Health
Vol. 4 No. 4 (2012) , Article ID: 18841 , 5 pages DOI:10.4236/health.2012.44027
Detection of health hazard insecticide dichlorodiphenyltrichloroethane (DDT) in some common marine dry fish samples from Bangladesh
![]()
1Norwegian College of Fishery Science, University of Tromso, Tromso, Norway;
*Corresponding Author: tigermomin@yahoo.com
2Institute of Marine Science and Fisheries, University of Chittagong, Chittagong, Bangladesh
Received 26 November 2011; revised 19 December 2011; accepted 29 December 2011
Keywords: Dry Fish; Dichlorodiphenyltrichloroethane (DDT); Organochlorine Insecticide; Bangladesh
ABSTRACT
Objectives: The general purpose of this study is to detection and determination of the concentration level of dichlorodiphenyltrichloroethane (DDT) in some commercially avail-able marine dry fishes and to investigate the contamination status of dichlorodip-henyltrichloroethane (DDT) of these dry fishes. Methods: Samples were collected from six largest dry fish markets (three from Chittagong district and three from Cox’s Bazar district) and four types of dry fishes were taken in this study are Ribbon fish (Lepturacanthus savala), Sin Croaker (Johnius dussumieri), Bombay duck (Harpodon nehereus) and Shrimp (mixed species). Total numbers of samples were 24 that were analyzed in the laboratory. Results: The results of the study show that the mean concentrations of dichlorodiphenyltrichloroethane (DDT) in the samples of Ribbon fish, Bombay duck and Sin croaker were ranged between 130.85 - 153.47 ppb, 125.21 - 181.4 ppb and 119.86 - 208.65 ppb respectively. The mean concentrations of dichlorodiphenyl-trichloroethane (DDT) were found at a lower amount in shrimp sp. than the other. Conclusions: This result indicates that the concentration of dichlorodiphenyltrichloroe-thane (DDT) in dry fish samples from Bangladesh are higher and may causes chronic disease and potential long-term risk for human health.
1. INTRODUCTION
Dry fish is low cost dietary protein source and used as a substitute of fish at the scarcity of fresh fish. About 15% of fishes are cured for mass people consumption at the scarcity of fresh fishes in Bangladesh [1]. It is also a very favourite food item among Bangladeshi people and has a very good market demand besides fish and seafood products. Dry fish consumption frequency is very satisfactory in the Southeast Asian countries where people in Bangladesh consume dry fish at least once a week in their daily meal [2]. The common dry fishes in Bangladesh include Ribbonfish, Bombay duck, Sin croaker, Shrimp etc.
Sundry is a common practice by the dry fish producing industries in the remote coastal isolated islands and in inland where chilling and freezing facilities are lacking. Most of the marine dry fishes are produced in remote areas and islands viz., Afatiar Chor, Dublar Chor, Kutubdia, Khuruskul, Moheskhali, Rangabali, Sonadia and St. Martin Island [3]. Dried fish products are generally stored in a dump warehouse either at the site or nearby coastal towns. During the storage period, sometimes fishers use insecticides to prevent dry fishes from insect infestation whatever they are getting within their reach. Most of the time, the fishers use organochlorine insecticides such as DDT, Heptachlor etc.
DDT is widely used commercial organochlorine insecticides that can persist in soil and sediments for more than 15 years and are known to bio accumulate in animal tissues. The half-life of DDT in human body is approximately 4 years [4]. However, these insecticides are health hazard both for users and for consumers and have longterm potential health risk [3].
Although it is banned in Bangladesh, DDT is used indiscriminately in our agriculture. During the monsoon season, it could be release with the fresh water discharge and could be bio-accumulated in fish. In the present study, we have considered only marine fishes and shrimp. Generally, they are retained from the deep sea by fishing boats and trawlers [3]. Therefore, there is very less probability of environmental bioaccumulation of DDT in these fish species. By physical inspection of the sampling sites (Teknaf, Moheskhali and Cox’s Bazar etc.) where the fishes are drying and processing by the anglers, we have observed that some anglers used some poisons (insecticides) without label or label with improper instructions.
In the developed world, people are more concern about the risk and health issues [5]. In Bangladesh, people are more aware about health issues [6] and higher income people are more concern about harmful and health hazardous food intake [2]. Like other developing countries, a great number of indiscriminate and dangerous insecticides are sold in the markets without names and proper labeling. Although DDT is banned in Bangladesh, nobody can draw a statistic on insecticide causalities in Bangladesh [7]. The Department of Environment controls the pesticide registration scheme in Bangladesh. However, there is no specific legislation for controlling the production and use of hazardous industrial chemicals into the food products [7].
The main objectives of this study is to detection and determination of the concentration level of DDT in some available marine dry fishes and the second objective is to elucidate the contamination status of using organochlorine insecticides (e.g. DDT) in Bangladesh.
2. MATERIALS AND METHODS
2.1. Sampling
Samples were collected from Chittagong New Market (Station 1), Asadgonj (Station 2), Reajuddin Bazaar (Station 3), Cox’s Bazar Sadar (Station 4), Moheshkhali (Station 5) and Teknaf (Station 6). Four commercially available species of dry fishes namely Ribbon fish (Lepturacanthus savala), Sin Croaker (Johnius dussumieri), Bombay duck (Harpodon nehereus) and Shrimp (Mixed species) were collected from each market. These dry fish species were selected for this experiment due to their greater market demand and availability. Total numbers of samples were 24. The control samples of three different fishes were collected from drying yards of Moheshkhali Island that are known sample treated with no insecticides and taken into account as blank.
2.2. Apparatus
Mincer fish chopper (Weisser No. 81 K), Soxhlet extractor, separatory funnels (500 ml and 200 ml), chromatographic tube (20 mm I.D 50 cm long), sample concentrator (Techne dry block DB.3), round bottomed flask (500 ml and 100 ml), volumetric flask (50 ml and 10 ml), gas chromatograph (GC-14B, Shimadzu), syringe (10 µl, Hamilton Co.).
2.3. Reagents
Acetone, diethyl ether, dimethyl formamide saturated with petroleum ether, nhexane, petroleum ether (30˚C 60˚C), petroleum ether (30˚C - 60˚C) saturated with dimethyl formamide, eluting mixture I (petroleum ether + diethyl ether 94:6 v/v), standard solutions, eosin solution (2 mg in 100 ml), sodium sulfate solution (2 g/100 ml NaSO4·10H2O), sodium sulfate anhydrous (heated for at least 2 hours at 550˚C), florisil 60 - 100 mesh (heated for at least 2 hours at 550˚C, cool and stored in tightly stopper container, prior to use heated for at least 5 hours at 130˚C, cool and add 5% w/w water, shake this mixture for at least 20 min and stored in a container for at least 10 hours), cotton wool. All the solvents used for the analysis purchased from MERCK, Germany. Dichlorodiphenyltrichloroethane (DDT) and heptachlor standards were obtained from Sigma Chemicals.
2.4. Sample Preparation
All the samples are finely comminuted in a mincer; heating of the samples during comminuting is avoided by briefly chopping several times [8].
2.5. Extraction
Triturate a sample of 25 g, with sodium sulfate to dry, powdery mixture, with the aid of an extraction thimble; extract the mixture exhaustively with Petroleum Ether in Soxhlet apparatus. Concentrate just to dryness the extract solution by a concentrator and dilute to 25 ml with petroleum ether saturated with dimethyl formamide [8]. Cleanup was done in two steps according to the procedure followed by Hans and Zeumer (1987).
2.6. Dimethyl Formamide-Petrolium Ether Partition
Transfer the solution (dissolved in 25 ml petroleum ether saturated with dimethyl formamide) to 250 ml separatory funnel. Rinse the flask with small portion of a previously measured amount of 75 ml dimethyl formamide. Then add the remainder of the dimethyl formamide to the separatory funnel, and shake vigorously for 1 min. Drain the dimethyl formamide phase, and again extract the petroleum ether phase with 10 ml dimethyl formamide. Transfer the combined dimethyl formamide phases to a 500 ml separatory funnel, and add 200 ml sodium sulfate solution. Add a few drops of eosin solution to achieve better recognition of phase separation in the subsequent partition. Then extract successively with a 40 ml portion and three 25 ml potions of petroleum ether for 1 min each time. Wash the combined petroleum ether phases with 10 ml water, dry on sodium sulfate, filter through a cotton wool plug, add 5 ml n-hexane, and concentrate to approximately 5 ml.
2.7. Florisil Column Chromatography
About half filled a chromatographic tube with petroleum ether, and sprinkle with 30 g florisil in small portions through a funnel with stopcock open, tapping the column in the process. Cover the florisil with an approx. 2 cm layer of sodium sulfate. Drain the supernatant solvent to the top of the column packing. Pipette the sample solution on to the column. Let the solution percolate to a level of 1 - 2 mm above the top of the column. Then rinse the flask with small portions of eluting mixture I, add the rinsing to the column, and let them percolate to a level of 1 - 2 mm above the top of the column. Next eluate the column with the remainder of the total 200 ml amount of eluting mixture I, at a flow rate of about 5 ml/min. Add 5 ml n-hexane to the eluate, concentrate the eluate to 5 ml and dilute with n-hexane to 10 ml.
2.8. Sample Analyses
The DDT residues were analyzed by GC-14B, Shimadzu with an electron capture detector (ECD), a manual sampler and GC solution software. A column of 3.1 m × 3.2 mm; I.D glass spiral; stationary phase silicon OV-17, 5%, aging 300˚C, support chromosorb-W-AW-DMCS, mesh 80/100, 1 µm film thickness was used for the chromatographic separation of insecticides. The temperature was fixed for the injector at 250˚C, column at 280˚C, detector at 280˚C. The carrier gas was nitrogen with a 60 ml/min-flow rate. 1.0 µl sample was injected for each run and the running time was 25 min. Standards’ peak were identified by injecting high concentration of the standard (0.5 ppm and 0.25 ppm) and the retention time for DDT was determined. Then calibration was done at 3 points (25 ppb, 50 ppb and 100 ppb) by composite stock standard solution. GC system was calibrated using external standard technique. Individual standard stock solution (100 mg/L) was prepared by weighting appropriate amounts of active ingredients in a brown bottle with a Teflon-lined screw cap and dissolving the weighed standard in HPLC grade hexane. Stock standard solution was used to prepare primary dilution standards. Appropriate volume of each individual stock solution was taken in a volumetric flask and mixed the solutions to obtain composite stock standard solution.
2.9. Analytical Quality Control
Gas chromatograph equipped with ECD was checked for linearity. Instrumental limit of detection for GC-ECD was 1.0 µg/L for organochlorine pesticides. An aliquot of dry fish samples which were collected as blank and treated exactly as a sample including exposure to all glassware, equipments, solvents and reagents used with the sample matrix. No analytic peak was detected in laboratory reagent blank. An aliquot of fortified samples matrix were prepared to which known quantities of the pesticides were added in the laboratory in ppb range. This laboratory fortified matrix was analyzed exactly like the sample. Extraction and clean up were done as mentioned and the recoveries from untreated control samples of dry fish fortified with the analyzed compounds at level of 25 ppb was 98% - 100% for DDT. Prior to injection of the first sample solution, a standard solution was injected at least three times to check the operating conditions and the constancy of the detector signals. Further linearity of the ECD signal was checked by injecting serial dilutions of DDT. A standard solution injected after at least every other sample solution so that any alterations of the gas chromatographic system recognized due to column contamination.
3. RESULTS
The results obtained from the samples from six different sampling sites are alarming for Bangladeshi consumers. Most of the samples contained DDT. Only samples collected from Station 5 and Station 3, DDT were not detected in Ribbon fish and shrimp sp. Samples respectively. The detailed results are presented in the Table 1.
The mean concentrations of DDT in the samples of Ribbon fish and Bombay duck collected from Station 1, Station 2, Station 3, Station 4, Station 5 and Station 6 were ranged from 130.85 - 153.47 ppb and 125.21 - 181.4ppb respectively. The highest concentration of DDT was found in Sin Croaker from Station 1.
Among the four types of samples, DDT was found at a higher concentration in Sin croaker from most of the sampling station. Compare with other dry fishes, lower amount of DDT was found in the shrimp. The range of DDT use in shrimp sp. was ranged between 24.37 ppb to 146.37 ppb.
The comparisons of DDT concentration among differrent types of dry fishes are presented in the Figure 1. The level of concentration of DDT in dry fish is a great concern but more concern is that such a dangerous poison is still using in some of our popular food items such as dry fish though it is banned in Bangladesh.
4. DISCUSSION AND CONCLUSIONS
Health hazard pesticides (e.g. dichlorodiphenyltrichloroethane) have been using in the dry fishes for last few years. However, very few studies have been conducted to investigate the concentration of DDT in dry fishes in Bangladesh. The present study has been done to investigate the concentration of DDT in some commercially available marine dry fishes. The study is found that the mean DDT concentration of Ribbon fish and Bombay duck ranged between 125.21 - 181.14 ppb. Although this study is not found DDT in Ribbon fish from Station 5 but the other sampling sites were found to be contained higher concentration of DDT. The contamination level is
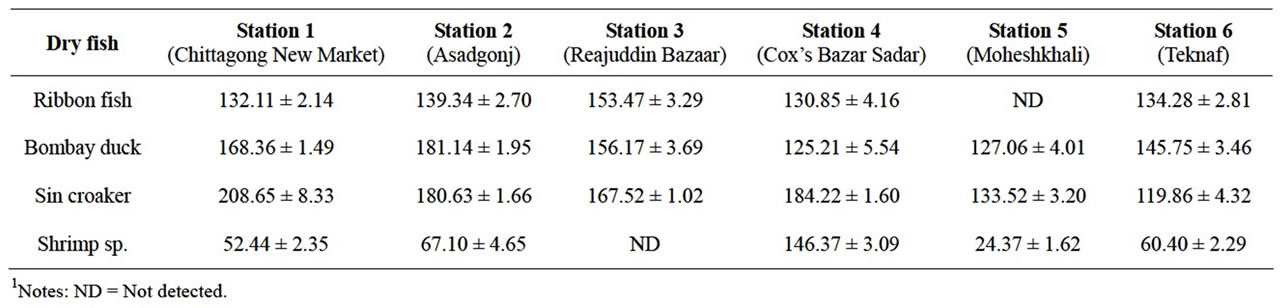
Table 1. Concentration of DDT (in ppb) in the dry fish samples collecting from six different sampling sites (mean concentration ± standard deviation).
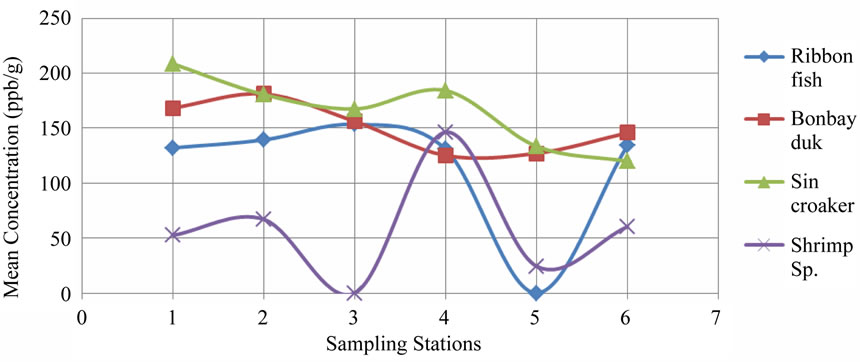
Figure 1. Comparisons of DDT concentration among different types of dry fishes collected from six sampling sites.
classified as “moderately toxic” by the US National Toxicological Program and “moderately hazardous” by WHO, based on the rat oral LD 50 of 113 mg/kg [9]. Among all the samples, maximum mean concentration of DDT was found in Sin croaker from Station 1. The mean concentration of DDT in Sin croaker was recorded ranged between 208.65 - 119.86 ppb. This concentration level of DDT is too much higher than that of contamination level.
Among all the samples, DDT was found comparatively at a lowest amount in shrimp sp. samples. The reason is that shrimp can dry very quickly and has a shell or body cover outside of their body which protects them from the quick insect infestation. Therefore, anglers used lower amount of DDT in dried shrimp samples. The mean concentrations of DDT in shrimp sp. were ranged between 24.37 - 146.37 ppb. However, it is found that the concentration of DDT is depends on sample size. Bigger size of shrimp was found to contain higher amount of DDT. The reason is that the fishers pay much more attention during conservation of bigger size shrimp and use higher amount of DDT than the smaller size shrimp.
To be consistent with previous studies on DDT, it is observed that DDT is a very slow poisoning substance [3, 4,8]. It could be transferred from generation to generation through breast milk [10]. According to the US National Toxicological Program, it could be referred as “moderately toxic” substance or could be considered as a “moderately hazardous” substance [9].
A number of studies showed that DDT is responsible for non-allergic asthma [11] and have direct link with diabetes [12]. A study found elevated risk of cancers of the liver and biliary tract for workers that handled DDT to control the malaria vector [13]. A number of studies argued that the accumulation of DDT in human body before puberty increases the risk of breast cancer for the women [14].
In some developing countries like Bangladesh due to the lacking of resources and infrastructure to implement and enforce the legislation DDT is still available. The government of Bangladesh should take all the necessary steps to combat the situation by implementing the legislation and improving the awareness among related people. The stocker should dry fishes correctly and should pack carefully so that the fish cannot absorb moisture in monsoon.
However, it could be concluded that the fishers and the dry fish stocker in Bangladesh have been using DDT as a compulsory preserver of dry fish without concerning health hazard issues. The concentration levels of DDT in the collected dry fish samples were higher which might causes health disease to the dry fish consumers. Other insecticides are also using as a composite mixture of insecticides to get good preservation. Due to budget restriction, this study is narrowed to focus on using DDT in the dry fishes. Further research should be focus on other organochlorine insecticides that are currently using in dry fish preservation.
5. ACKNOWLEDGEMENTS
The authors want to express their gratitude to the Director, Institute of Marine Sciences and Fisheries, University of Chittagong and BCSIR, Chittagong for providing the necessary facilities pertaining to the work.
![]()
![]()
REFERENCES
- Ashraful, M.A.K. and Khan, Y.S.A. (2001) Insect’s infestation and preventive measures in dry fish storage of Chittagong, Bangladesh. Online Journal of Biological Science, 1, 963-965.
- Siddique, M.A.M. (2011) The role of perceived risk, knowledge, price and cost in explaining dry fish consumption in Bangladesh within the theory of planned behaviour (TPB). Master’s Thesis, University of Tromso, Norway.
- Bhuiyan, M.N.H., Bhuiyan, H.R., Rahim, M., Ahmed, K., Haque, K.M.F., Hassan, M.T. and Bhuiyan, M.N.I. (2008) Screening of organchlorine insecticides (DDT and heptachlor) in dry fish available in Bangladesh. Bangladesh Journal of Pharmacology, 3, 114-120.
- Noren, K. and Meironyte, D. (2000) Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20 - 30 years. Chemosphere, 40, 1111-1123. doi:10.1016/S0045-6535(99)00360-4
- Redmond, E.C. and Griffith, C.J. (2005) Consumer perceptions of food safety education sources Implications for effective strategy development. British Food Journal, 107, 467-483. doi:10.1108/00070700510606882
- Hossain, M.M., Heinonen, V. and Islam, K.M.Z. (2008) Consumption of foods and foodstuffs processed with hazardous chemicals: A case study of Bangladesh. International Journal of Consumer Studies, 32, 588-595. doi:10.1111/j.1470-6431.2008.00690.x
- UNEP (2002) United Nations environment program chemicals. Indian Ocean regional report. UNEP Chemicals is a part of UNEP’s Technology. Industry and Economics Division, 15-67.
- Thierand, H.-P. and Zeumer, H. (1987) Manual of pesticides residue analysis. Vol. I. Working group “analysis”. Pesticide Commission, Germany, 298-319.
- WHO (2005) The WHO recommended classification of pesticides by hazard. British Journal of Psychiatrist, 187, 583-584.
- Solomon, G. and Weiss, P. (2001) Healthy milk, healthy baby. Natural Resources Defense Council, New York.
- Brow, A.J. (2007) Pesticide exposure linked to asthma. Scientific American, 162, 890-897.
- Jones, O.A., Maguire, M.L. and Griffin, J.L. (2008) Environmental pollution and diabetes: A neglected association. Lancet, 371, 287-288. doi:10.1016/S0140-6736(08)60147-6
- Rogan, W.J. and Chen, A. (2005) Health risks and benefits of bis(4-chlorophenyl)-1, 1, 1-trichloroethane (DDT). Lancet, 366, 763-773. doi:10.1016/S0140-6736(05)67182-6
- Clapp, R.W., Jacobs, M.M. and Loechler, E.L. (2008) Environmental and occupational causes of cancer: New evidence 2005-2007. Reviews on Environmental Health, 23, 1- 37.

