Health
Vol.5 No.7A4(2013), Article ID:34204,6 pages DOI:10.4236/health.2013.57A4003
The influence of different contraceptive methods on vaginal microbiota: Clinical study*
![]()
1Biochemist-Specialist in Clinical Bacteriology, Servicio de Atención Médica, Ministerio de Salud, 25 de Mayo 254 Sa Pereira, Santa Fe, Argentina; #Corresponding Author: sonialab1@gmail.com
2Facultad de Ingeniería y Ciencias Hídricas, Universidad Nacional del Litoral, Pje El Pozo, Santa Fe, Argentina
3Programa de Salud Sexual y Reproductiva en el Servicio de Atención Medica, Ministerio de Salud, 25 de Mayo 254 Sa Pereira, Santa Fe, Argentina
4Servicio de Atención Médica, Ministerio de Salud, 25 de Mayo 254 Sa Pereira, Santa Fe, Argentina
Copyright © 2013 Sonia E. Fosch et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 13 May 2013; revised 14 June 2013; accepted 29 June 2013
Keywords: Vaginal Dysfunction; Vaginal Content; Vaginal States; Contraception
ABSTRACT
Objective: The aim of this work was to evaluate the vaginal content of women attending family planning controls and its relationship with the contraceptive practice used (oral contraceptive pills, intrauterine device, condoms, or rhythm method) as well as its relationship with the lack of regular contraception. Design: Observational, descriptive study. Setting: Servicio de Atención Mé- dica—Ministerio de Salud—Sa Pereira—Santa Fe. Argentina. Population: A total of 250 women were studied following the BAVACO (balance of the vaginal content). Methods: Standard method, which included the wet mount test, and Giemsa and Gram stain under the Nugent score. Results: Obtained from the vaginal microbiota, the vaginal inflammatory response, presence of yeast and Trichomonas allowed defining five basic vaginal microbial states which were statistically related to contraceptive methods. Main Outcome Measures: The association of each of the five vaginal states with the different contraceptive practices was determined by X2 test, considering p < 0.05 as a significant difference (Program EPI-INFO 6. Version 6.04). Results: A significantly positive association was found between oral contraceptives and normal microbiota (OR 3.98 – p = 0.000); intrauterine device and bacterial vaginosis (OR 10.1 – p = 0.000); whereas a negative relationship (OR 0.18 – p = 0.005) was found between oral contraceptives and bacterial vaginosis (OR 0.21 – p = 0.000). Conclusions: a) the positive association of combined oral contraceptives with a normal microbiota indicates a positive trend of protection, b) intrauterine devices increase whereas oral contraceptives decrease the risk for bacterial vaginosis, while oral contraceptives reduce the frequency.
1. INTRODUCTION
The balance of the Vaginal Microbiota (VM) can be disturbed by physiological and non-physiological changes mediated by the hormonal status, sexual behavior, contraception practice used, vaginal blood, vaginal showers, presence of foreign bodies and/or concomitant use of medicines [1].
Recent studies have revealed that the vagina, where the vaginal microbiota is housed, is actually an autonomous internal sexual organ, whose functional importance is crucial in sexual and reproductive health [2]. The clinical concept of vaginal dysfunction involves a large number of pathologies (such as vaginosis and vaginitis), within a frame of common signs and symptoms that decrease the predictive value of the syndromic diagnosis [2].
Whether expressed in one or another individual syndrome, or as a whole (vaginosis, vaginitis), the microbial etiology of vaginal dysfunction entails a high risk factor for the acquisition of sexually transmitted infections, surgical risk, gestational alterations and mother-to-baby infections [3-5].
Although the microbial etiology has been extensively studied, the etiology of bacterial vaginosis and nonspecific microbial vaginitis still remains a mystery, and thus needs further research since empirical results are key when deciding which treatment is to be used. Systemic factors, mainly from the hormone-immune system, together with the psychic component, are also involved, although the remaining question to be solved is how the phenomenon is generated, that is, whether it responds to exclusively systemic changes (per se) or to external factors such as sexual activity, contraception, vaginal invasion by habitual/exogenous (intestinal, oral) or environmental microbiota, and/or to typical agents of sexual transmission, and hygienic habits, among others [6,7].
However, the etiology of most of these basic vaginal states, is still poorly known and a better definition of the risk factors associated with their acquisition needs to be optimized. It is also necessary to find out how sensitive to external factors this global physiological system controlling the vaginal function is.
As regards contraception methods, significant differences have been observed in the frequencies of vaginal dysfunction syndromes as a function of the adoption of combined oral contraceptives, use of intrauterine devices, condoms, natural ovulation rhythm and women who use no contraceptive method periodically [1].
With the general purpose of studying the risk factors associated with Vaginal Dysfunction, this work focused particularly on analyzing the influence of contraceptive methods used by women belonging to a socially uniform community, with a similar educational and economic profile, attending family planning controls of a Sexual and Reproductive Health Program.
2. MATERIALS AND METHODS
2.1. Population under Study
He joined the study group with the inclusion of 250 women who signed informed consent approved by the Ethics Committee of the Faculty of Biochemistry (August 23, 2007-UNL) and were selected according to the following conditions: age between 17 and 45, sexually active one to two average sex per week, none of the patients were on immunosuppressive therapy, had received antibiotic or antifungal therapy within the month prior to sampling, there had been no douching in the last 48 h.
A minimum of 6 months before the study, the following contraceptive practices were adopted: 51 women (20.4%) used an intrauterine device; 132 (52.8%) used combined oral contraceptives (Levonorgestrel-Ethynilestradiol); 23 (9.2%) used condoms; 15 (6%) women practised the rhythm method (sexual abstinence between days 10 and 18 of the cycle) and 29 (11.6%) did not use any contraceptive method periodically.
All women included in the study showed no symptoms and/or evident signs of vaginal dysfunction. Although low confidence can be attributed to this information, most women declared an average of two sexual intercourses per week. This assertion, however, validates the regularity among the group of selected women and that no particular cases, such as sexually inactive women, lesbians, or sex workers, were considered.
All the selected patients were subjected to the following techniques: vaginal content study, according to the guidelines of BAVACO Procedure Handbook [8,9], in particular wet mount test, and Giemsa and Gram stain. The basic criteria for the diagnosis are the Numerical Value obtained according to Nugent’s criteria [8,9] and the vaginal inflammatory response. The former assigns numbers from 0 to 10, which result from estimating the proportion of bacterial morphotypes: values from 0 to 3 indicate normal microbiota (prevalence of lactobacilli); from 4 to 6, intermediate microbiota (decreased lactobacilli—increased habitual anaerobic microbiota), and from 7 to 10, altered microbiota (absence of lactobacilli, prevalence of anaerobic bacteria) [8].
The vaginal inflammatory response determines the number of leukocytes found in the vaginal content, measured at the same time as the Numerical Value and expressed as a sole figure, being the cut off value for this work five leukocytes per field (using 1000× microscope magnification).
The occurrence of yeast and/or Trichomonas and the significant presence of bacterial morphotypes of predictive importance are expressed using a qualitative approach.
Integration of the results from vaginal microbiota, vaginal inflammatory response, presence of yeast and Trichomonas allowed defining five basic vaginal states which were statistically related to contraceptive methods.
Only the standardized microscopic study of the balance of vaginal content (BAVACO) allows defining five basic vaginal states: I) normal microbiota state (NM) and absence of vaginal inflammatory response (VIR); II) normal microbiota with VIR (NM + VIR); III) intermediate microbiota without VIR (IM); IV) bacterial vaginosis (BV) (alteration of the vaginal microbiota without VIR); V) nonspecific microbial vaginitis (NMV) (alteration of the vaginal microbiota with VIR) [9].
2.2. Data Processing
The program EPI-INFO 6.version 6.04 was used for data processing. The association of each of the five vaginal states with the different contraceptive practices was determined by X2 test, considering p < 0.05 as a significant difference.
3. RESULTS
Table 1 shows the distribution of the five basic vaginal states detected with the BAVACO methodology (9) and “defined” by the algorithm which included bacterial vaginal microbiota through its numerical value, presence or absence of vaginal inflammatory response, and morphological detection of yeast and Trichomonas.
The relationship between the results of the five basic vaginal states obtained by morphological diagnosis and the contraceptive and protection method adopted by the women under study, at least during the six months prior to the study, is shown in Table 2.
A significantly positive association was found between oral contraceptives and normal microbiota (OR 3.98 – p = 0.000); intrauterine device and bacterial vaginosis (OR 10.1 – p = 0.000); condoms and normal microbiota + vaginal inflammatory response (OR 16.1 – p = 0.000); the rhythm method and normal microbiota (OR 9.92 – p = 0.000), and without periodic contraception and microbial nonspecific vaginitis (OR 5.9 – p= 0.000), whereas a negative relationship (OR 0.18 – p = 0.005) was found between oral contraceptives and normal microbiota + vaginal inflammatory response (OR 0.27 – p 0.000) and bacterial vaginosis (OR 0.21 – p = 0.000), intrauterine device and normal microbiota (OR 0.34 – p = 0.002), and condoms and normal microbiota.
When analyzing vaginal contents considering the presence of yeast, yeast + vaginal inflammatory response, Trichomonas and Trichomonas + vaginal inflammatory response, a positive association was observed between oral contraceptives and yeast (OR 2.17 – p= 0.036), and
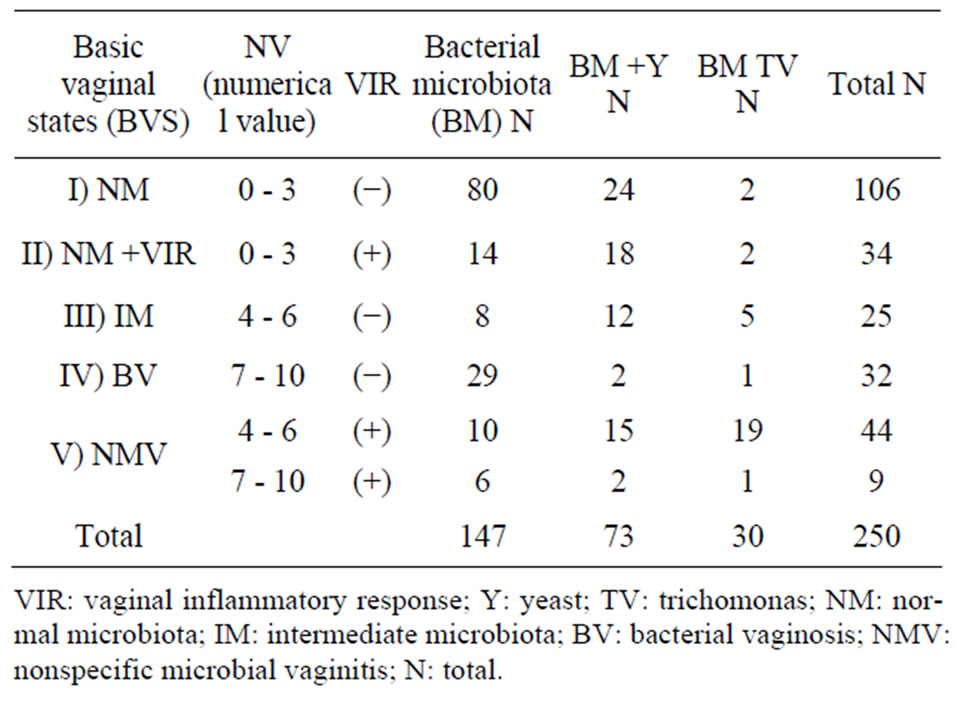
Table 1. Content classification according to the numerical value of bacterial microbiota, vaginal inflammatory response, yeast and trichomonas.
between the lack of periodic contraception and Trichomonas + vaginal inflammatory response (OR 24 – p = 0.000). In contrast, this association was negative between oral contraceptives and Trichomonas + vaginal inflammatory response (OR 0.23 – p = 0.003) (Table 3).
4. DISCUSSION
Results showed undoubted signs of an alarmingly high abnormal state of the vaginal function, namely, 68% of the women studied.
The group studied comprised asymptomatic women regularly adopting preventive medical attention, motivated mainly by their concern about birth control.
When analyzing the various contraception methods and their effect on the vaginal microbiota, a significant positive association was found between oral contraceptives and normal microbiota, and a negative one between normal microbiota + vaginal inflammatory response and bacterial vaginosis, which confirms that the intake of oral contraceptives resulted in a protective action, in agreement with other studies [1,10,11].
The use of oral contraceptives also affected the relationship between bacterial vaginosis and the endocrinal environment [1,10,11]. This effect, also observed by other authors, can probably be attributed to the increase in the glycogen content of epithelial cells due to estrogens, a better lactic acid production and a decrease in vaginal pH favorable for lactobacilli [1,10].
As regards the relationship between oral contraceptives and yeast colonization, the idea of it being a predisposing factor relevant for recurrent vulvovaginitis cannot be rejected. In fact, oral contraceptives cause anovulation, with the subsequent absence of estrogen and progesterone peaks, usually present in the normal sexual cycle but, on the other hand, they add hormonal aggregates responsible for a significant change in the behavior of yeast [12,13].
It is well known that physiological and pharmacological hyperestrogenemia is associated with a higher yeast colonization in the vagina, which could result from certain mechanisms directly mediated by estrogens, mainly the increase in vaginal glycogen, the reduction of vaginal pH and easier adhesion to epithelial cells [1,12,14]. Although this subject has been under study for many years, it still remains as a controversial point.
Many factors still need to be adjusted, although an association between oral contraceptives and Trichomonas, and a different susceptibility to sexually transmitted infections has been observed.
When considering the relationship between the intrauterine device as a contraceptive method and the vaginal microenvironment, the negative association with normal microbiota and positive association with bacterial vaginosis found in this work are in agreement with the
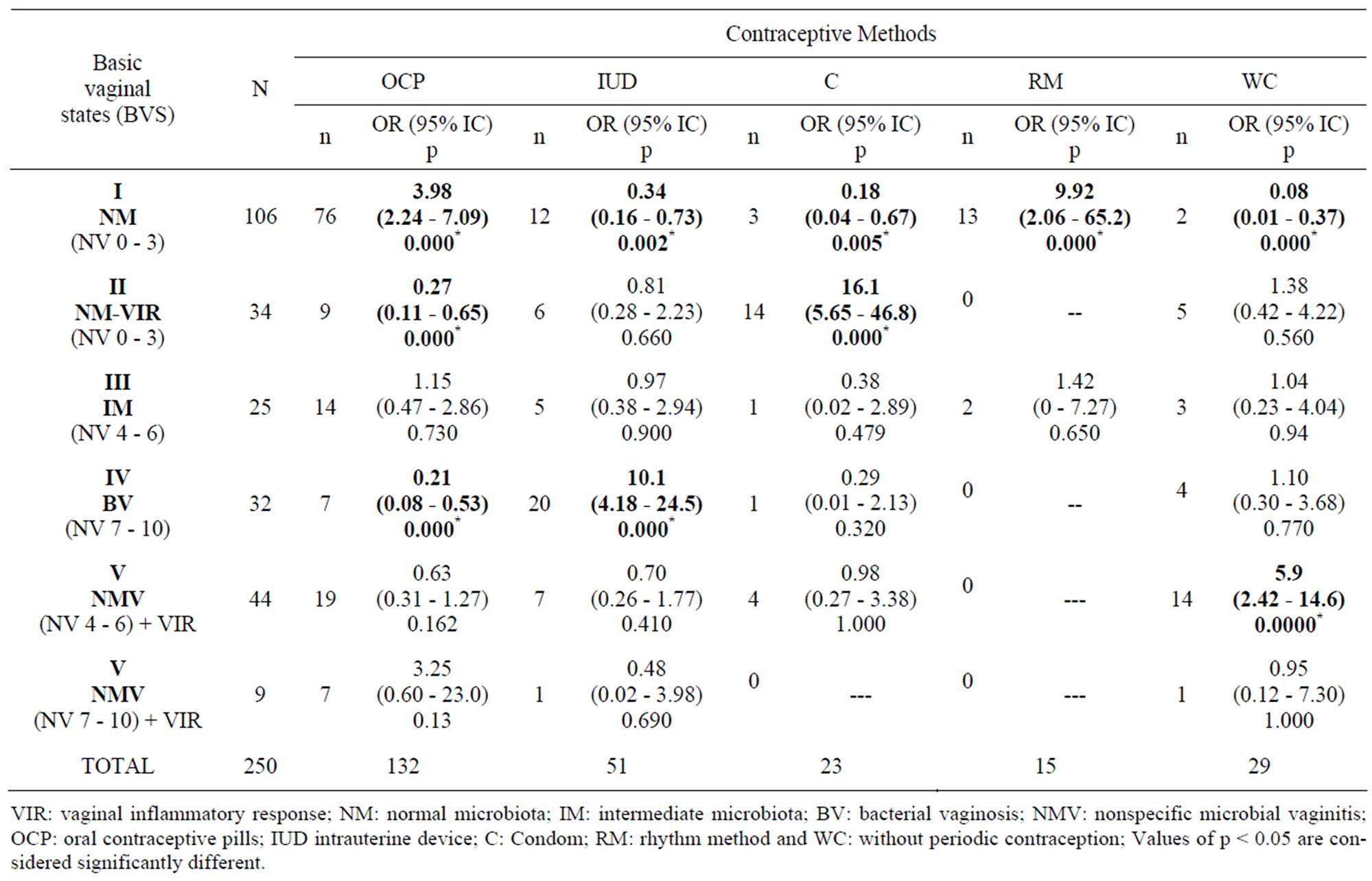
Table 2. Association between vaginal contents and contraceptive methods.
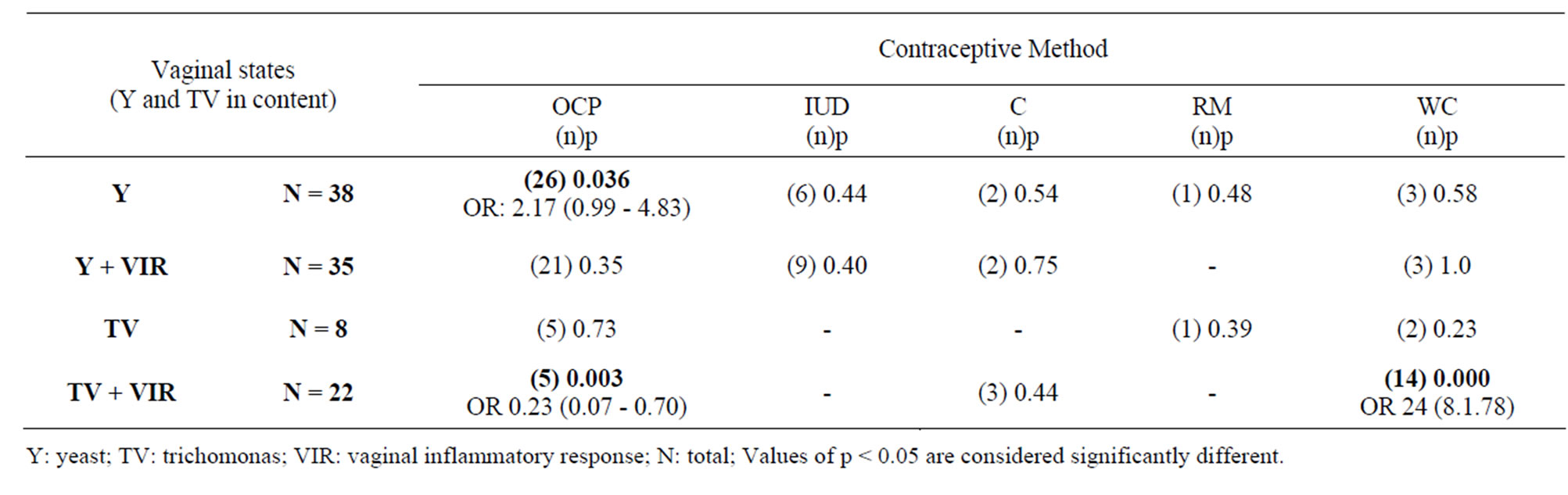
Table 3. Association of yeast and trichomonas in vaginal contents with contraceptive methods.
findings of other authors, which have shown that there is a higher risk of an altered microbiota with Numerical value 7 - 10 (defined criteria of bacterial vaginosis according to Nugent) [1,10,14].
Unlike women using oral contraceptives, patients using an intrauterine device do not have systemic hormonal factors affecting them, but the presence of a foreign body. Furthermore, the influence of sexual relations at any time in the cycle should be recognized as a factor modifying the numerical value of the microbiota [1,10,14].
The fact that condoms were used by a low percentage of the women under study did not allow a complete analysis. However, the positive association with normal microbiota and vaginal inflammatory response are indicators of a possible inflammation of the cervical state or the upper genital tract, urinary infection, and potential yeast vaginitis. Previous studies have reported dermatitis vaginal, allergic and irritant vulvovaginitis and inflammatory conditions associated with the use of condoms [15]. Although latex is mentioned as a possible cause of these conditions, spermicides (nonoxynol-9) seem to be the most probable cause [15].
As regards the rhythm method, the reduced number of women (15/250) adopting this contraceptive procedure (sexual abstinence between days 10 to 18 of the cycle) makes it difficult to draw a general conclusion or to make reference to other works. However, the balance value turned out to be surprising [10,11,14].
It is evident that this practice is not so frequent and requires differentiated personal levels; if the results obtained were confirmed, the psychological factor would be playing a significant role in the function balance. The vaginal microenvironment of these women is only under the systemic hormonal influence during the ovulation stage, which favors the growth of lactobacilli, hence the association with a normal microbiota. Besides, during this sexual abstinence period, there is no exposure to semen or leukocytes as environment modifiers, so their normal environment is kept longer [10,11,14].
On the other hand, a positive association with Trichomonas + vaginal inflammatory response was found in the group not using contraceptive methods. These women had no additional hormones or foreign bodies but were exposed to sexually transmitted infections due to sexual relationships without protection [13].
The nature of the vaginal microenvironment fights in the search for balance, but it is so susceptible that it is permanently exposed to different levels of gynecologicalobstetric risk [10,16].
The present work allowed investigating the complex vaginal microenvironment, evaluating the effect of contraceptive methods, defining normal and pathological states and identifying women under gynecologicalob-stetric risk, from two public health centers. In addition, it contributed epidemiological local data aimed to optimize the institutional attention.
5. CONCLUSION
The prevalence of abnormal states (68% of total cases under study) exceeds most of the reported data (about 50%) for Vaginal Dysfunction in asymptomatic women in their reproductive age. The influence of contraceptive methods can be summarized as follows: a) there is a positive association between oral contraceptives and normal microbiota, b) the use of an intrauterine device generates a higher risk for bacterial vaginosis, whereas the use of oral contraceptives reduces it, c) condom use of periodic manner increases the risk of vaginal inflammatory states but with normal microbiota, d) although the number of cases considered is low, the rhythm method leads to the highest values of normal microbiota in all the series under study in this work and e) in patients without contraception an increase in the risk of microbial vaginitis is detected.
6. ACKNOWLEDGEMENTS
We thank Dr. Ramón de Torres—Director of the BAVACO Project, PROECO Program of Fundación Bioquímica Argentina, for his invaluable collaboration.
REFERENCES
- Tibaldi, C., Capello, N., Latino, M.A., Masuelli, G., Marini, S. and Benedetto, C. (2009) Vaginal and endocervical microorganisms in symptomatic and asymptomatic non-pregnant females: Risk factors and rates of occurrence. Clinical Microbiology and Infectious Diseases, 15, 670-679.
- Andrews, W.W., Hauth, J.C., Cliver, S.P., Conner, M., Goldenberg, R.L. and Goepfert, A.R. (2006) Association of asymptomatic bacterial vaginosis with endometrial microbial colonization and plasma cell endometritis in non pregnant women. American Journal of Obstetic Gynecology, 6, 1611-1616. doi:10.1016/j.ajog.2006.04.010
- McGregor, J.A. and French, J.L. (2000) Bacterial vaginosis in pregnancy. Obstetrical & Gynecological Survey, 55, S1-S19. doi:10.1097/00006254-200005001-00001
- Balaka, B., Agbere, A., Dagnra, A., Baeta, S., Jessie, K. and Assimadi, K. (2005) Genital bacterial carriage during the last trimestre of pregnancy and early-onset neonatal sepsis. Archives of Pediatry, 12, 514-519. doi:10.1016/j.arcped.2005.02.010
- Cohen, C.R., Duerr, A., Pruithithada, N., Rugpao, S., Hillier, S., Garcia, P., et al. (1995) Bacterial vaginosis and HIV seroprevalence among female commercial sex workers. Chiang Mai Thailand AIDS, 9, 1093-1097.
- Spiegel, C.A. (1991) Bacterial vaginosis. Clinical Microbiological Reviews, 4, 485-502.
- Marrazzo, J.M. (2006) A persistent enigmatic ecological mistery: Bacterial vaginosis. Journal of Infectious Disease, 193, 1475-1477. doi:10.1086/503783
- Nugent, R.P., Krohn, M.A. and Hillier, S.L. (1991) Reliability of diagnosting bacterial vaginosis is improved by a standardized method of Gram stain interpretation. Journal of Clinical Microbiology, 29, 297-301.
- Proyecto BACOVA and Programa PROSAR (2010) Fundación Bioquímica Argentina. Manual de Procedimientos BACOVA. http://www.fba.org.ar/PROSAR
- Gupta, K., Hillier, S.L., Hooton, T.M., Roberts, P.L. and Stamm, W.E. (2000) Effects of contraceptive method on the vaginal microbial flora: A prospective evaluation. The Journal of Infectious Diseases, 181, 595-601. doi:10.1086/315267
- Holzman, C., Leventhal, J., Qui, H., et al. (2001) Factors linked to bacterial vaginosis in non pregnant women. American Journal of Public Health, 91, 1664-1670. doi:10.2105/AJPH.91.10.1664
- Sobel, J.D. (1993) Candidal vulvovaginitis. Clinical Obstetrics & Gynecology, 36, 153-165. doi:10.1097/00003081-199303000-00021
- Fosch, S., Fogolin, N., Azzaroni, E., Pairetti, N., Dana, L., Minacori, H., et al. (2006) Vulvovaginitis: Correlation with predisposing factors, clinical and microbiological studies. Revista de Argentina de Microbiología, 38, 202-205.
- Martin, R., Soberon, N., Vazquez, F. and Suarez, J.E. (2008) The vaginal microbiota: Composition, protective role, associated pathology and therapeutic perspectives. Enfermedades Infecciosas y Microbiología Clínica, 26, 160-167.
- Schreiber, C.A., Meyn, L.A., Creinin, M.D., Barnhart, K.T. and Hillier, S.L. (2006) Effects of long-term use of nonoxynol-9 on vaginal flora. Obstetrics & Gynecology, 107, 136-143. doi:10.1097/01.AOG.0000189094.21099.4a
- Wilson, J. (2004) Managing recurrent bacterial vaginosis. Sexually Transmitted Infections, 80, 8-11. doi:10.1136/sti.2002.002733
NOTES
*Disclosure of interest: There are no conflicts of interest.
Contribution to authorship: SF conducted the study, designed and performed the analyses and led the writing of the article. CY contributed to the study design and analyzed the data, helped with statistical methods. MT assisted with data collection. OG conceived and supervised the study. All authors assisted in critical revision of the manuscript and have read and approved the final version of the article.
Details of ethics approval: Approval for the study was given by the Ethics Committee of the Faculty of Biochemistry (August 23, 2007) and all participants gave their informed consent to participate.
Funding: There were no sources of funding for this study.

