Engineering
Vol.5 No.2(2013), Article ID:27729,6 pages DOI:10.4236/eng.2013.52025
Synergistic Effect of Allium cepa-Zn2+ System on the Corrosion of Carbon Steel in Ground Water
1Post Graduate and Research Department of Chemistry, Periyar E. V. R. College (Autonomous), Trichy, India
2Department of Chemistry, Jeppiaar Engineering College, Chennai, India
3Post Graduate and Research Department of Chemistry, St. Joseph’s College (Autonomous), Trichy, India
4Post Graduate Department of Chemistry, Nehru Memorial College (Autonomous), Trichy, India
5Bharathiar Matric Higher Secondary School, Madurai, India
Email: amalrajevr@gmail.com
Received October 25, 2012; revised November 26, 2012; accepted December 24, 2012
Keywords: Carbon Steel; Allium cepa; Corrosion Inhibition Efficiency; Ground Water
ABSTRACT
The corrosion inhibition efficiency (IE) of an aqueous extract Allium cepa (onion) in controlling the corrosion of carbon steel ground water in absence and presence of with Zn2+ has been studied by weight loss method. The formulation consisting of 3 mL Allium cepa extract, 50 ppm of Zn2+ and 50 ppm of sodium pattassium tartarate which offers 97% inhibition efficiency. The synergistic effect exists between onion-Zn2+-tartarate system. The addition of N-cetyl-N,N,Ntrimethylammonium bromide on onion-Zn2+-tartarate system does not change the excellent inhibition efficiency. Polarization study shows that the onion-Zn2+-tartarate system functions as a cathodic inhibitor. AC impedance spectra reveal that a protective film is formed on the metal surface. The UV fluorescent spectra indicate the possibility of formation of Fe2+-onion complex and also Zn2+-onion complex in solution. Thus the protective film is found to be UV fluorescent.
1. Introduction
The toxic inhibitors like chromate based inhibitors were used to control corrosion process but it creates the environmental hazards. The use of chromates at high concentration has declined in recent years because of health and safety considerations. Natural products are nontoxic, biodegradable and readily available. The recent trend in research on environmental friendly corrosion inhibitors is taking us back to exploring the use of natural products as possible sources of cheap, nontoxic, and eco-friendly corrosion inhibitors. These natural products are either synthesized or extracted from aromatic herbs, spices, and medicinal plants [1-7]. Of increasing interest is the use of medicinal plant extracts as corrosion inhibitors for metals in acid solutions. This is because these plants serve as incredibly rich sources of naturally synthesized chemical compounds that are environmentally acceptable, inexpensive, readily available, and renewable sources of materials [8,9]. These chemicals include alkaloids, flavonoids, terpenoids, glycosides, tannins, saponins, fats and oils, and carbohydrates, and so forth [10-18]. Several plants extracts [19-23] and eco-friendly inhibitors attracted the researchers. Investigation of natural inhibitors is particularly interesting because they are non-expensive, ecologically friendly/acceptable and possess no threat to the environment. Onions not only provide flavor; they also provide health-promoting phytochemicals as well as nutrients. Onion contains an acrid, volatile principle that stimulates the tear glands and the mucous membranes of the respiratory tract. All the healthy compounds present in onions, two stand out: sulfur and quercentin-both being strong antioxidants. The present work is undertaken:
1) To evaluate the inhibition efficiency (IE) of an aqueous extract onion (AC) in controlling the corrosion of carbon steel in ground water, in the absence and presence of Zn2+.
2) To examine the influence of N-cetyl-N,N,N-trimethylammonium bromide (CTAB) on the IE of the ACZn2+ system.
3) To analyze the protective film formed on the carbon steel by UV Fluorescence spectra.
4) To understand the mechanistic aspects of corrosion inhibition by polarization studies and AC impedance analysis and;
5) To propose a suitable mechanism for corrosion inhibition.
2. Experimental
2.1. Preparation of Plant Extract
An aqueous extract of Allium cepa (onion) was prepared by grinding 10 g of onion with double distilled water, filtering the suspending impurities, and making up to 500 mL. The extract was used as corrosion inhibitor in the present study.
2.2. Preparation of Specimens
The carbon steel specimens were drilled a hole at one end and numbered by punching. Carbon steel specimens (0.0267% S, 0.06% P, 0.4% Mn, 0.1% C and the rest iron) of dimensions 1.0 cm × 4.0 cm × 0.2 cm were polished with 400 grade emery paper to a mirror finish and degreased with trichloroethylene.
2.3. Weight-Loss Method
Relevant data on the ground water used in this study are given in Table 1. Carbon steel specimens in triplicate were immersed in 100 mL of the solutions containing various concentrations of the inhibitor in the presence and absence of Zn2+ for one day. The weight of the specimens before and after immersion was determined using Shimadzu balance model AY 62. The corrosion products were cleansed with Clarke’s solution [24]. The inhibition efficiency (IE) was then calculated using the equation
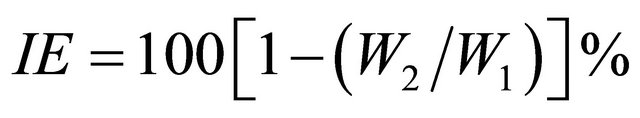
where W1 and W2 are the corrosion rates in the absence and presence of the inhibitor, respectively.
2.4. Polarization Study
Polarization studies were carried out in an H & CH electrochemical work station impedance analyzer model CHI 660 A. A three electrode cell assembly was used. The working electrode was carbon steel. A saturated calomel electrode (SCE) was used as the reference electrode and a rectangular platinum foil was used as the counter electrode. According to the Stern-Geary equation, the steps of the linear polarization plot are substituted to get corrosion current
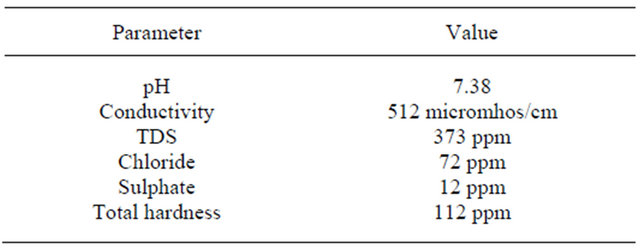
Table 1. Parameters of ground water.
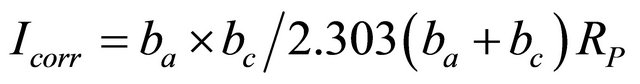
Where, RP is polarization resistance.
Determination of inhibition efficiency.
By Tafel method
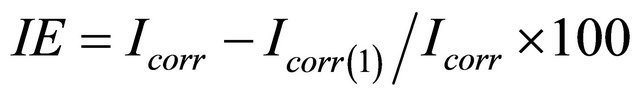
whereIcorr is corrosion current without inhibitor.
Icorr(1) is corrosion current with inhibitor.
The results, such as Tafel slopes, and Icorr and Ecorr values, were calculated. During the polarization study, the scan rate (v/s) was 0.01; hold time at Ef(s) was zero and quiet time(s) was 2.
2.5. AC Impedance Study
The instrument used polarization was also used for AC impedance study. The cell set up was the same as that used for polarization measurements. The real part and imaginary part of the cell impedance were measured in ohms at various frequencies. The values of the charge transfer resistance Rt and the double layer capacitance Cdl were calculated.
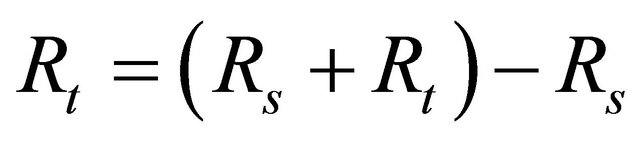
where Rs = solution resistance
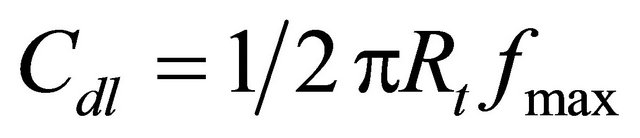
where fmax = maximum frequency AC impedance spectra were recorded with initial E(v) = 0; high frequency (Hz) = 1 × 105; low frequency (Hz) = 1; amplitude (v) = 0.05; and quiet time (s) = 2.
2.6. Surface Examination
The carbon steel specimens were immersed in various test solutions for a period of one day, after one day, the specimen were taken out and dried. The nature of the film formed on the surface of metal specimens was analyzed by UV-Fluorescence spectra.
Fluorescence Spectra
These spectra were recorded in a Hitachi F-4500 fluorescence spectrophotometer.
3. Results and Discussion
3.1. Analysis of Results of Mass Loss Method
The corrosion inhibition efficiency of carbon steel immersed in ground water in the absence and presence of inhibitor systems are given in Tables 3. The inhibition efficiencies (IE) are also given in these Tables. It is seen from Table 2 that the aqueous extract of Allium cepa (AC) alone is poor inhibitor. As concentration AC in-
Table 2. Corrosion inhibition efficiency (IE) of Carbon steel in aqueous solution in the presence of inhibitor obtained by weight loss method.
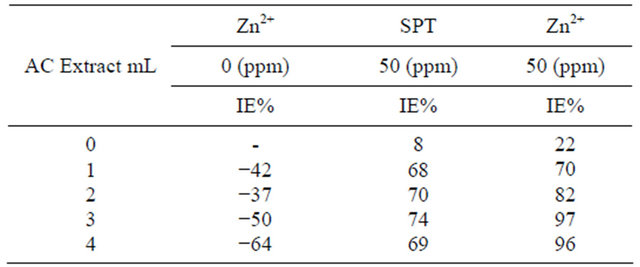
Inhibitor system: AC + SPT + Zn2+ system.
Table 3. Corrosion inhibition efficiency (IE) of Carbon steel in aqueous solution in the presence of inhibitor obtained by weight loss method.
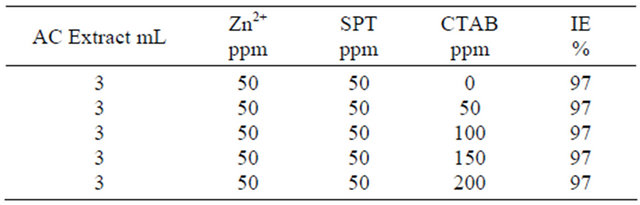
Inhibitor system: AC + SPT + CTAB + Zn2+ system.
creases, IE slowly decreases. That is, at higher concentrations, AC accelerates corrosion. It favours dissolution of carbon steel in ground water. For example, 3 mL of AC shows −50% IE; But addition of 50 ppm of Sodium Potassium Tartarate (SPT) with allium cepa shows 74% IE. The formulation consisting 3 mL of AC, 50 ppm Zn2+ and 50 ppm SPT shows 97% IE. This suggests that a synergistic effect exists between AC-SPT-Zn2+ system [25].
3.2. Influence of CTAB on AC-SPT-Zn2+ System
The Influence of CTAB on AC-SPT-Zn2+ system is given in Table 3. The N-cetyl-N,N,N-trimethylammonium bromide (CTAB) is a biocide. It can control the corrosion caused by microorganism [26]. When various concentration of CTAB added to the AC-SPT-Zn2+ system, the inhibition efficiency does not altered. The AC-SPT-Zn2+ system are much transported towards the metal surface, hence protective film is stable.
3.3. Analysis of Polarization Curves
The potentiodynamic polarization curves of carbon steel immersed in ground water in the absence and presence of inhibitors are shown in Figure 1.
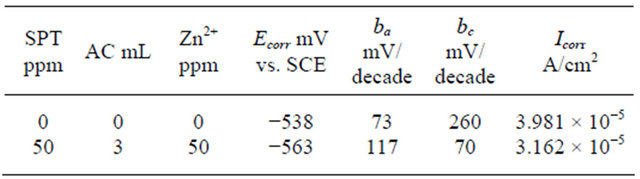
The corrosion parameters are given in Table 4. When carbon steel is immersed in ground water the corrosion potential is −538 mV vs. SCE (Saturated Calomel Electrode). The formulation consisting of 3 mL of AC, 50 ppm of SPT and 50 ppm of Zn2+ shifts the corrosion potential to −563 mV vs SCE.
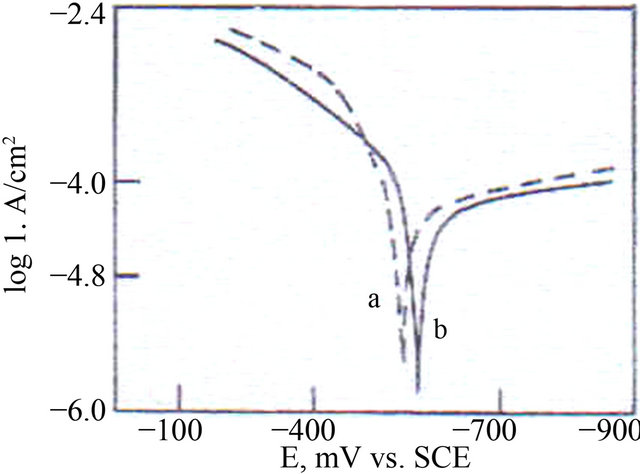
a) Ground water; b) 3 mL AC + 50 ppm of Zn2+ + 50 ppm SPT.
Figure 1. Polarization curves of carbon steel immersed in various solutions.
Table 4. Corrosion parameters of carbon steel immersed in ground water in the absence and presence of inhibitors.
Inhibitor system: AC + SPT + Zn2+ system.
This suggests that the cathodic reaction is controlled predominantly. But Tafel slopes (ba & bc) are not shifted equally [27,28]. The corrosion current is 3.981 × 10−5 A/cm2 to 3.162 × 10−5 A/cm2. This suggests the inhibitive nature of this inhibitor system.
3.4. Analysis of AC Impedance Spectra
The AC impedance spectra of carbon steel immersed in ground water, in the absence and presence of inhibitors are shown in Figure 2.
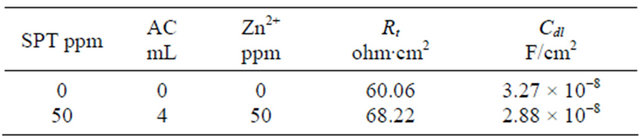
The AC impedance parameters such as charge transfer resistance (Rt) and double layer capacitance (Cdl) are given in Table 5. When carbon steel is immersed in ground water, the charge transfer resistance (Rt) is 60.06 ohm∙cm2; the double layer capacitance Cdl is 3.27 × 10−8 F/cm2. When carbon steel is immersed in the formulation consisting of AC-SPT-Zn2+, the Rt value increases and Cdl value decreases. This confirms that a protective film is formed on the metal surface. This accounts for very high inhibition efficiency [29-32].
3.5. Analysis of Fluorescence Spectra
A few drops of the AC extract were dried on a glass palte. A red solid mass was obtained. Its emission spectrum (lex = 300 nm) is shown in Figure 3(a). two prominent peaks appeared at 361 nm and 505 nm. A few drops of the AC extract were mixed with a few drops of freshly prepared Fe2+ ions (ferrous sulphate). Fe2+-AC complex
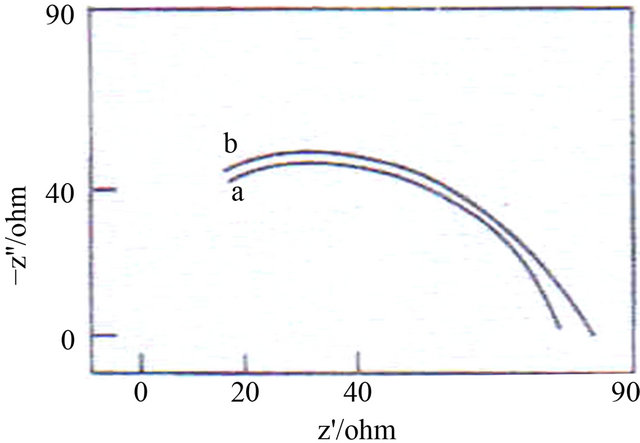
a) Ground water; b) 3 mL AC + 50 ppm of Zn2+ + 50 ppm + SPT.
Figure 2. AC impedance spectra of carbon steel immersed in various solutions.
Table 5. AC impedance parameters of carbon steel immersed in ground water in the absence and presence of inhibitors.
was stormed. It was dried. Its emission spectrum (lex = 300 nm) is shown in Figure 3(b). The intensity of the peak at 361 nm decreased. The peak at 505 nm disappeared.
The emission spectrum (lex = 300 nm) of the ground film formed on surface of the metal after immersion in the solution containing ground water, 3 mL of AC and 50 ppm of Zn2+, is shown in Figure 3(c). The nature of the spectrum matched well with that of the Fe2+-AC complex prepared. The excitation spectra (lex = 361 nm) corresponding to Figures 3(a)-(c), are shown in Figures 3(d)-(f), respectively. A peak appeared at 265 nm, in all the cases. This confirmed the presence of Fe2+-AC complex formed on the anodic sites of the metal surface [33-35].
4. Mechanism
1) When the environment consisting of 50 ppm of Zn2+ and 50 ppm of SPT + 3 mL of AC is prepared, there is a formation of Zn2+-AC complex and Zn2+-SPT complex in solution.
2) When Carbon steel is introduced in this solution, there is diffusion of Zinc complex towards the metal surface.
3) On the metal surface Zinc complex is converted into iron complex on the anodic site.


4) The released Zn2+ combined with OH− to form Zn(OH)2 on the cathodic sites.
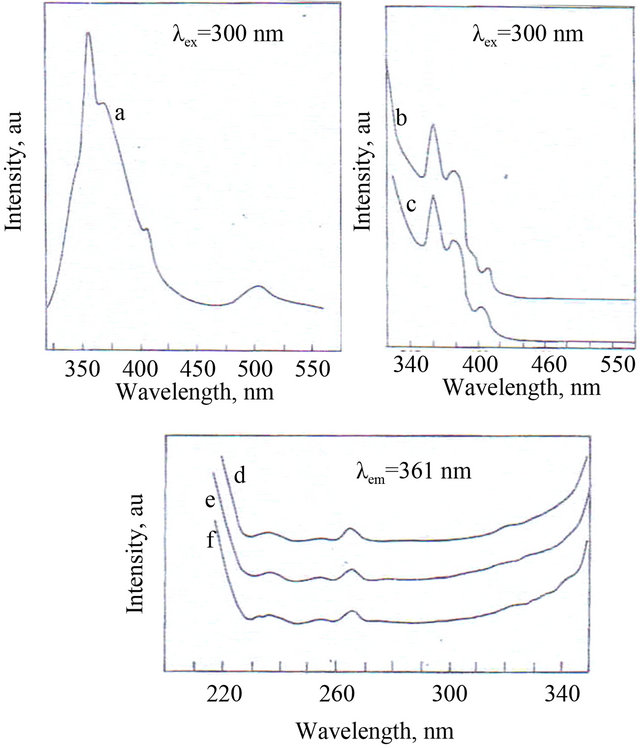
a) and d) Emission and excitation spectra of solid mass obtained by evaporating onion extract; b) and e) Emission and excitation spectra of solid Fe2+-AC complex prepared; c) and f) Emission and excitation spectra of film formed on surface of carbon steel specimen after immersion in ground water containing 3 mL onion extract and 50 ppm of Zn2+ + 50 ppm SPT.
Figure 3. Fluorescence spectra.
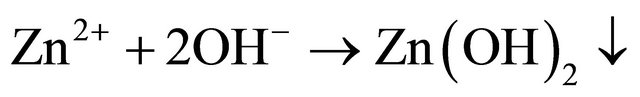
5) Thus protective film consists of Fe2+-SPT complex, Fe2+-AC complex and Zn (OH)2 [36].
5. Conclusions
The present study leads to the following conclusions:
Ø The formulation consisting of 3 mL allium cepa (onion) extract, 50 ppm of Zn2+ and 50 ppm of SPT offers 97% inhibition efficiency;
Ø The synergistic effect exists between onion-Zn2+-tartarate system;
Ø The addition of CTAB (a biocide) on onion-Zn2+-tartarate system does not change the excellent inhibition efficiency;
Ø Polarization study reveals that this formulation controls the cathodic reaction predominantly;
Ø AC impedance spectra reveal that a protective film is formed on the metal surface;
Ø The film is found to be UV-fluorescent.
6. Acknowledgements
The Authors are thankful to their respective management for their help and encouragement.
REFERENCES
- J. Dubey, N. Jeenger, R. K. Upadhyay and A. Chaturvedi, “Corrosion Inhibitive Effects of Withania Somnifera(A Medicinal Plant) on Aluminium in HCl Solution,” Research Journal of Recent Sciences, Vol. 1, 2012, pp. 73- 78.
- A. Singh, E. E. Ebenso and M. A. Quraishi, “Corrosion Inhibition of Carbon Steel in HCl Solution by Some Plant Extracts,” International Journal of Corrosion, Vol. 2012, 2011, pp. 1-20. doi:10.1155/2012/897430
- S. Ambrish and M. A. Quraishi, “Azwain (Trachyspermum Copticum) Seed Extract as an Efficient Corrosion Inhibitor for Aluminium in NaOH Solution,” Research Journal of Recent Sciences, Vol. 1, 2012, pp. 57- 61.
- A. I. Ali and N. Foaud, “Inhibition of Aluminium corRosion in Hydrochloric Acid Solution Using Black Mulberry Extract,” Journal of Material Environmental Science, Vol. 3, No. 5, 2012, pp. 917-924.
- H. Cang, Z. H. Fei, H. R. Xiao, J. L. Huang and Q. Xu, “Inhibition Effect of Reed Leaves Extract on Steel in Hydrochloric Acid and Sulphuric Acid Solutions,” International Journal of Electrochemical Sciences, Vol. 7, 2012, pp. 8869-8882.
- K. Rajam, S. Rajendran and R. Saranya, “Allium sativum(Garlic) Extract as Nontoxic Corrosion Inhibitor,” Journal of Chemistry, Vol. 2013, 2012, pp. 1-4. doi:10.1155/2013/743807
- K. Rajam, S. Rajendran, M. Manivannan and R. Saranya, “Corrosion Inhibition by Allium sativum(Garlic) Extract,” Journal of Chemical, Biological and Physical Sciences, Vol. 2, No. 3, 2012, pp. 1223-1233.
- R. M. Saleh, A. A. Ismail and A. H. El Hosary “Corrosion Inhibition by Naturally Occurring Substances. The Effect of Aqueous Extracts of Some Leaves and Fruit Peels on the Corrosion of Steel, Al, Zn and Cu in Acids,” British Corrosion Journal, Vol. 17, No. 3, 1982, pp. 131- 135.
- O. K. Abiola and A. O. James, “The Effects of Aloe vera Extract on Corrosion and Kinetics of Corrosion Process of Zinc in HCl Solution,” Corrosion Science, Vol. 52, No. 2, 2010, pp. 661-664. doi:10.1016/j.corsci.2009.10.026
- A. Y. El-Etre, “Inhibition of Acid Corrosion of Carbon Steel Using Aqueous Extract of Olive Leaves,” Journal of Colloid and Interface Science, Vol. 314, No. 2, 2007, pp. 578-583. doi:10.1016/j.jcis.2007.05.077
- P. C. Okafor and E. E. Ebenso, “Inhibitive Action of Carica papaya Extracts on the Corrosion of Mild Steel in Acidic Media and Their Adsorption Characteristics,” Pigment and Resin Technology, Vol. 36, No. 3, 2007, pp. 134- 140. doi:10.1108/03699420710748992
- P. C. Okafor, M. E. Ikpi, I. E. Uwah, E. E. Ebenso, U. J. Ekpe and S. A. Umoren, “Inhibitory Action of Phyllanthus amarus Extracts on the Corrosion of Mild Steel in Acidic Media,” Corrosion Science, Vol. 50, No. 8, 2008, pp. 2310-2317. doi:10.1016/j.corsci.2008.05.009
- P. C. Okafor, E. E. Ebenso and U. J. Ekpe, “Azadirachta indica Extracts as Corrosion Inhibitor for Mild Steel in Acid Medium,” International Journal of Electrochemical Science, Vol. 5, No. 7, 2010, pp. 978-993.
- I. B. Obot, S. A. Umoren and N. O. Obi-Egbedi, “Corrosion Inhibition and Adsorption Behavior for Aluminuim by Extract of Aningeria robusta in HCl Solution: Synergistic Effect of Iodide Ions,” Journal of Materials and Environmental Science, Vol. 2, No. 1, 2011, pp. 60-71.
- M. Lebrini, F. Robert and C. Roos, “Alkaloids Extract from Palicourea guianensis Plant as Corrosion Inhibitor for C38 Steel in 1 M Hydrochloric Acid Medium,” International Journal of Electrochemical Science, Vol. 6, No. 3, 2011, pp. 847-859.
- M. Lebrini, F. Robert, P. A. Blandinières and C. Roos, “Corrosion Inhibition by Isertia coccinea Plant Extract in Hydrochloric Acid Solution,” International Journal of Electrochemical Science, Vol. 6, No. 7, 2011, pp. 2443- 2460.
- A. O. James and O. Akaranta, “Inhibition of Corrosion of Zinc in Hydrochloric Acid Solution by Red Onion Skin Acetone Extract,” Research Journal of Chemical Sciences, Vol. 1, No. 1, 2011, pp. 31-37.
- P. C. Okafor, U. J. Ekpe, E. E. Ebenso, E. E. Oguzie, N. S. Umo and A. R. Etor, “Extract of Allium cepa and Allium sativum as Corrosion Inhibitors of Mild Steel in HCl Solution,” Transactions of the SAEST, Vol. 41, No. 2, 2006, pp. 82-87.
- O. James and O. Akaranta “The Inhibition of Corrosion of Zinc in 2. 0 M Hydrochloric Acid Solution with Acetone Extract of Red Onion Skin” African Journal of Pure and Applied Chemistry, Vol. 3, No. 11, 2009, pp. 212-217.
- P. C. Okafor, M. E. Ikpi, I. E. Uwah, E. E. Ebenso, U. J. Ekpe and S. A. Umoren, “Inhibitory Action of Phyllanthus amarus Extracts on the Corrosion of Mild Steel in Acidic Media,” Corrosion Science, Vol. 50, No. 8, 2008, pp. 2310-2317.
- J. C. da Rocha, J. A. da Cunha Ponciano Gomes and E. D’Elia, “Corrosion Inhibition of Carbon Steel in Hydrochloric Acid Solution by Fruit Peel Aqueous Exta,” Corrosion Science, Vol. 52, No. 7, 2010, pp 2341-2348. doi:10.1016/j.corsci.2010.03.033
- L. G. da Trindade and R. S. Goncalves, “Evidence of Caffeine Adsorption on a Low-Carbon Steel Surface in Ethanol,” Corrosion Science, Vol. 51, No. 8, 2009, pp 1578-1583. doi:10.1016/j.corsci.2009.03.038
- E. A. Noor, “Potential of Aqueous Extract of Hibiscus sabdariffa Leaves for Inhibiting the Corrosion of Aluminum in alkaline Solutions,” Journal of Applied Electrochemistry, Vol. 39, No. 9, 2009, pp. 1465-1475. doi:10.1007/s10800-009-9826-1
- G. Wranglen, “Introduction to Corrosion and Protection of Metals,” Chapman and Hall, London, 1985, p. 236. doi:10.1007/978-94-009-4850-1
- J. W. Sahayaraj, A. J. Amalraj, S. Rajendran and N. Vijaya, “Synergistic and Antogonistic Effect of Sodium Molybdate-Zn2+ System,” E-Journal of Chemistry, Vol. 9, No. 4, 2012, pp. 1746-1752.
- S. Rajendran, S. Mary Reenkala, N. Antony and R. Ramaraj, “Synergistic Corrosion Inhibition by the Sodium Dodecyl Sulphate-Zn2+ System,” Corrosion Science, Vol. 44, 2002, pp. 2243-2252. doi:10.1016/S0010-938X(02)00052-5
- J. W. Sahayaraj, A. J. Amalraj , S. Rajendran and T. S. Muthumegala, “Eco-Friendly Inhibitor L-Valine-Zn2+ System Controlling Corrosion of Carbon Steel in Rain Water,” Zastita Materijala, Vol. 51, No. 4, 2010, pp. 231- 236.
- N. Antony, H. B. Sherine and S. Rajendran, “Evaluation of Co-Inhibition Characteristics of Caffeine-Zn2+ System in Preventing Carbon Steel Corrosion,” International Journal of Engineering Science and Technology, Vol. 2, No. 7, 2010, pp. 2774-2782.
- V. Sribharathy and S. Rajendran, “Influence of Melonic Acid on the Corrosion Inhibition of Sodium Metavanadate in Chloride Medium,” Research Journal of Chemical Sciences, Vol. 2, No. 6, 2012, pp. 72-81.
- N. Antony, H. B. Sherine and S. Rajendran, “Investigation of the Inhibiting Effect of Carboxy Methyicellulose—Zn2+ System on the Corrosion of Carbon Steel in Neutral Chloride Solution,” The Arabian Journal of Science and Engineering, Vol. 35, No. 2A, 2010, pp. 41-53.
- T. H. Ibrahim, Y. Chehade and M. A. Zour, “Corrosion Inhibition of Mild Steel Using Potato Peel Extract in 2 M HCl Solution,” International Journal of Electrochemical Sciences, Vol. 6, 2011, pp. 6542-6556.
- G. Ji, S. K. Shukla, P. Dwivedi, S. Sundaram, E. E. Ebenso and R. Prakash, “Parthenium hysterophorus Plant Extract as in Efficient Green Corrosion Inhibitor for Mild Steel in Acidic Environment,” International Journal of Electrochemical Sciences, Vol. 7, No. 10, 2012, pp. 9933- 9945.
- K. Nakamoto, “Infrared and Raman Spectra of Inorganic and Coordination Compounds,” 4th Edition, Wiley and Sons, New York, 1986, p. 95.
- R. M. Silverstein, G. C. Bassler and T. C. Morril, “Spectrometric Identification of Organic Compounds,” John Wiley and Sons, New York, 1986, pp. 72-110.
- N. Antony, H. B. Sherine and S. Rajendran, “Inhibition and Biocide Actions of Sodium Dodecyl Sulfate-Zn2+ System for the Carbon Steel in Chloride Solution,” Portugaliae Electrochimica Acta, Vol. 28, No. 1, 2010, pp. 1-14. doi:10.4152/pea.201001001
- N. Manimaran, S. Rajendran, M. Manivannan and S. John Mary, “Corrosion Inhibition of Carbon Steel by Polyacrylamide,” Research Journal of Chemical Sciences, Vol. 2, No. 3, 2012, pp. 52-57.

