervals between sets in our study were less than 15 seconds. Considering the training stimulus, we did not record the HR at any point during the sessions, so we do not have any information about this parameter. Nevertheless, it appears that the volume of training (number of repetitions, sets, and workload) provided adequate training stimulus to eliciting a significant improvement in aerobic capacity of the untrained subjects.
is concordant with the findings of several studies [19] - [21] [23] . Harris & Holly [23] noted that short rest intervals and adequate training stimulus appear to be keys to eliciting changes in aerobic capacity. Rest intervals between sets in our study were less than 15 seconds. Considering the training stimulus, we did not record the HR at any point during the sessions, so we do not have any information about this parameter. Nevertheless, it appears that the volume of training (number of repetitions, sets, and workload) provided adequate training stimulus to eliciting a significant improvement in aerobic capacity of the untrained subjects.
4.5. UBME, AHFME, BS and HS
The current results indicate that CT may increase UBME and AHFME confirming the particular tested hypotheses. The present findings are consistent with the positive effect of CT on muscular endurance observed by Wilmore et al. [19] and Kaikkonen et al. [21] . In addition, the present results are confirmed by several previous studies (e.g. [20] - [24] [26] ), which were found that a circuit regime is an ideal methods for muscular endurance training. According to Rose and Rothstein [44] muscular endurance training (either aerobic endurance or low resistance weight training with several repetitions) was found to improve the oxidative capacity of the muscle.
The back strength improvements are consistent with whole body strength improvements found in other circuit weight programs [19] [20] [22] - [24] . Consequently, the hypothesis that CT would have a positive effect on back strength was confirmed. The circuit training-induced improvement in back strength could be explained by an increase in myofibrillar proteins, resulting in enlarged or hypertrophic muscle fibers [44] . The training-induced muscle hypertrophy can be supported by the fact that there was a significant increase in lean body weight.
Contrary to our findings regarding HS, two studies by Taniguchi [47] and Saito et al. [48] found increases in maximal handgrip force after resistance training. It should be noted, however, that the training interventions in these studies consisted of isometric handgrip training. In our study there was no handgrip exercise training involved so most probably that was the reason why this variable did not change.
4.6. Fasting Blood Variables
A review study by Hurley [45] reported that most longitudinal studies investigating the effect of resistive training on lipid profiles have showed that lipid profiles, especially HDL and LDL, are improved as a result of this intervention. Regarding fasting blood glucose, there is very little data examining this dose-response relationship between resistance exercise and this variable in healthy subjects. Williams et al. [46] found that community based resistance training significantly decreased fasting blood glucose levels in healthy older individuals. Our results on fasting blood variables are in disagreement with these findings; hence the hypotheses about these variables were not confirmed. Nevertheless, due to methodological limitations and design flaws from many of the studies involving resistive training there is not enough information to determine whether this type of exercise is effective for risk factor intervention [45] .
5. Conclusion
In conclusion, the present study suggests that CT involving bodyweight exercises, as opposed to aerobic endurance training has no significant impact on resting HRV time-, frequency-domain and nonlinear measures. However, the fact that this type of training caused a significant reduction in arterial blood pressure, suggested that it might prevent the development of CVD through other mechanisms. This means that aerobic endurance and CT, or resistance training in general, should complement each other, as they seem to prevent the development of CVD through different mechanisms. Increases in aerobic capacity, lean body weight, muscular endurance and strength indicate additional benefits.
6. Study Limitations
Some limitations should be taken into consideration when interpreting the results of the current study. A) The present findings may not be applicable to older adults or clinical populations, due to the fact that age and cardiovascular diseases appear to be interrelated to resting HRV [7] [8] [49] ; B) In most of the studies investigating the effect of resistance training on resting HRV measures, data was collected with the subjects resting in supine position. However in the current study, data was collected in seated position. This could partially influence the results. A study by Ribeiro et al. [50] however, showed no significant differences in HRV indexes in the supine or seated position in both young and postmenopausal women. C) In the present study, breathing patterns were not controlled during the HRV measurements since we aimed at obtaining data from a condition as close to real life as possible. However, a study by Bloomfield et al. [51] showed that when collecting HRV data in both healthy subjects and/or patients with heart disease, there is no need to control breathing.
Acknowledgements
We would like to thank Dr Jackie Dabinett for her valuable contribution, Mr. Claudio De Marco for his assistance in the HRV analysis and Ms. Maria Lazarou for her assistance in the blood sampling analysis. We also thank the participants for their excellent cooperation and commitment.
Cite this paper
AdamosVrachimis,MariosHadjicharalambous,ChrisTyler, (2016) The Effect of Circuit Training on Resting Heart Rate Variability, Cardiovascular Disease Risk Factors and Physical Fitness in Healthy Untrained Adults. Health,08,144-155. doi: 10.4236/health.2016.82017
References
- 1. WHO (2012) Cardiovascular Diseases. World Health Organization, Geneva.
http://www.who.int/ - 2. Molgaard, H., Sorensen, K.E. and Bjerregaard, P. (1991) Attenuated 24-h Heart Rate Variability in Apparently Healthy Subsequently Suffering Sudden Cardiac Death. Clinical Autonomic Research, 1, 233-237.
http://dx.doi.org/10.1007/BF01824992 - 3. Tsuji, H., Venditti Jr., F.J., Manders, E.S., Evans, J.C., Larson, M.G. and Feldman, C.L., et al. (1994) Reduced Heart Rate Variability and Mortality Risk in an Elderly Cohort. The Framingham Heart Study. Circulation, 90, 878-883.
http://dx.doi.org/10.1161/01.CIR.90.2.878 - 4. Liao, D., Sloan, R.P., Cascio, W.E., Folsom, A.R., Liese, A.D., Evans, G.W., et al. (1998) Multiple Metabolic Syndrome Is Associated with Lower Heart Rate Variability. Diabetes Care, 21, 2116-2122.
http://dx.doi.org/10.2337/diacare.21.12.2116 - 5. Achten, J. and Jeukendrup, A.E. (2003) Heart Rate Monitoring Applications and Limitations. Sports Medicine, 33, 517-538.
http://dx.doi.org/10.2165/00007256-200333070-00004 - 6. Kuusela, T.A., Jartti, T.T., Tahvanainen, K.U. and Kaila, T.J. (2002) Nonlinear Methods of Biosignal Analysis in Assessing Terbutaline-Induced Heart Rate and Blood Pressure Changes. American Journal of Physiology-Heart and Circulatory Physiology, 282, H773-H783.
http://dx.doi.org/10.1152/ajpheart.00559.2001 - 7. Tsuji, H., Larson, M.G., Venditti Jr., F.J., Manders, E.S., Evans, J.C., Feldman, C.L., et al. (1996) Impact of Reduced Heart Rate Variability on Risk for Cardiac Events. The Framingham Heart Study. Circulation, 94, 2850-2855.
http://dx.doi.org/10.1161/01.CIR.94.11.2850 - 8. Liao, D., Cai, J., Rosamond, W.D., Barnes, R.F., Hutchinson, R.G., Whitsel, E.A., et al. (1997) Cardiac Autonomic Function and Incident Coronary Heart Disease: A Population-Based Case-Cohort Study. American Journal of Epidemiology, 145, 696-706.
http://dx.doi.org/10.1093/aje/145.8.696 - 9. Dekker, J.M., Schouten, E.G., Klootwijk, P., Pool, J., Swenne, C.A. and Kromhout, D. (1997) Heart Rate Variability from Short Electrocardiographic Recording Predicts Mortality from All Causes in Middle-Aged and Elderly Men. The Zutphen Study. American Journal of Epidemiology, 145, 899-908.
http://dx.doi.org/10.1093/oxfordjournals.aje.a009049 - 10. De Meersman, R.E. (1992) Respiratory Sinus Arrhythmia Alteration Following Training in Endurance Athletes. European Journal of Applied Physiology, 64, 434-436.
http://dx.doi.org/10.1007/BF00625063 - 11. Levy, W.C., Cerqueira, M.D., Harp, G.D., Johannessen, K.A., Abrass, I.B., Schwartz, R.S., et al. (1998) Effect of Endurance Exercise Training on Heart Rate Variability at Rest in Healthy Young and Older Men. American Journal of Cardiology, 82, 1236-1241.
http://dx.doi.org/10.1016/S0002-9149(98)00611-0 - 12. Melanson, E.L. and Freedson, P.S. (2001) The Effect of Endurance Training on Resting Heart Rate Variability in Sedentary Adult Males. European Journal of Applied Physiology, 85, 442-449.
http://dx.doi.org/10.1007/s004210100479 - 13. Carter, J.R., Ray, C.A., Downs, E.M. and Cooke, W.H. (2003) Strength Training Reduces Arterial Blood Pressure but Not Sympathetic Neural Activity in Young Normotensive Subjects. Journal of Applied Physiology, 94, 2212-2216.
http://dx.doi.org/10.1152/japplphysiol.01109.2002 - 14. Van Hoof, R., Macor, F., Lijnen, P., Staessen, J., Thijs, L., Vanhees, L., et al. (1996) Effect of Strength Training on Blood Pressure Measured in Various Conditions in Sedentary Men. International Journal of Sports Medicine, 17, 415- 422.
http://dx.doi.org/10.1055/s-2007-972871 - 15. Cooke, W.H. and Carter, J.R. (2005) Strength Training Does Not Affect Vagal-Cardiac Control or Cardio-Vagal Baroreflex Sensitivity in Young Healthy Subjects. European Journal of Applied Physiology, 93, 719-725.
http://dx.doi.org/10.1007/s00421-004-1243-x - 16. Heffernan, K.S., Fahs, C.A., Shinsako, K.K., Jae, S.Y. and Fernball, B. (2007) Heart Rate Recovery and Heart Rate Complexity Following Resistance Exercise Training and Detraining in Young Men. American Journal of Physiology-Heart and Circulatory Physiology, 293, H3180-H3186.
http://dx.doi.org/10.1152/ajpheart.00648.2007 - 17. Tatro, D.L., Dudley, G.A. and Convertino, V.A. (1992) Carotid-Cardiac Baroreflex Response and LBNP Tolerance Following Resistance Training. Medicine and Science in Sports Exercise, 24, 789-796.
http://dx.doi.org/10.1249/00005768-199207000-00009 - 18. Taylor, A.C., McCartney, N., Kamath, M.V. and Wiley, R.L. (2003) Isometric Training Lowers Resting Blood Pressure and Modulates Autonomic Control. Medicine and Science in Sports Exercise, 35, 251-256.
http://dx.doi.org/10.1249/01.MSS.0000048725.15026.B5 - 19. Wilmore, J.H., Parr, R.B., Vodak, P.A., Barstow, T.J., Pipes, T.V., Ward, P., et al. (1976) Strength, Endurance, BMR, and Body Composition Changes with Circuit Weight Training. Medicine and Science in Sports Exercise, 8, 59-60.
http://dx.doi.org/10.1249/00005768-197621000-00073 - 20. Gettman, L.R., Ward, P. and Hagan, R.D. (1982) A Comparison of Combined Running and Weight Training with Circuit Weight Training. Medicine and Science in Sports Exercise, 14, 229-234.
http://dx.doi.org/10.1249/00005768-198203000-00014 - 21. Kaikkonen, H., Yrjama, M., Siljander, E., Byman, P. and Laukkanen, R. (2000) The Effect of Heart Rate Controlled Low Resistance Circuit Weight Training and Endurance Training on Maximal Aerobic Power in Sedentary Adults. Scandinavia Journal of Medicine and Science in Sports, 10, 211-215.
http://dx.doi.org/10.1034/j.1600-0838.2000.010004211.x - 22. Gettman, L.R., Ayres, J.J., Pollock, M.L. and Jackson, A. (1978) The Effect of Circuit Weight Training on Strength, Cardiorespiratory Function, and Body Composition of Adult Men. Medicine and Science in Sports Exercise, 10, 171-176.
- 23. Harris, K.A. and Holly, R.G. (1987) Physiological Response to Circuit Weight Training in Borderline Hypertensive Subjects. Medicine and Science in Sports Exercise, 19, 246-252.
http://dx.doi.org/10.1249/00005768-198706000-00011 - 24. Harber, M.P., Fry, A.C., Rubin, M.R., Smith, J.C. and Weiss, L.W. (2004) Skeletal Muscle and Hormonal Adaptations to Circuit Weight Training in Untrained Men. Scandinavia Journal of Medicine and Science in Sports, 14, 176-185.
http://dx.doi.org/10.1111/j.1600-0838.2003.371.x - 25. Willardson, J.M. (2006) A Brief Review: Factors Affecting the Length of the Rest Interval between Resistance Exercise Sets. Journal of Strength Conditioning Research, 20, 978-984.
http://dx.doi.org/10.1519/00124278-200611000-00040 - 26. Klika, B. and Jordan, C. (2013) High-Intensity Circuit Training Using Body Weight: Maximum Results with Minimal Investment. ACSM’s Health and Fitness Journal, 17, 8-14.
http://dx.doi.org/10.1249/FIT.0b013e31828cb1e8 - 27. Thalheimer, W. and Cook, S. (2002) How to Calculate Effect Sizes from Published Research Articles: A Simplified Methodology. Work-Learning Research.
http://www.work-learning.com/ - 28. American College of Sports Medicine (2005) ACSM’s Guidelines for Exercise Testing and Prescription. 7th Edition, Lippincott Williams & Wilkins, Philadelphia.
- 29. Augustsson, S.R., Bersas, E., Thomas, E.M., Sahlberg, M., Augustsson, J. and Svantesson, U. (2009) Gender Differences and Reliability of Selected Physical Performance Tests in Young Women and Men. Advances in Physiotherapy, 11, 64-70.
http://dx.doi.org/10.1080/14038190801999679 - 30. Altunkan, S. (2007) Validation of the Omron M6 (HEM-7001-E) Upper-Arm Blood Pressure Measuring Device According to the International Protocol in adults and Obese Adults. Blood Pressure Monitoring, 12, 219-225.
http://dx.doi.org/10.1097/MBP.0b013e3280f813d0 - 31. Durnin, J. and Womersley, J. (1974) Body Fat Assessed from Total Body Density and Its Estimation from Skinfold Thickness: Measurements on 481 Men and Women Aged 16 to 72 Years. British Journal of Nutrition, 32, 77-97.
http://dx.doi.org/10.1079/BJN19740060 - 32. Duz, S., Kocak, M. and Korkusuz, F. (2009) Evaluation of Body Composition Using Three Difference Methods Compared to Dual-Energy X-Ray Absorptiometry. European Journal of Sport Science, 9, 181-190.
http://dx.doi.org/10.1080/17461390902763425 - 33. McArdle, W.D., Katch, F.I., Pechar, G.S., Jacobson, L. and Ruck, S. (1972) Reliability and Interrelationships between Maximal Oxygen Intake, Physical Work Capacity and Step-Test Scores in College Women. Medicine and Science in Sports, 4, 182-186.
http://dx.doi.org/10.1249/00005768-197200440-00019 - 34. Coldwells, A., Atkinson, G. and Reilly, T. (1994) Sources of Variation in Back and Leg Dynamometry. Ergonomics, 37, 79-86.
http://dx.doi.org/10.1080/00140139408963625 - 35. Juricek, J., Derek, L., Unic, A., Serdar, T., Marijancevic, D., Zivkovic, M., et al. (2010) Analytical Evaluation of the Clinical Chemistry Analyzer Olympus AU2700 Plus. Biochemia Medica, 20, 334-340.
http://dx.doi.org/10.11613/bm.2010.043 - 36. Heffernan, K.S., Kelly, E.E., Collier, S.R. and Fernhall, B. (2006) Cardiac Autonomic Modulation during Recovery from Acute Endurance versus Resistance Exercise. European Journal of Cardiovascular Prevention and Rehabilitation, 13, 80-86.
http://dx.doi.org/10.1097/00149831-200602000-00012 - 37. Heffernan, K.S., Sosnoff, J.J., Jae, S.Y., Gates, G.J. and Fernhall, B. (2008) Acute Resistance Exercise Reduces Heart Rate Complexity and Increases QTc Interval. International Journal of Sports Medicine, 29, 289-293.
http://dx.doi.org/10.1055/s-2007-965363 - 38. Hurley, B.F., Hagberg, J.M., Goldberg, A.P., Seals, D.R., Ehsani, A.A., Brennan, R.E., et al. (1988) Resistive Training Can Reduce Coronary Risk Factors without Altering VO2max or Percent Body Fat. Medicine and Science in Sports and Exercise, 20, 150-154.
http://dx.doi.org/10.1249/00005768-198820020-00008 - 39. Fagard, R.H. (2006) Exercise Is Good for Your Blood Pressure: Effects of Endurance Training and Resistance Training. Clinical and Experimental Pharmacology and Physiology, 33, 853-856.
http://dx.doi.org/10.1111/j.1440-1681.2006.04453.x - 40. Ray, C.A. and Carrasco, D.I. (2000) Isometric Handgrip Training Reduces Arterial Pressure at Rest without Changes in Sympathetic Nerve Activity. American Journal of Physiology-Heart and Circulatory Physiology, 279, H245-H249.
- 41. Reid, C.M., Yeater, R.A. and Ullrich, I.H. (1987) Weight Training and Strength, Cardiorespiratory Functioning and Body Composition of Men. British Journal of Sports Medicine, 21, 40-44.
http://dx.doi.org/10.1136/bjsm.21.1.40 - 42. Pollock, M.L. (1973) The Quantification of Endurance Training Programs. In: Wilmore, J.H., Ed., Exercise and Sports Science Reviews, Academic Press, New York, 155-188.
http://dx.doi.org/10.1249/00003677-197300010-00010 - 43. Wilmore, J.H., Parr, R.B., Ward, T.J., Vodak, P.A., Barstow, T.J., Pipes, T.V., et al. (1978) Energy Cost of Circuit Weight Training. Medicine and Science in Sports and Exercise, 10, 75-78.
- 44. Rose, S.J. and Rothstein, J.M. (1982) General Concepts and Adaptations to Altered Patterns of Use. Physical Therapy, 62, 1773-1787.
- 45. Hurley, B. (1982) Effects of Resistive Training on Lipoprotein-Lipid Profiles: A Comparison to Aerobic Exercise Training. Medicine and Science in Sports Exercise, 21, 689-693.
http://dx.doi.org/10.1249/00005768-198912000-00012 - 46. Williams, A.D., Almond, J., Ahuja, K.D.K., Beard, D.C., Robertson, I.K. and Ball, M.J. (2011) Cardiovascular and Metabolic Effects of Community Based Resistance Training in an Older Population. Journal of Science and Medicine in Sport, 14, 331-337.
http://dx.doi.org/10.1016/j.jsams.2011.02.011 - 47. Taniguchi, Y. (1997) Lateral Specificity in Resistance Training: The Effect of Bilateral and Unilateral Training. European Journal of Applied Physiology, 75, 144-150.
http://dx.doi.org/10.1007/s004210050139 - 48. Saito, M., Iwase, S. and Hachiya, T. (2009) Resistance Exercise Training Enhances Sympathetic Nerve Activity during Fatigue-Inducing Isometric Handgrip Trials. European Journal of Applied Physiology, 105, 225-234.
http://dx.doi.org/10.1007/s00421-008-0893-5 - 49. Aubert, A.E., Seps, B. and Beckers, F. (2003) Heart Rate Variability in Athletes. Sports Medicine, 33, 889-919.
http://dx.doi.org/10.2165/00007256-200333120-00003 - 50. Ribeiro, T.F., Azevedo, G.D., Crescencio, J.C., Maraes, V.R.F.S., Papa, V., Catai, A.M., et al. (2001) Heart Rate Variability under Resting Conditions in Postmenopausal and Young Women. Brazilian Journal of Medical Biology Research, 34, 871-877.
http://dx.doi.org/10.1590/S0100-879X2001000700006 - 51. Bloomfield, D.M., Magnano, A., Bigger, J.R., Rivadeneira, H., Parides, M. and Steinman, R.C. (2001) Comparison of Spontaneous vs. Metronome-Guided Breathing on Assessment of Vagal Modulation Using RR Variability. American Journal of Physiology, 280, H1145-H1150.
NOTES
*Corresponding author.



